Studies of Interactions between Beta-Cyfluthrin and BSA Based on Fluorescence Spectrometry and Ultraviolet Degradation
Abstract
:1. Introduce
2. Experiment
2.1. Experimental Sample
2.2. Experimental Instrument
2.3. Experimental Process
- (1)
- Prediction model construction of the beta-cyfluthrin concentration
- (2)
- Ultraviolet degradation and water-bath heating of samples
- (3)
- Parameters calculation of the interaction between beta-cyfluthrin and BSA
3. Results and Analysis
3.1. Concentration Prediction of Beta-Cyfluthrin after UV Degradation
3.1.1. Content Prediction Modeling of Beta-Cyfluthrin
3.1.2. Equivalent Concentration of Beta-Cyfluthrin after UV Degradation
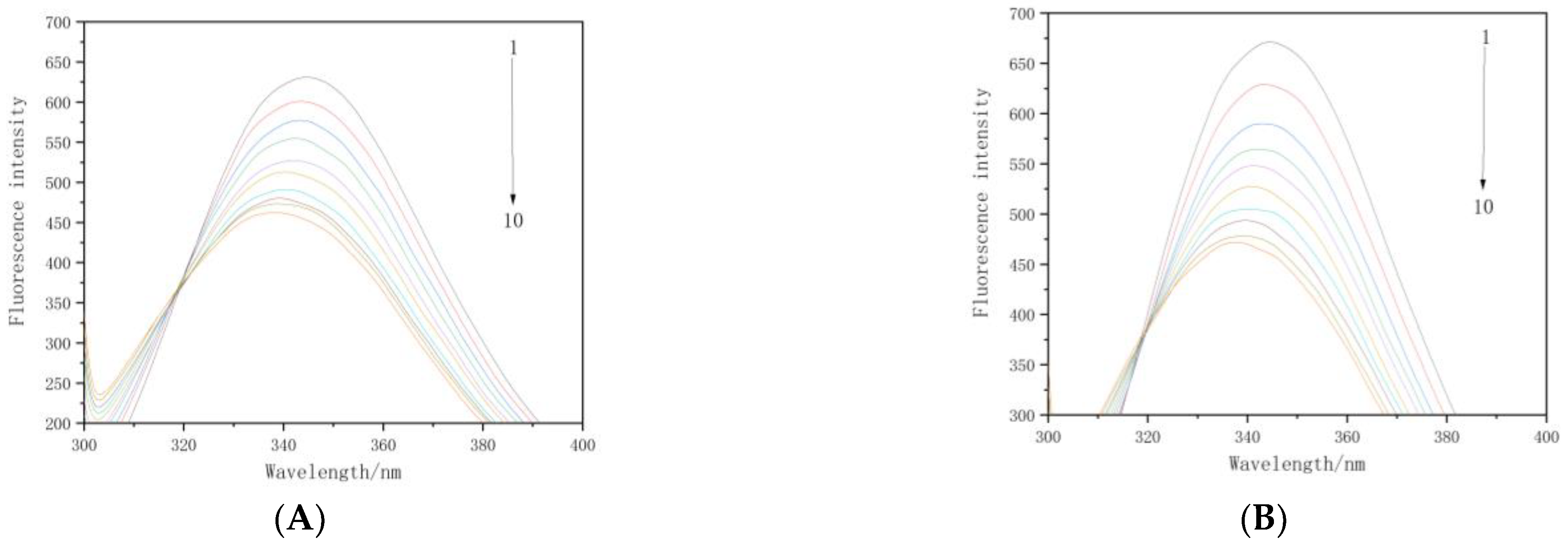
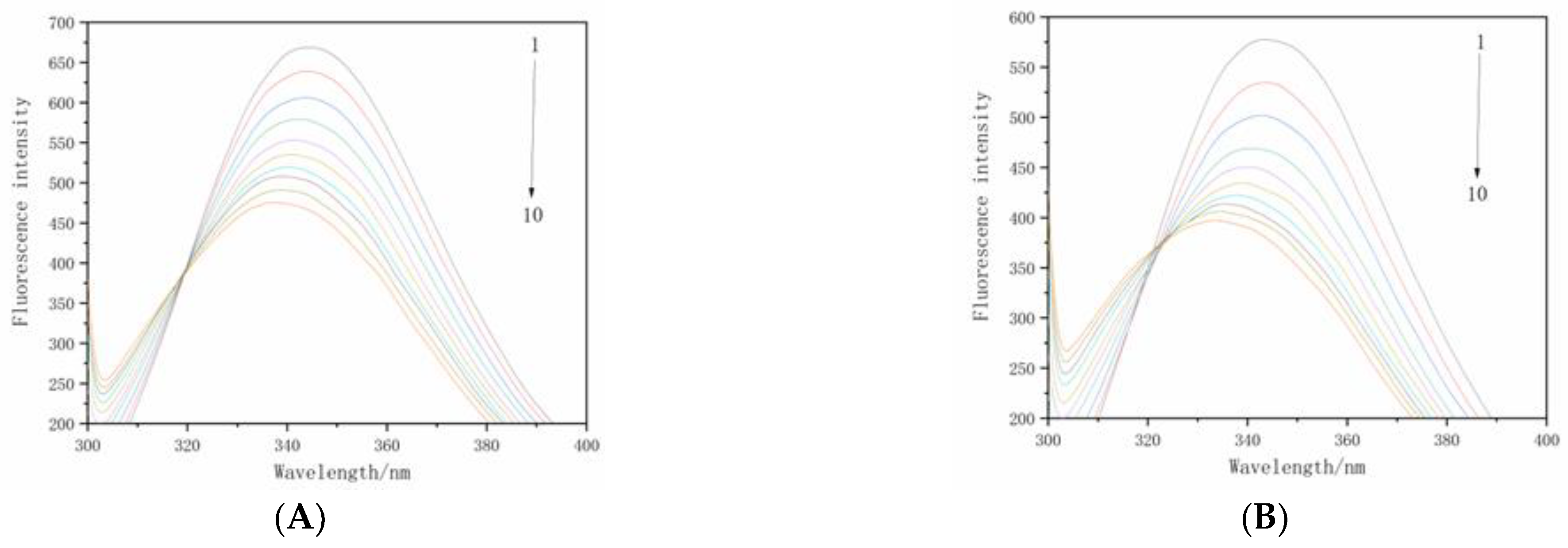
3.2. Fluorescence Quenching of Beta-Cyfluthrin on BSA
3.3. Binding Constants and Number of Binding Sites for the Interaction between Beta-Cyfluthrin and BSA
3.4. Thermodynamic Parameters and Force Types of Interaction between Beta-Cyfluthrin and BSA
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, S.Q.; Yu, F.L.; Wang, X. Determination of Beta-cyfluthrin Residue in Pear and Soil by Gas Chromatography. Pestic. Sci. Adm. 2014, 35, 29–32. (In Chinese) [Google Scholar]
- Rodríguez, J.; Ares, I.; Martínez, M.; Martínez, M.; Anadón, A.; Martínez, M. Bioavailability and nervous tissue distribution of pyrethroid insecticide cyfluthrin in rats. Food Chem. Toxicol. 2018, 118, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Sinha, C.; Agrawal, A.K.; Islam, F.; Seth, K.; Chaturvedi, R.K.; Shukla, S.; Seth, P.K. Mosquito repellent (pyrethroid-based) induced dysfunction of blood–brain barrier permeability in developing brain. Int. J. Dev. Neurosci. 2003, 22, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.Z.; Zhou, D.S.; Wang, X.J.; Cao, J.W.; Zhou, L.; Peng, Y.X.; Ju, D.C. Preparation of porous adsorbent and separation of beta-cyfluthrin. J. Jiangsu Univ. Sci. Technol. (Nat. Sci. Ed.) 2019, 33, 110–117. (In Chinese) [Google Scholar]
- Tian, Z.Y.; Ding, T.L.; Niu, H.J.; Wang, T.; Zhang, Z.Z.; Gao, J.H.; Kong, M.; Ming, L.; Tian, Z.H.; Ma, J.; et al. 2-Phenylquinoline-polyamine conjugate (QPC): Interaction with bovine serum albumin (BSA). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 300, 122875. [Google Scholar] [CrossRef]
- Qi, H.Y.; Wang, Y.; Wang, X.W.; Su, L.Q.; Wang, Y.; Wang, S. The different interactions of two anticancer drugs with bovine serum albumin based on multi-spectrum method combined with molecular dynamics simulations. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 259, 119809. [Google Scholar] [CrossRef]
- Sharma, S.; Takkella, D.; Kumar, P.; Gavvala, K. Spectroscopic analysis to identify the binding site for Rifampicin on Bovine Serum Albumin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 283, 121721. [Google Scholar] [CrossRef]
- Dahiya, V.; Chaubey, B.; Chaubey, A.K.; Pal, S. Solvent-dependent binding interactions of the organophosphate pesticide, chlorpyrifos (CPF), and its metabolite, 3,5,6-trichloro-2-pyridinol (TCPy), with Bovine Serum Albumin (BSA): A comparative fluorescence quenching analysis. Pestic. Biochem. Physiol. 2017, 139, 92–100. [Google Scholar] [CrossRef]
- Yuan, S.F.; Yang, F.W.; Yu, H.; Xie, Y.F.; Guo, Y.H.; Yao, W.R. Degradation mechanism and toxicity assessment of chlorpyrifos in milk by combined ultrasound and ultraviolet treatment. Food Chem. 2022, 383, 132550. [Google Scholar] [CrossRef]
- Ma, Y.N.; Wang, Z.Y.; Yang, W.Y.; Chen, C.Y.; Li, J.F.; He, R.N.; Liu, S.L. Insights into the radical and nonradical oxidation degradation of ciprofloxacin in peroxodisulfate activation by ultraviolet light. J. Water Process Eng. 2022, 49, 103184. [Google Scholar] [CrossRef]
- Ayare, S.D.; Gogate, P.R. Degradation of Tricyclazole fungicide using combined oxidation strategies based on ultrasound, ultraviolet irradiation and microwave. Environ. Technol. Innov. 2022, 26, 102533. [Google Scholar] [CrossRef]
- Wei, X.L.; Xiao, J.B.; Wang, Y.; Bai, Y. Which model based on fluorescence quenching is suitable to study the interaction between trans-resveratrol and BSA? Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2010, 75, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Hu, Y.X.; Li, Y.C.; Zhang, L.; Ai, H.X.; Liu, H.S.; Liu, Y.F.; Sang, Y.L. Study on the interaction of tussilagone with human serum albumin (HSA) by spectroscopic and molecular docking techniques. J. Mol. Struct. 2017, 1149, 645–654. [Google Scholar] [CrossRef]
- Ben Salem, I.; Errami, M.; Mezni, M.; Salghi, R.; Ebenso, E.E.; Hammouti, B.; Fattouch, S.; Raboudi, F. Biological, Ionizing and Ultraviolet Radiation and Electrochemical Degradation of Chlorpyrifos Pesticide in Aqueous Solutions. Int. J. Electrochem. Sci. 2014, 9, 342–351. [Google Scholar] [CrossRef]
- Zhang, G.W.; Ma, Y.D.; Wang, L.; Zhang, Y.P.; Zhou, J. Multispectroscopic studies on the interaction of maltol, a food additive, with bovine serum albumin. Food Chem. 2012, 133, 264–270. [Google Scholar] [CrossRef]
- Peng, X.; Wang, X.C.; Qi, W.; Su, R.X.; He, Z.M. Affinity of rosmarinic acid to human serum albumin and its effect on protein conformation stability. Food Chem. 2016, 192, 178–187. [Google Scholar] [CrossRef]
- Sinisi, V.; Forzato, C.; Cefarin, N.; Navarini, L.; Berti, F. Interaction of chlorogenic acids and quinides from coffee with human serum albumin. Food Chem. 2015, 168, 332–340. [Google Scholar] [CrossRef]
- Shahabadi, N.; Hadidi, S.; Abdoli, Z.; Mardani, Z. Interaction of a cobalt(III) complex containing β-amino alcohol with human serum albumin (HSA): Spectroscopic and molecular docking methods. J. Mol. Liq. 2023, 384, 122187. [Google Scholar] [CrossRef]
- Bagheri, M.; Fatemi, M.H. Fluorescence spectroscopy, molecular docking and molecular dynamic simulation studies of HSA-Aflatoxin B1 and G1 interactions. J. Lumin. 2018, 202, 345–353. [Google Scholar] [CrossRef]
- Paz, E.R.S.; Isoppo, V.G.; Isoppo, F.S.; Machado, L.A.; de Freitas, R.P.; Silva Junior, H.C.; Chaves, O.A.; Iglesias, B.A.; Rodembusch, F.S.; da Silva Júnior, E.N. Imidazole-based optical sensors as a platform for bisulfite sensing and BSA/HSA interaction study. An experimental and theoretical investigation. J. Mol. Liq. 2023, 387, 122666. [Google Scholar] [CrossRef]
- Wang, X.X.; Wu, H.; Nie, Z.H.; MA, L.T.; Cui, J.L.; Sai, H.Z.; Cheng, J.G. Study on the interaction between fulvic acid and bovine serum albumin by multispectral and molecular docking. Spectrosc. Spectr. Anal. 2021, 41, 2904–2910. (In Chinese) [Google Scholar]
- Liu, Q.Q.; Zhu, K.X.; Guo, X.N.; Peng, W.; Zhou, H.M. Spectroscopic analysis of interaction between tea polyphenol and soy protein isolate. Food Sci. 2015, 36, 43–47. (In Chinese) [Google Scholar]
- Vidhyapriya, P.; Divya, D.; Manimaran, B.; Sakthivel, N. Molecular interaction of manganese based carbon monoxide releasing molecule (MnCORM) with human serum albumin (HSA). Bioorganic Chem. 2019, 92, 103078. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yang, H.T.; Hu, R.X.; Liang, Y.H.; Li, Y.C.; Xu, W.L.; Fan, X.Y.; Liu, Y.F. Comparing the interaction of four structurally similar coumarins from Fraxinus Chinensis Roxb. with HSA through multi-spectroscopic and docking studies. J. Mol. Liq. 2021, 340, 117234. [Google Scholar] [CrossRef]
- Sun, Q.M.; Yang, H.Q.; Tang, P.X.; Liu, J.Y.; Wang, W.; Li, H. Interactions of cinnamaldehyde and its metabolite cinnamic acid with human serum albumin and interference of other food additives. Food Chem. 2018, 243, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Shinde, M.; Kale, K.; Yadav, P.; Suryawanshi, M.; Ottoor, D. Influence of beta cyclodextrin on amlodipine-BSA interaction: A spectroscopic and molecular docking study. Chem. Data Collect. 2022, 42, 100945. [Google Scholar] [CrossRef]
- Lakshmi, T.P.; Mondal, M.; Ramadas, K.; Natarajan, S. Molecular interaction of 2,4-diacetylphloroglucinol (DAPG) with human serum albumin (HSA): The spectroscopic, calorimetric and computational investigation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 183, 90–102. [Google Scholar]
- Mu, H.T.; Chen, S.H.; Liu, F.Y.; Xiao, J.B.; Huang, H.; Zhang, Y.H.; Sun, Y.M.; Gao, X.Y.; Lei, H.T.; Yuan, X.W. Stereoselective interactions of lactic acid enantiomers with HSA: Spectroscopy and docking application. Food Chem. 2019, 270, 429–435. [Google Scholar] [CrossRef]



| The Actual Concentration of Beta-Cyfluthrin (mol/L) | The Predicted Concentration of Beta-Cyfluthrin (mol/L) | Recovery Rate (%) |
|---|---|---|
| 1.4380 × 10−4 | 1.4466 × 10−4 | 100.6 |
| 1.2327 × 10−4 | 1.2349 × 10−4 | 100.18 |
| 1.1506 × 10−4 | 1.1484 × 10−4 | 99.81 |
| 1.0460 × 10−4 | 1.0264 × 10−4 | 98.13 |
| 9.5883 × 10−5 | 9.5347 × 10−5 | 99.44 |
| 8.5230 × 10−5 | 8.5747 × 10−5 | 100.61 |
| 7.4232 × 10−5 | 7.4721 × 10−5 | 100.66 |
| 5.7102 × 10−5 | 5.732 × 10−5 | 100.38 |
| 4.3666 × 10−5 | 4.4049 × 10−5 | 100.88 |
| 3.0930 × 10−5 | 3.0966 × 10−5 | 100.12 |
| The Concentration of Beta-Cyfluthrin before Degradation (mol/L) | The Concentration of Beta-Cyfluthrin after Degradation for 10 min (mol/L) | The Concentration of Beta-Cyfluthrin after Degradation for 20 min (mol/L) |
|---|---|---|
| 0 | 0 | 0 |
| 3.56 × 10−6 | 3.41 × 10−6 | 2.34 × 10−6 |
| 7.16 × 10−6 | 6.87 × 10−6 | 6.72 × 10−6 |
| 10.80 × 10−6 | 10.35 × 10−6 | 10.14 × 10−6 |
| 13.57 × 10−6 | 13.02 × 10−6 | 12.75 × 10−6 |
| 16.42 × 10−6 | 15.47 × 10−6 | 15.41 × 10−6 |
| 19.33 × 10−6 | 18.53 × 10−6 | 18.15 × 10−6 |
| 21.33 × 10−6 | 20.46 × 10−6 | 20.03 × 10−6 |
| 23.42 × 10−6 | 22.46 × 10−6 | 22.00 × 10−6 |
| 25.60 × 10−6 | 24.55 × 10−6 | 24.04 × 10−6 |
| Temperature (K) | Degradation Time (min) | Ksv (L∗mol−1) | Kq (L∗mol−1∗s−1) | R |
|---|---|---|---|---|
| 303 K | 0 | 1.36 × 104 | 1.36 × 1012 | 0.996 |
| 10 | 1.52 × 104 | 1.52 × 1012 | 0.998 | |
| 20 | 1.64 × 104 | 1.64 × 1012 | 0.998 | |
| 309 K | 0 | 1.56 × 104 | 1.56 × 1012 | 0.997 |
| 10 | 1.72 × 104 | 1.72 × 1012 | 0.998 | |
| 20 | 1.84 × 104 | 1.84 × 1012 | 0.993 |
| Temperature (K) | Degradation Time (min) | KA (L∗mol−1) | N | R |
|---|---|---|---|---|
| 303 K | 0 | 1.41 × 103 | 0.785 | 0.995 |
| 10 | 2.29 × 104 | 1.04 | 0.997 | |
| 20 | 8.63 × 103 | 0.943 | 0.995 | |
| 309 K | 0 | 4.00 × 103 | 0.869 | 0.997 |
| 10 | 8.22 × 103 | 0.93 | 0.998 | |
| 20 | 1.59 × 103 | 0.768 | 0.995 |
| Degradation Time (min) | Temperature (K) | ∆G (KJ/mol) | ∆H (KJ/mol) | ∆S (J/(mol∗K) |
|---|---|---|---|---|
| 0 | 303 | −18.27 | 135.45 | 507.33 |
| 309 | −21.31 | 507.31 | ||
| 10 | 303 | −25.29 | −132.97 | −355.38 |
| 309 | −23.16 | −355.38 | ||
| 20 | 303 | −22.83 | −219.92 | −650.46 |
| 309 | −22.08 | −650.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Wang, X.; Ji, R.; Bian, H.; Guo, X.; He, Y.; Chen, H.; Abdalla, A.N. Studies of Interactions between Beta-Cyfluthrin and BSA Based on Fluorescence Spectrometry and Ultraviolet Degradation. Photonics 2023, 10, 1079. https://doi.org/10.3390/photonics10101079
Wang X, Wang X, Ji R, Bian H, Guo X, He Y, Chen H, Abdalla AN. Studies of Interactions between Beta-Cyfluthrin and BSA Based on Fluorescence Spectrometry and Ultraviolet Degradation. Photonics. 2023; 10(10):1079. https://doi.org/10.3390/photonics10101079
Chicago/Turabian StyleWang, Xiaoyan, Xuyang Wang, Rendong Ji, Haiyi Bian, Xinyue Guo, Ying He, Huichang Chen, and Ahmed N. Abdalla. 2023. "Studies of Interactions between Beta-Cyfluthrin and BSA Based on Fluorescence Spectrometry and Ultraviolet Degradation" Photonics 10, no. 10: 1079. https://doi.org/10.3390/photonics10101079
APA StyleWang, X., Wang, X., Ji, R., Bian, H., Guo, X., He, Y., Chen, H., & Abdalla, A. N. (2023). Studies of Interactions between Beta-Cyfluthrin and BSA Based on Fluorescence Spectrometry and Ultraviolet Degradation. Photonics, 10(10), 1079. https://doi.org/10.3390/photonics10101079





