Photonic Crystal-Based Water Concentration Estimation in Blood Using Machine Learning for Identification of the Haematological Disorder
Abstract
1. Introduction
2. Numerical Analysis
3. Design Structure
4. Result and Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Malekshahi, M. Evolution of intense laser pulse spot size propagating in collisional plasma embedded in a magnetic field with variable direction. Phys. Plasmas 2017, 25, 012302. [Google Scholar] [CrossRef]
- Naumenko, E.K. Back-scattered radiation from the capillary network under the influence of low-power laser heating (Invited Paper). In Saratov Fall Meeting 2004: Optical Technologies in Biophysics and Medicine VI; SPIE: Bellingham, WA, USA, 2005; pp. 195–201. [Google Scholar]
- Lijnema, T.H.; Huizenga, J.R.; Jager, J.; Mackor, A.J.; Gips, C.H. Gravimetric determination of the water concentration in whole blood, plasma and erythrocytes and correlations with hematological and clinic chemical parameters. Clin. Chim. Acta 1993, 214, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Kim, S.H.; Ryu, J.K. Changes in the Blood Components Caused by Water Intake. Korean J. Clin. Lab. Sci. 2007, 49, 227–232. [Google Scholar] [CrossRef]
- Rodak, B.F.; Frisma, G.A.; Keohane, E.M. Hematology: Clinical Principles and Applications, 4th ed.; Elesvier: Amsterdam, The Netherlands, 2012; pp. 1–6. [Google Scholar]
- Coban, E.; Ozdogan, M.; Yazicioglu, G.; Akcit, F. The mean platelet volume in patients with obesity. Int. J. Clin. Pract. 2005, 59, 981–982. [Google Scholar] [CrossRef]
- Elblbesy, M.A. The refractive index of human blood measured at the visible spectral region by single-fiber reflectance spectroscopy. AIMS Biophys. 2021, 8, 57–65. [Google Scholar] [CrossRef]
- Meinke, M.C.; Müller, G.J.; Helfmann, J.; Friebel, M. Optical properties of platelets and blood plasma and their influence on the optical behavior of whole blood in the visible to near-infrared wavelength range. J. Biomed. Opt. 2007, 12, 014024. [Google Scholar] [CrossRef]
- Liu, S.; Deng, Z.; Li, J.; Wang, J.; Huang, N.; Cui, R.; Zhang, Q.; Mei, J.; Zhou, W.; Zhang, C.; et al. Measurement of the refractive index of whole blood and its components for a continuous spectral region. J. Biomed. Opt. 2019, 24, 1–5. [Google Scholar] [CrossRef]
- Gienger, J.; Smuda, K.; Müller, R.; Bär, M.; Neukammer, J. Refractive index of human red blood cells between 290 nm and 1100 nm determined by optical extinction measurements. Sci. Rep. 2019, 9, 4623. [Google Scholar] [CrossRef]
- Jin, Y.L.; Chen, J.Y.; Xu, L.; Wang, P.N. Refractive index measurement for biomaterial samples by total internal reflection. Phys. Med. Biol. 2006, 51, N371–N379. [Google Scholar] [CrossRef]
- Friebel, M.; Meinke, M.C. Determination of the complex refractive index of highly concentrated hemoglobin solutions using transmittance and reflectance measurements. J. Biomed. Opt. 2005, 10, 064019. [Google Scholar] [CrossRef]
- Heller, W. Remarks on refractive index mixture rules. J. Phys. Chem. 1965, 69, 1123–1129. [Google Scholar] [CrossRef]
- Tuchin, V.V. Handbook of Optical Biomedical Diagnostics: Methods; Society of Photo-Optical Instrumentation Engineers (SPIE): Bellingham, WA, USA, 2016. [Google Scholar]
- Kohl, M.; Cope, M.; Essenpreis, M.; BÖCker, D. Influence of glucose concentration on light scattering in tissue-simulating phantoms. Opt. Lett. 1994, 19, 2170–2172. [Google Scholar] [CrossRef] [PubMed]
- Oduncuoğlu, M. Refractive Index Formula of Blood as a Function of Temperature and Concentration. An. Acad. Bras. Ciênc. 2021, 93, e20201634. [Google Scholar] [CrossRef] [PubMed]
- Yablonovitch, E. Inhibited spontaneous emission in solid-state physics and electronics. Phys. Rev. Lett. 1987, 58, 2059. [Google Scholar] [CrossRef] [PubMed]
- John, S. Strong localization of photons in certain disordered dielectric superlattices. Phys. Rev. Lett. 1987, 58, 2486–2489. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Wu, X.; Xiao, S.; Liu, G.; Li, H. Broadband wide-angle multilayer absorber based on a broadband omnidirectional optical Tamm state. Opt. Express. 2021, 29, 23976–23987. [Google Scholar] [CrossRef]
- Nair, R.V.; Vijaya, R. Photonic crystal sensors: An overview. Prog. Quantum Electron. 2010, 34, 89–134. [Google Scholar] [CrossRef]
- Scherer, A.; Painter, O.; Vuckovic, J.; Loncar, M.; Yoshie, T. Photonic crystals for confining, guiding, and emitting light. IEEE Trans. Nanotechnol. 2002, 1, 4–11. [Google Scholar] [CrossRef]
- Biswas, U.; Rakshit, J.K.; Bharti, G.K. Design of photonic crystal microring resonator based all-optical refractive-index sensor for analyzing different milk constituents. Opt. Quantum Electron. 2020, 52, 19. [Google Scholar] [CrossRef]
- Joannopoulos, J.D.; Villeneuve, P.R.; Fan, S. Photonic crystals: Putting a new twist on light. Nature 1997, 386, 143–149. [Google Scholar] [CrossRef]
- Joannopoulos, J.D.; Johnson, S.G.; Winn, J.N.; Meade, R.D. Photonic Crystals: Molding the Flow of Light, 2nd ed.; Princeton University Press: Princeton, NJ, USA, 2008. [Google Scholar]
- Troia, B.; Paolicelli, A.; Leonardis, F.D.; Passaro, V.M.N. Photonic Crystals for Optical Sensing: A Review. Adv. Photonic Cryst. 2013, 241–295. [Google Scholar] [CrossRef]
- Agarwal, A.; Sahu, S.; Mudgal, N.; Singh, G.; Bhatnagar, S.K. Photonic Crystal Cavities based Biosensors: A Review. In Proceedings of the 2020 International Conference on Emerging Trends in Communication, Control and Computing (ICONC3), Lakshmangarh, India, 21–22 February 2020; pp. 1–5. [Google Scholar]
- Agarwal, A.; Mudgal, N.; Sahu, S.; Singh, G.; Bhatnagar, S.K. Design of a Nanocavity Photonic Crystal Structure for Biosensing Application. Opt. Wirel. Technol. Proc. OWT 2021, 771, 321–330. [Google Scholar]
- Agarwal, A.; Mudgal, N.; Saharia, A.; Singh, G.; Bhatnagar, S.K. Silicon nitride based photonic biosensor for analyzing blood diseases. Mater. Today Proc. 2022, 66, 3507–3510. [Google Scholar] [CrossRef]
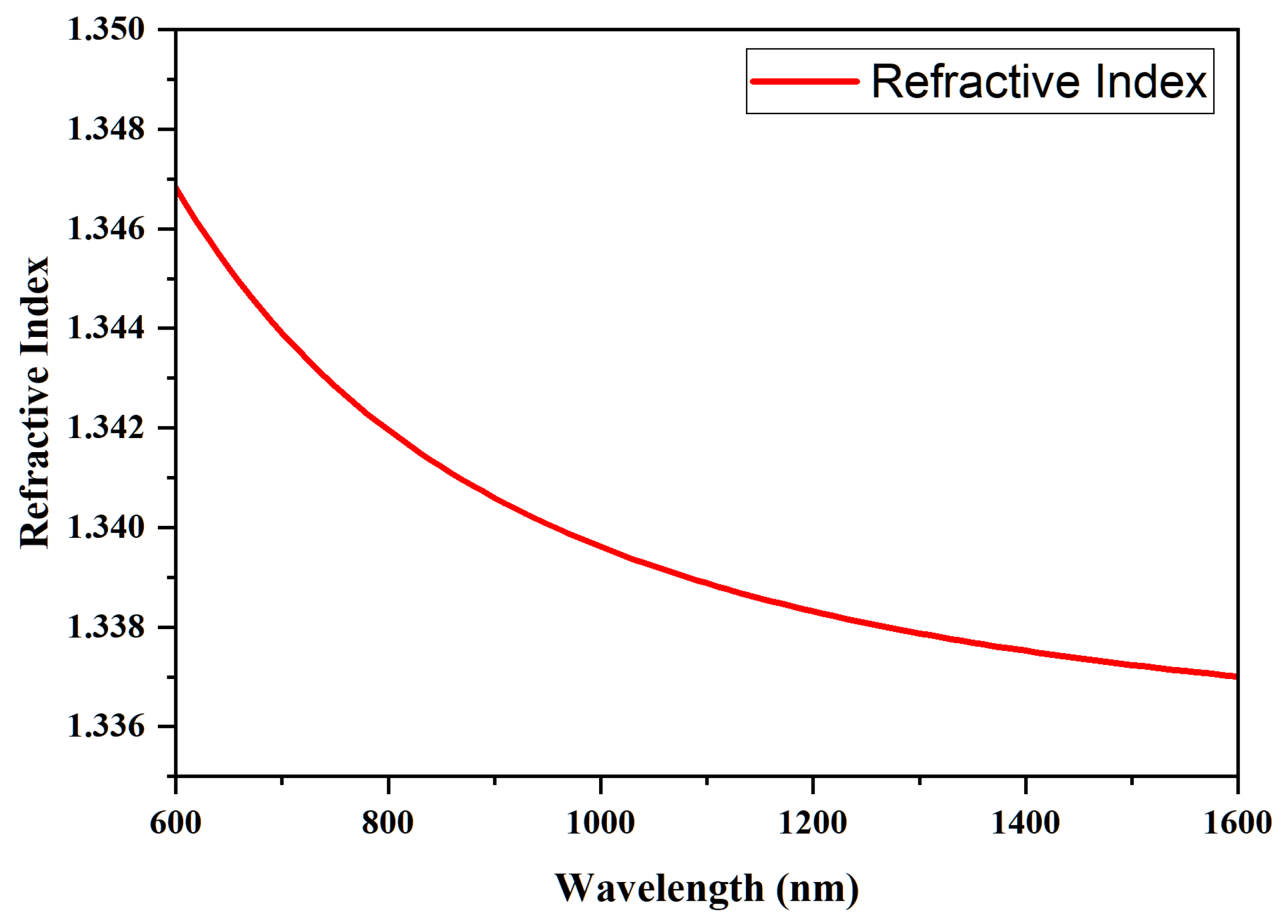



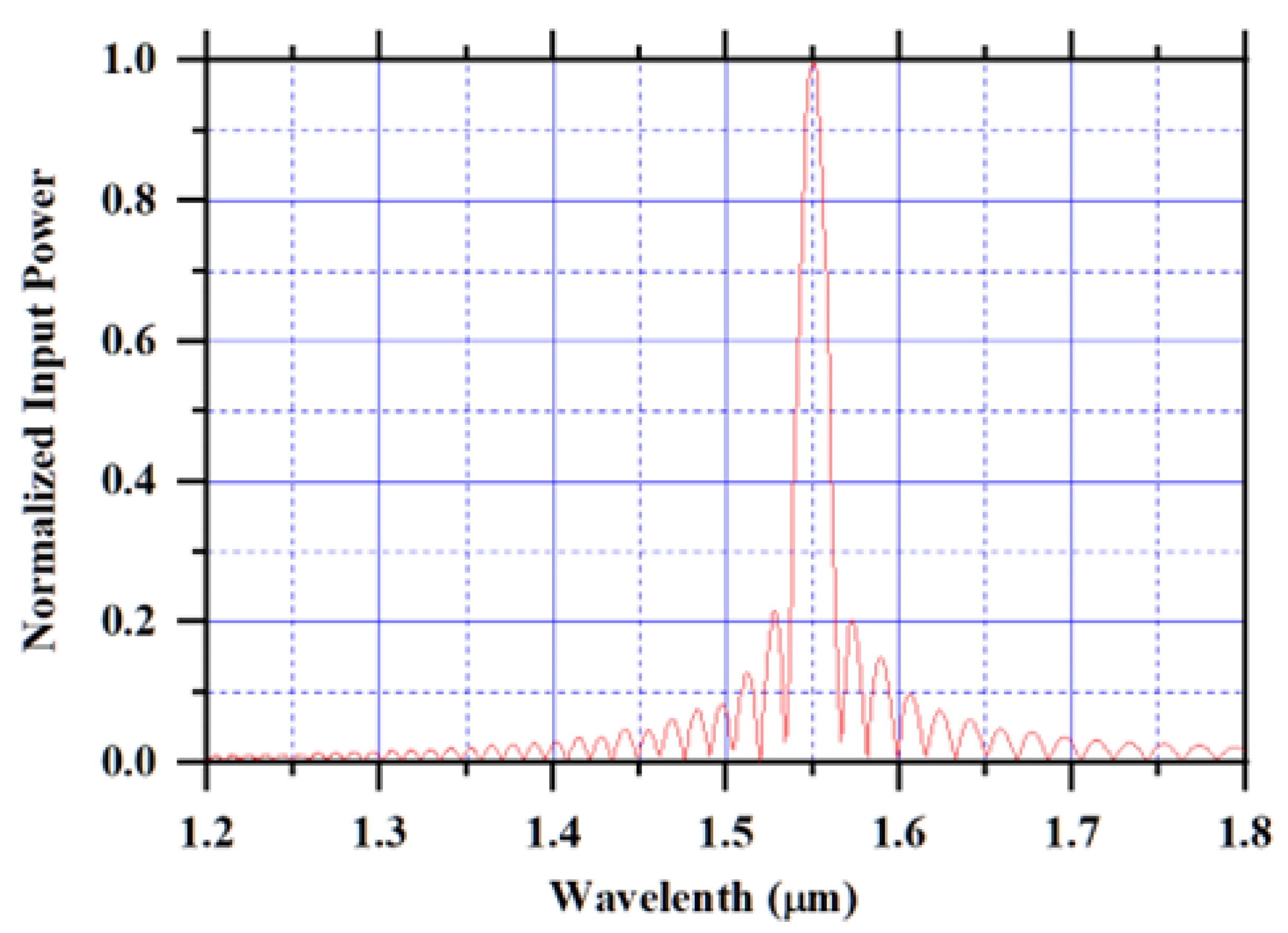
| Hematologic Parameters | Less Water Concentration | Normal Water Concentration |
|---|---|---|
| WBC (L) | 6.975 | 6.63 |
| RBC (L) | 4.792 | 4.78 |
| Hgb (g/dL) | 12.238 | 10.9 |
| MCV (fL) | 87.63 | 82.67 |
| MCH (pg) | 26.54 | 24.67 |
| MCHC (g/dL) | 30.1 | 29.067 |
| MPV (fL) | 10.95 | 11.6 |
| Design Parameter | Parameter Value |
|---|---|
| Substrate material | Silicon (Si) |
| Refractive index of the substrate | 3.45 |
| Refractive index of sensing cavity | Refractive index of plasma |
| Lattice constant | 0.48 μm |
| Radius of air holes | 0.14 μm |
| Radius of point defect a | 0.20 μm |
| Radius of point defect b | 0.08 μm |
| Radius of point defect c | 0.05 μm |
| Lattice constant for point defect c | 0.405 μm |
| Photonic band gap region | 0.305–0.446 |
| 0.686–0.723 |
| f (Water) | (Water) | f (Albumin) | (Albumin) for 55 g/L | (pl) |
|---|---|---|---|---|
| 90% | 1.33 | 10% | 1.3387 | 1.3309 |
| 89% | 1.33 | 10% | 1.3387 | 1.3243 |
| 88% | 1.33 | 10% | 1.3387 | 1.3177 |
| 87% | 1.33 | 10% | 1.3387 | 1.3110 |
| 86% | 1.33 | 10% | 1.3387 | 1.3044 |
| 85% | 1.33 | 10% | 1.3387 | 1.2978 |
| 84% | 1.33 | 10% | 1.3387 | 1.2912 |
| 83% | 1.33 | 10% | 1.3387 | 1.2846 |
| 82% | 1.33 | 10% | 1.3387 | 1.2780 |
| 81% | 1.33 | 10% | 1.3387 | 1.2714 |
| Water Percentage | f (RBC) | (RBC) | f (Plasma) | (Plasma) | (Blood) |
|---|---|---|---|---|---|
| 90% | 45% | 1.33 | 55% | 1.3309 | 1.3305 |
| 89% | 45% | 1.33 | 55% | 1.3243 | 1.3267 |
| 88% | 45% | 1.33 | 55% | 1.3177 | 1.3232 |
| 87% | 45% | 1.33 | 55% | 1.311 | 1.3196 |
| 86% | 45% | 1.33 | 55% | 1.3044 | 1.3159 |
| 85% | 45% | 1.33 | 55% | 1.2978 | 1.3123 |
| 84% | 45% | 1.33 | 55% | 1.2912 | 1.3087 |
| 83% | 45% | 1.33 | 55% | 1.2846 | 1.3050 |
| 82% | 45% | 1.33 | 55% | 1.278 | 1.3014 |
| 81% | 45% | 1.33 | 55% | 1.2714 | 1.2978 |
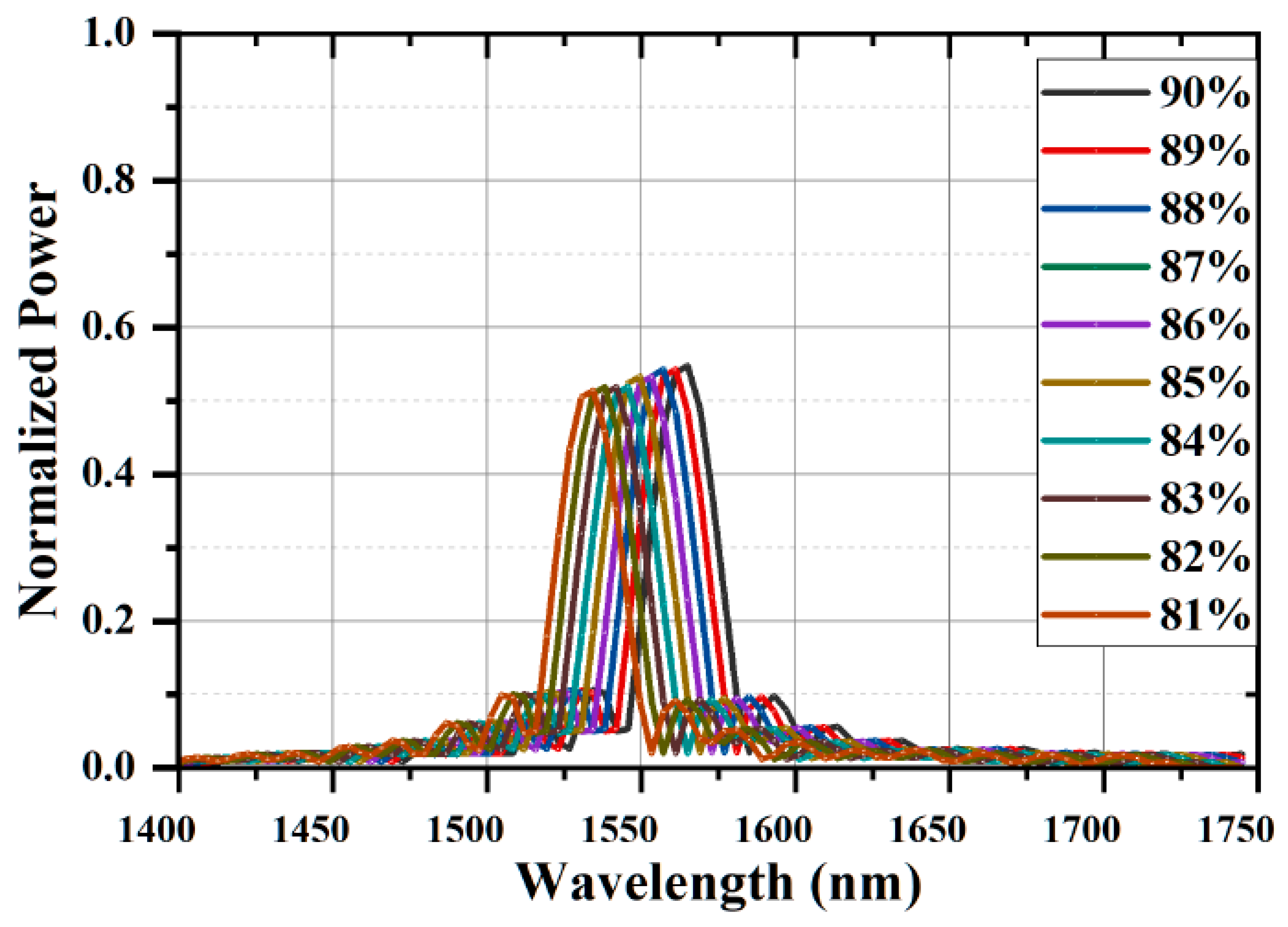
| Water Concentration | Refractive Index of Plasma (pl) | Position of Resonant Peak (nm) | Sensitivity (nm/RIU) |
|---|---|---|---|
| 90% | 1.3309 | 1571.25 | Reference |
| 89% | 1.3243 | 1567.52 | 565 |
| 88% | 1.3177 | 1563.79 | 565 |
| 87% | 1.311 | 1559.82 | 574 |
| 86% | 1.3044 | 1556.23 | 567 |
| 85% | 1.2978 | 1552.15 | 577 |
| 84% | 1.2912 | 1548.67 | 569 |
| 83% | 1.2846 | 1544.84 | 571 |
| 82% | 1.278 | 1541.23 | 567 |
| 81% | 1.2714 | 1537.90 | 560 |
| Water Concentration | Refractive Index of blood (Blood) | Position of Resonant Peak (nm) | Sensitivity (nm/RIU) |
|---|---|---|---|
| 90% | 1.3305 | 1571.10 | Reference |
| 89% | 1.3269 | 1568.97 | 570 |
| 88% | 1.3232 | 1566.92 | 571 |
| 87% | 1.3196 | 1564.73 | 579 |
| 86% | 1.3159 | 1562.76 | 575 |
| 85% | 1.3123 | 1560.51 | 575 |
| 84% | 1.3087 | 1558.60 | 572 |
| 83% | 1.3050 | 1556.49 | 578 |
| 82% | 1.3014 | 1554.51 | 574 |
| 81 % | 1.2978 | 1552.68 | 573 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agarwal, A.; Mudgal, N.; Choure, K.K.; Pandey, R.; Singh, G.; Bhatnagar, S.K. Photonic Crystal-Based Water Concentration Estimation in Blood Using Machine Learning for Identification of the Haematological Disorder. Photonics 2023, 10, 71. https://doi.org/10.3390/photonics10010071
Agarwal A, Mudgal N, Choure KK, Pandey R, Singh G, Bhatnagar SK. Photonic Crystal-Based Water Concentration Estimation in Blood Using Machine Learning for Identification of the Haematological Disorder. Photonics. 2023; 10(1):71. https://doi.org/10.3390/photonics10010071
Chicago/Turabian StyleAgarwal, Ankit, Nitesh Mudgal, Kamal Kishor Choure, Rahul Pandey, Ghanshyam Singh, and Satish Kumar Bhatnagar. 2023. "Photonic Crystal-Based Water Concentration Estimation in Blood Using Machine Learning for Identification of the Haematological Disorder" Photonics 10, no. 1: 71. https://doi.org/10.3390/photonics10010071
APA StyleAgarwal, A., Mudgal, N., Choure, K. K., Pandey, R., Singh, G., & Bhatnagar, S. K. (2023). Photonic Crystal-Based Water Concentration Estimation in Blood Using Machine Learning for Identification of the Haematological Disorder. Photonics, 10(1), 71. https://doi.org/10.3390/photonics10010071





