Laser Intensity Effect on Polyyne Synthesis in Liquid Hydrocarbons
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
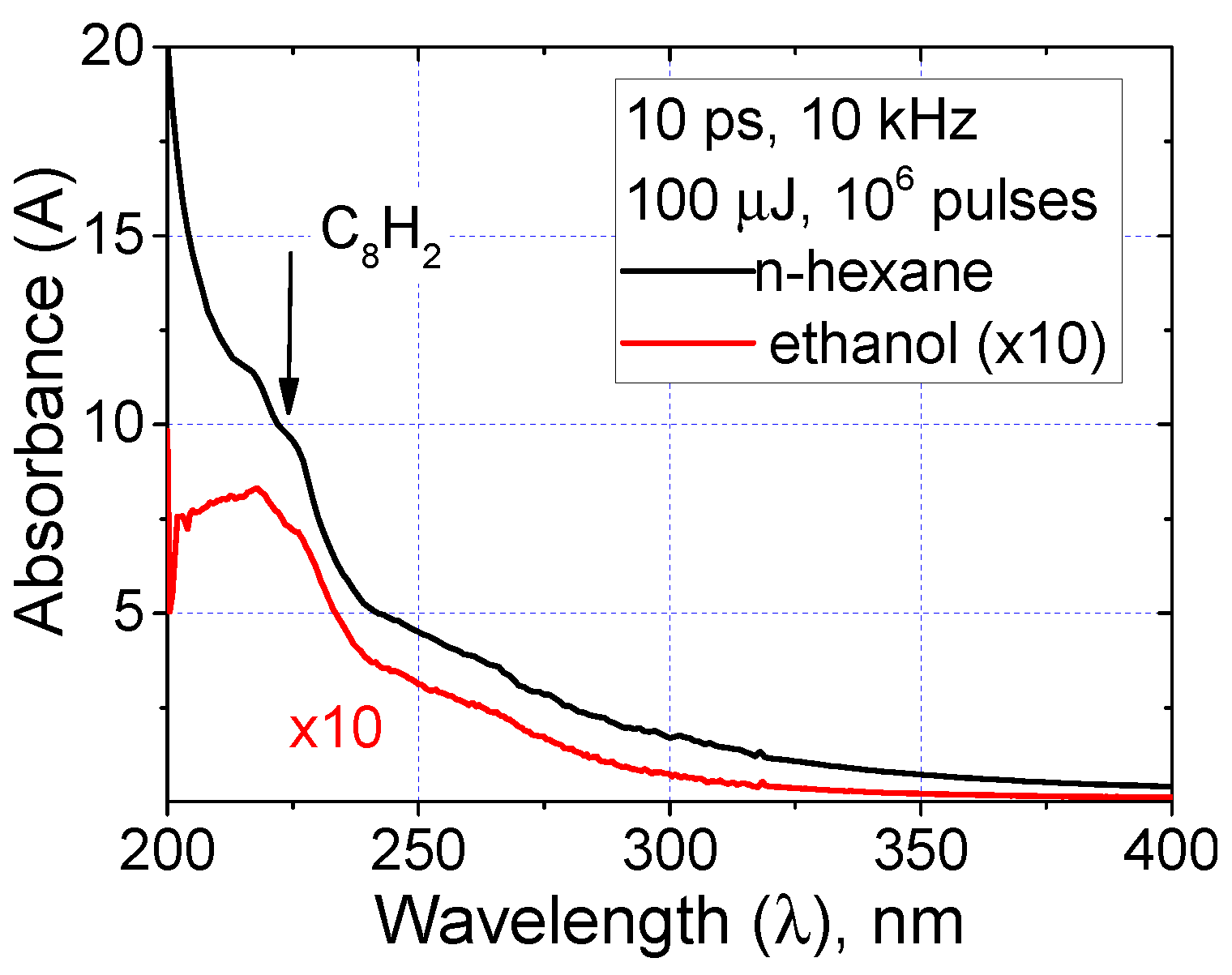
3.1. Polyyne Synthesis in Pure Hydrocarbons
3.2. Polyyne Synthesis in Graphitic Suspension
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Symbols and Abbreviations
| N | laser pulse number |
| E | laser pulse energy |
| A | optical absorbance |
| number of electron–hole pairs | |
| PLAL | pulsed laser ablation in liquid |
| LIB | laser-induced breakdown |
| LCC | linear carbon chain |
References
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Paul, F.; Lapinte, C. Organometallic molecular wires and other nanoscale-sized devices: An approach using the organoiron (dppe) Cp* Fe building block. Coord. Chem. Rev. 1998, 178, 431–509. [Google Scholar] [CrossRef]
- Bryce, M.R. A review of functional linear carbon chains (oligoynes, polyynes, cumulenes) and their applications as molecular wires in molecular electronics and optoelectronics. J. Mater. Chem. C 2021, 9, 10524–10546. [Google Scholar] [CrossRef]
- Eisler, S.; Slepkov, A.D.; Elliott, E.; Luu, T.; McDonald, R.; Hegmann, F.A.; Tykwinski, R.R. Polyynes as a model for carbyne: Synthesis, physical properties, and nonlinear optical response. J. Am. Chem. Soc. 2005, 127, 2666–2676. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.; Walton, D. Silylation as a protective method in acetylene chemistry: Polyyne chain extensions using the reagents, Et3Si (C-C) mH (m= 1, 2, 4) in mixed oxidative couplings. Tetrahedron 1972, 28, 5221–5236. [Google Scholar] [CrossRef]
- Januszewski, J.A.; Tykwinski, R.R. Synthesis and properties of long [n] cumulenes (n>5). Chem. Soc. Rev. 2014, 43, 3184–3203. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, F. Synthesis of polyynes in a submerged electric arc in organic solvents. Carbon 2004, 42, 129–142. [Google Scholar] [CrossRef]
- Heath, J.; Zhang, Q.; O’brien, S.; Curl, R.; Kroto, H.; Smalley, R. The formation of long carbon chain molecules during laser vaporization of graphite. J. Am. Chem. Soc. 1987, 109, 359–363. [Google Scholar] [CrossRef]
- Taguchi, Y.; Endo, H.; Kodama, T.; Achiba, Y.; Shiromaru, H.; Wakabayashi, T.; Wales, B.; Sanderson, J. Polyyne formation by ns and fs laser induced breakdown in hydrocarbon gas flow. Carbon 2017, 115, 169–174. [Google Scholar] [CrossRef]
- Tsuji, M.; Tsuji, T.; Kuboyama, S.; Yoon, S.H.; Korai, Y.; Tsujimoto, T.; Kubo, K.; Mori, A.; Mochida, I. Formation of hydrogen-capped polyynes by laser ablation of graphite particles suspended in solution. Chem. Phys. Lett. 2002, 355, 101–108. [Google Scholar] [CrossRef]
- Tsuji, M.; Kuboyama, S.; Matsuzaki, T.; Tsuji, T. Formation of hydrogen-capped polyynes by laser ablation of C60 particles suspended in solution. Carbon 2003, 41, 2141–2148. [Google Scholar] [CrossRef]
- Arutyunyan, N.R.; Fedotov, P.V.; Kononenko, V.V. Laser synthesis and stability of one-dimensional polyynic carbon chains in liquid media. J. Nanophotonics 2016, 10, 012519. [Google Scholar] [CrossRef]
- Sato, Y.; Kodama, T.; Shiromaru, H.; Sanderson, J.; Fujino, T.; Wada, Y.; Wakabayashi, T.; Achiba, Y. Synthesis of polyyne molecules from hexane by irradiation of intense femtosecond laser pulses. Carbon 2010, 48, 1673–1676. [Google Scholar] [CrossRef]
- Wesolowski, M.J.; Kuzmin, S.; Moores, B.; Wales, B.; Karimi, R.; Zaidi, A.A.; Leonenko, Z.; Sanderson, J.H.; Duley, W.W. Polyyne synthesis and amorphous carbon nano-particle formation by femtosecond irradiation of benzene. Carbon 2011, 49, 625–630. [Google Scholar] [CrossRef]
- Shin, S.K.; Song, J.K.; Park, S.M. Preparation of polyynes by laser ablation of graphite in aqueous media. Appl. Surf. Sci. 2011, 257, 5156–5158. [Google Scholar] [CrossRef]
- Peggiani, S.; Facibeni, A.; Marabotti, P.; Vidale, A.; Scotti, S.; Casari, C.S. A single liquid chromatography procedure to concentrate, separate and collect size-selected polyynes produced by pulsed laser ablation in water. Fullerenes Nanotub. Carbon Nanostructures 2023, 31, 224–230. [Google Scholar] [CrossRef]
- Yogesh, G.K.; Shukla, S.; Sastikumar, D.; Koinkar, P. Progress in pulsed laser ablation in liquid (PLAL) technique for the synthesis of carbon nanomaterials: A review. Appl. Phys. A 2021, 127, 810. [Google Scholar] [CrossRef]
- Marabotti, P.; Peggiani, S.; Vidale, A.; Casari, C.S. Pulsed laser ablation in liquid of sp-carbon chains: Status and recent advances. Chin. Phys. B 2022. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, Y.; Shi, L. A review of linear carbon chains. Chin. Chem. Lett. 2020, 31, 1746–1756. [Google Scholar] [CrossRef]
- Zaidi, A.; Hu, A.; Henneke, D.; Duley, W. Femtosecond laser irradiation of liquid alkanes: Mechanism of polyyne formation. Chem. Phys. Lett. 2019, 723, 151–154. [Google Scholar] [CrossRef]
- Matsutani, R.; Inoue, K.; Wada, N.; Kojima, K. Wavelength dependence of polyyne preparation by liquid-phase laser ablation using pellet targets. Chem. Commun. 2011, 47, 5840–5842. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.E.; Shin, S.K.; Park, S.M. The physical effects on the formation of polyynes by laser ablation. Chem. Phys. Lett. 2013, 568, 112–116. [Google Scholar] [CrossRef]
- Eastmond, R.; Johnson, T.; Walton, D. Silylation as a protective method for terminal alkynes in oxidative couplings: A general synthesis of the parent polyynes H (C-C) nH (n = 4–10, 12). Tetrahedron 1972, 28, 4601–4616. [Google Scholar] [CrossRef]
- Kononenko, V.V.; Gololobov, V.M.; Konov, V.I. Dynamics of optical polarizability of liquid water exposed to intense laser light. Opt. Lett. 2020, 45, 256–259. [Google Scholar] [CrossRef]
- Liu, W.; Petit, S.; Becker, A.; Aközbek, N.; Bowden, C.; Chin, S. Intensity clamping of a femtosecond laser pulse in condensed matter. Opt. Commun. 2002, 202, 189–197. [Google Scholar] [CrossRef]
- Meader, V.K.; John, M.G.; Rodrigues, C.J.; Tibbetts, K.M. Roles of Free Electrons and H2O2 in the Optical Breakdown-Induced Photochemical Reduction of Aqueous AuCl4. J. Phys. Chem. A 2017, 121, 6742–6754. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kononenko, V.V.; Arutyunyan, N.R.; Ashikkalieva, K.K.; Zavedeev, E.V.; Kononenko, T.V.; Akhlyustina, E.V.; Konov, V.I. Laser Intensity Effect on Polyyne Synthesis in Liquid Hydrocarbons. Photonics 2023, 10, 1100. https://doi.org/10.3390/photonics10101100
Kononenko VV, Arutyunyan NR, Ashikkalieva KK, Zavedeev EV, Kononenko TV, Akhlyustina EV, Konov VI. Laser Intensity Effect on Polyyne Synthesis in Liquid Hydrocarbons. Photonics. 2023; 10(10):1100. https://doi.org/10.3390/photonics10101100
Chicago/Turabian StyleKononenko, Vitali V., Natalia R. Arutyunyan, Kuralay K. Ashikkalieva, Evgeny V. Zavedeev, Taras V. Kononenko, Ekatherina V. Akhlyustina, and Vitaly I. Konov. 2023. "Laser Intensity Effect on Polyyne Synthesis in Liquid Hydrocarbons" Photonics 10, no. 10: 1100. https://doi.org/10.3390/photonics10101100
APA StyleKononenko, V. V., Arutyunyan, N. R., Ashikkalieva, K. K., Zavedeev, E. V., Kononenko, T. V., Akhlyustina, E. V., & Konov, V. I. (2023). Laser Intensity Effect on Polyyne Synthesis in Liquid Hydrocarbons. Photonics, 10(10), 1100. https://doi.org/10.3390/photonics10101100




