A Case Report on Skin Sebum Extraction Using High Lateral Resolution Spectral-Domain Optical Coherence Tomography
Abstract
1. Introduction
2. Materials and Methods
2.1. Subject Preparation for Imaging
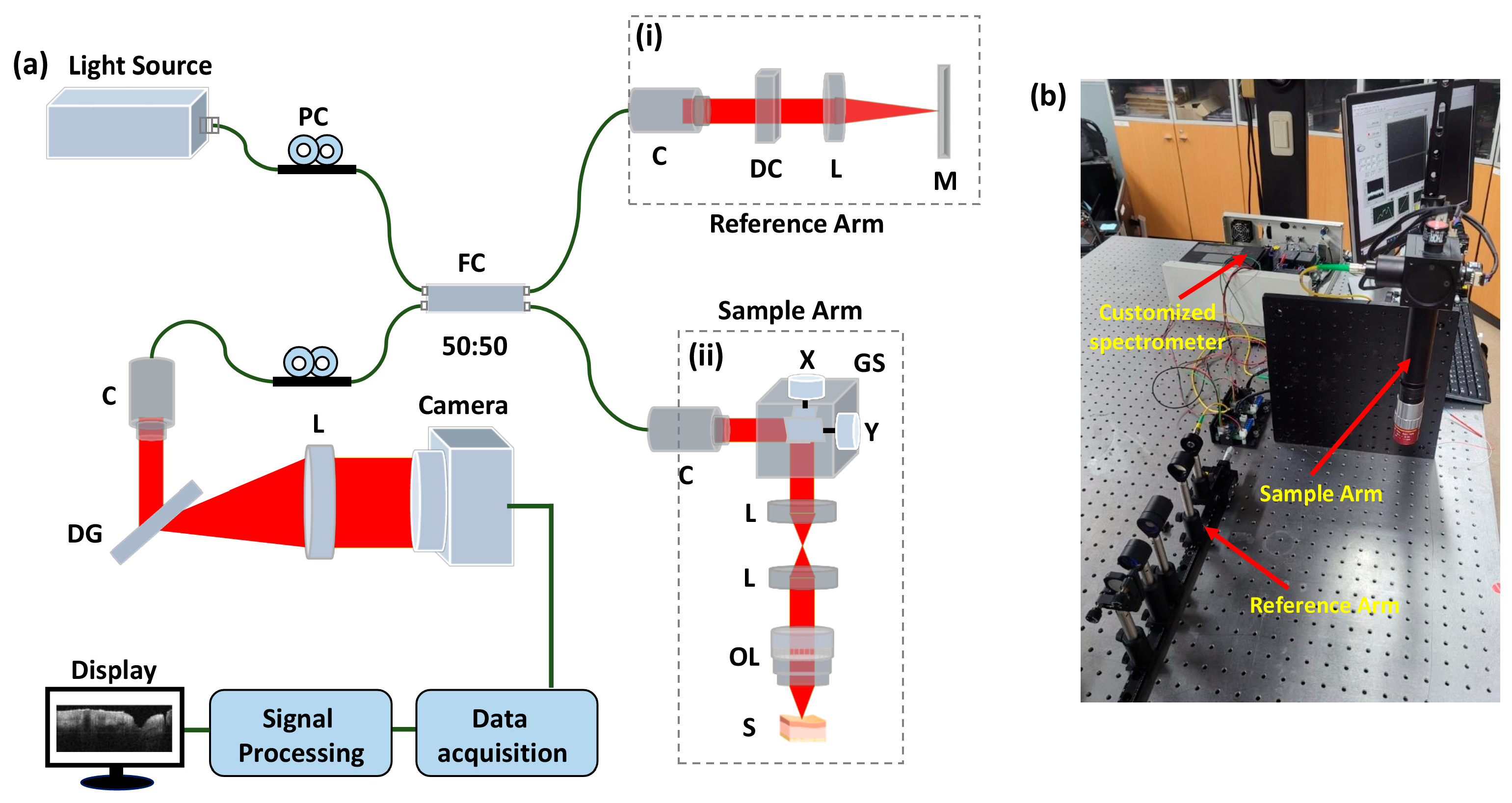
2.2. OCT System Configuration
3. Results
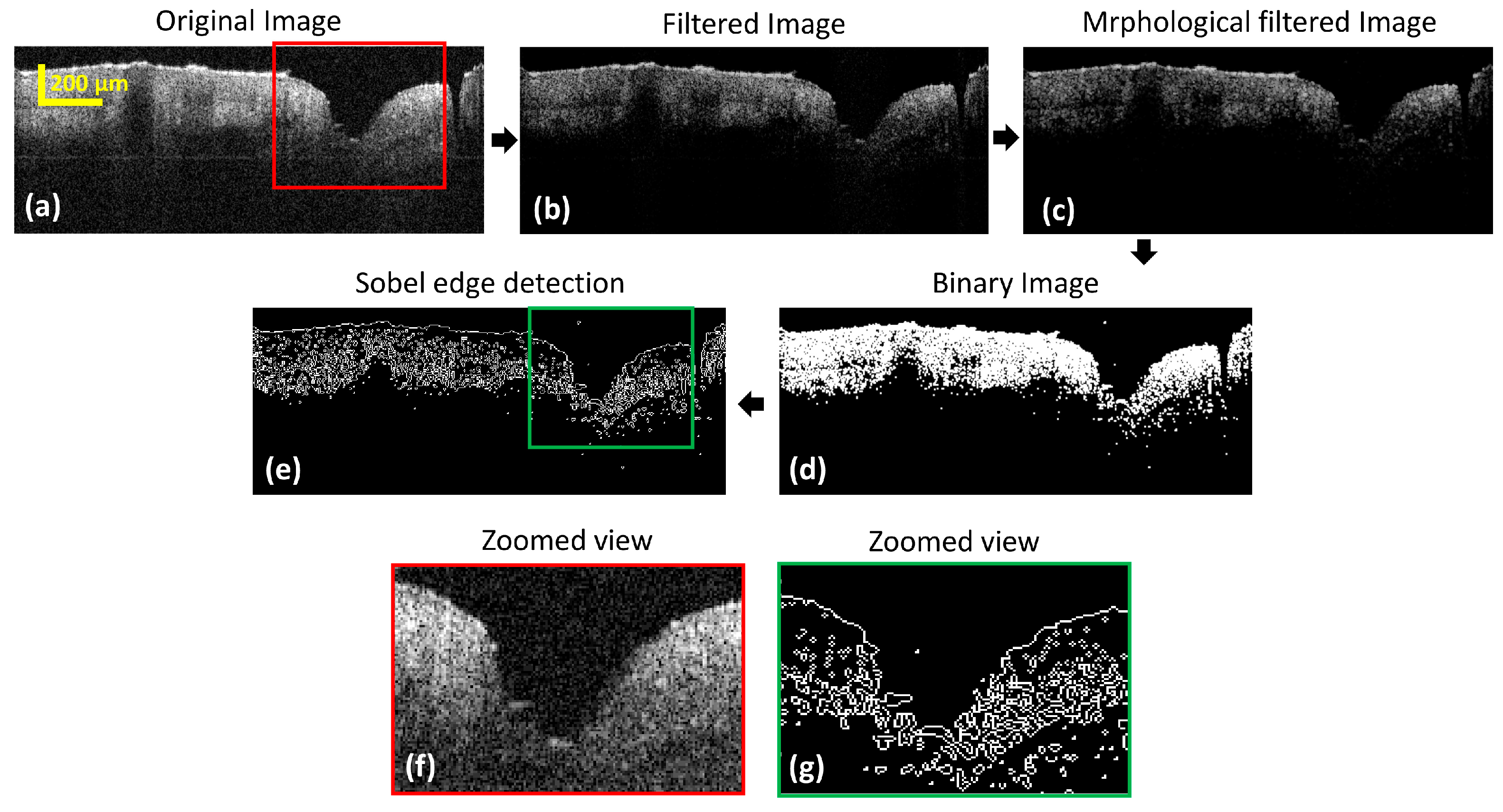
3.1. Evaluation of the Cross-Sectional Skin Pore Images with Edge Detection
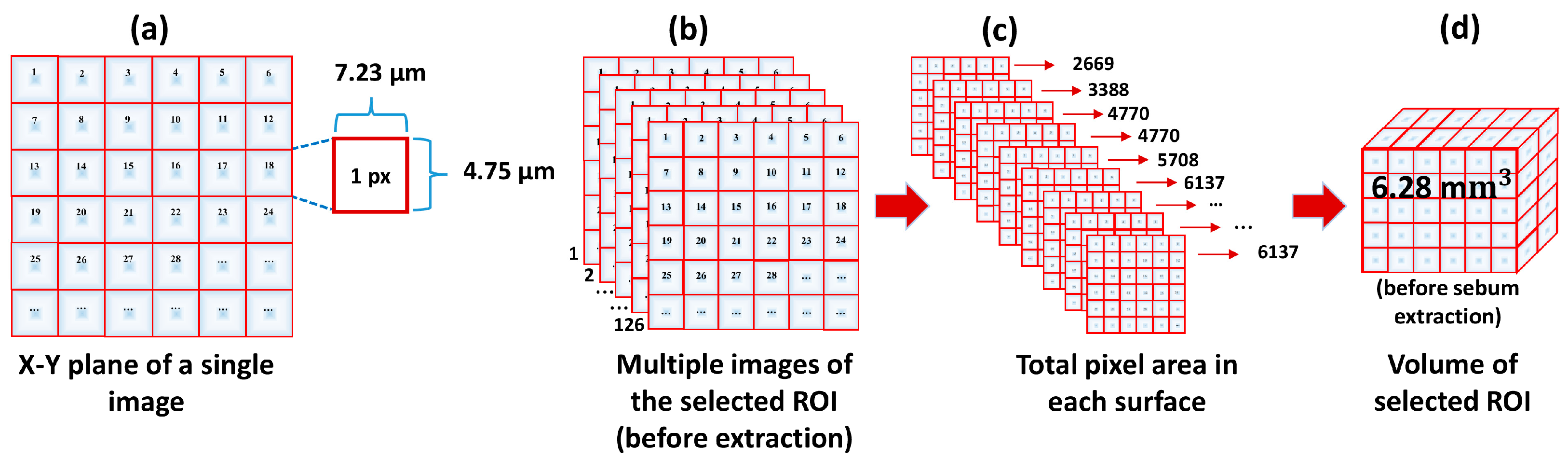
3.2. Image Processing and Volume Calculation Algorithm
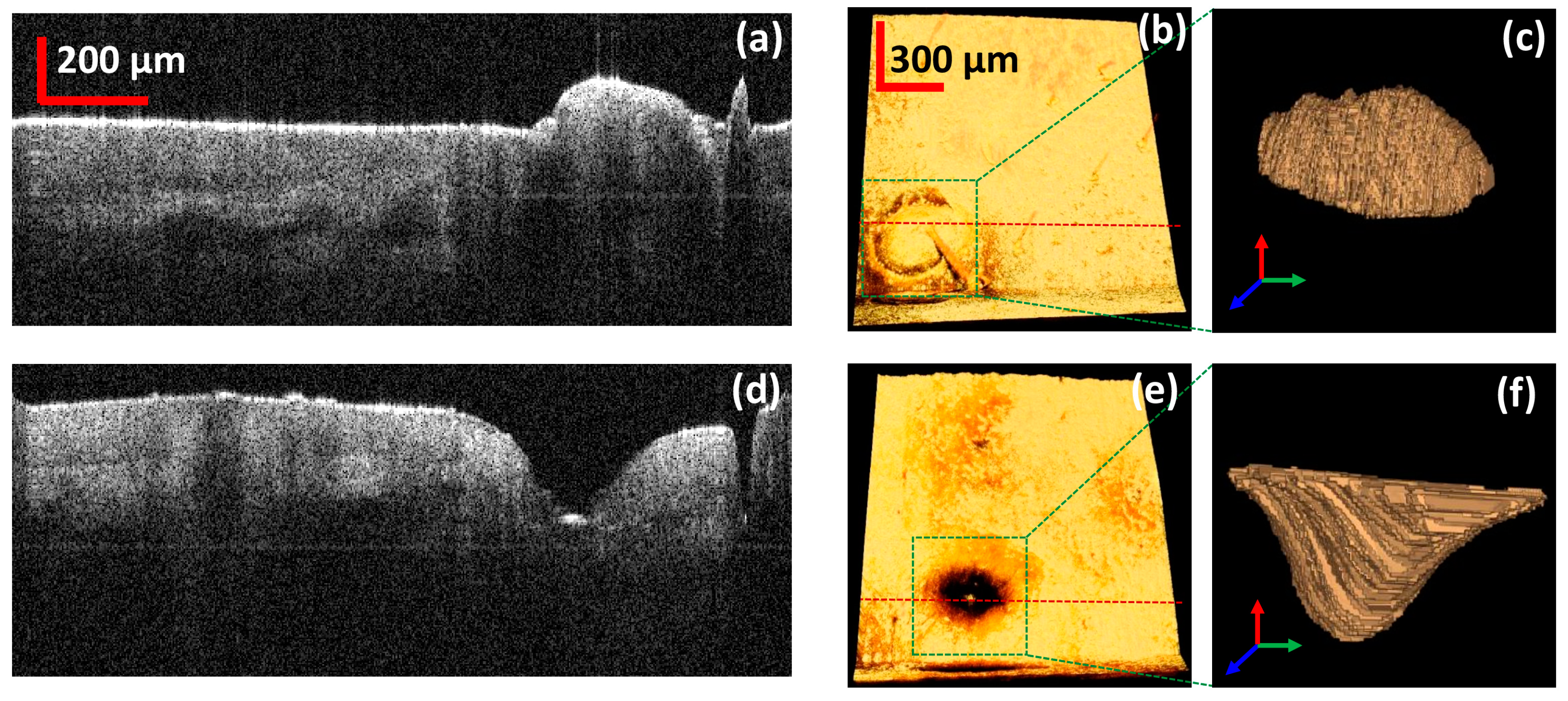
3.3. Quantitative Analysis of the 3D Volume of the Skin Pore
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Camera, E.; Ludovici, M.; Galante, M.; Sinagra, J.-L.; Picardo, M. Comprehensive analysis of the major lipid classes in sebum by rapid resolution high-performance liquid chromatography and electrospray mass spectrometry. J. Lipid Res. 2010, 51, 3377–3388. [Google Scholar] [CrossRef] [PubMed]
- Shetage, S.S.; Traynor, M.J.; Brown, M.B.; Raji, M.; Graham-Kalio, D.; Chilcott, R.P. Effect of ethnicity, gender and age on the amount and composition of residual skin surface components derived from sebum, sweat and epidermal lipids. Ski. Res. Technol. 2014, 20, 97–107. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Schagen, S.; Alestas, T. The sebocyte culture: A model to study the pathophysiology of the sebaceous gland in sebostasis, seborrhoea and acne. Arch. Dermatol. Res. 2008, 300, 397–413. [Google Scholar] [CrossRef] [PubMed]
- Capitanio, B.; Lora, V.; Ludovici, M.; Sinagra, J.L.; Ottaviani, M.; Mastrofrancesco, A.; Ardigò, M.; Camera, E. Modulation of sebum oxidation and interleukin-1α levels associates with clinical improvement of mild comedonal acne. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Ottaviani, M.; Camera, E.; Picardo, M. Lipid mediators in acne. Mediat. Inflamm. 2010, 2010, 858176. [Google Scholar] [CrossRef]
- Leung, A.K.; Barankin, B.; Lam, J.M.; Leong, K.F.; Hon, K.L. Dermatology: How to manage acne vulgaris. Drugs Context 2021, 10, 1–18. [Google Scholar]
- Dong, J.; Lanoue, J.; Goldenberg, G. Enlarged facial pores: An update on treatments. Cutis 2016, 98, 33–36. [Google Scholar]
- Maia Campos, P.M.; Melo, M.O.; Mercurio, D.G. Use of advanced imaging techniques for the characterization of oily skin. Front. Physiol. 2019, 10, 254. [Google Scholar] [CrossRef]
- Ki, V.; Rotstein, C. Bacterial skin and soft tissue infections in adults: A review of their epidemiology, pathogenesis, diagnosis, treatment and site of care. Can. J. Infect. Dis. Med. Microbiol. 2008, 19, 173–184. [Google Scholar] [CrossRef]
- Fritzsche, A.; Arevalo, A.; Connolly, A.; Moore, M.; Elings, V.; Wu, C. The structure and morphology of the skin of polyethersulfone ultrafiltration membranes: A comparative atomic force microscope and scanning electron microscope study. J. Appl. Polym. Sci. 1992, 45, 1945–1956. [Google Scholar] [CrossRef]
- Sidiq, A.; Gravina, R.J.; Setunge, S.; Giustozzi, F. High-efficiency techniques and micro-structural parameters to evaluate concrete self-healing using X-ray tomography and Mercury Intrusion Porosimetry: A review. Constr. Build. Mater. 2020, 252, 119030. [Google Scholar] [CrossRef]
- Han, W.; Zhou, G.; Gao, D.; Zhang, Z.; Wei, Z.; Wang, H.; Yang, H. Experimental analysis of the pore structure and fractal characteristics of different metamorphic coal based on mercury intrusion-nitrogen adsorption porosimetry. Powder Technol. 2020, 362, 386–398. [Google Scholar] [CrossRef]
- Fathima, N.N.; Dhathathreyan, A.; Ramasami, T. Mercury intrusion porosimetry, nitrogen adsorption, and scanning electron microscopy analysis of pores in skin. Biomacromolecules 2002, 3, 899–904. [Google Scholar] [CrossRef] [PubMed]
- ElHadidy, A.M.; Peldszus, S.; Van Dyke, M.I. Development of a pore construction data analysis technique for investigating pore size distribution of ultrafiltration membranes by atomic force microscopy. J. Membr. Sci. 2013, 429, 373–383. [Google Scholar] [CrossRef]
- Sánchez-Pellicer, P.; Navarro-Moratalla, L.; Núñez-Delegido, E.; Ruzafa-Costas, B.; Agüera-Santos, J.; Navarro-López, V. Acne, Microbiome, and Probiotics: The Gut–Skin Axis. Microorganisms 2022, 10, 1303. [Google Scholar] [CrossRef] [PubMed]
- Chilicka, K.; Rogowska, A.M.; Szyguła, R.; Rusztowicz, M.; Nowicka, D. Efficacy of oxybrasion in the treatment of acne vulgaris: A preliminary report. J. Clin. Med. 2022, 11, 3824. [Google Scholar] [CrossRef] [PubMed]
- Chilicka, K.; Rusztowicz, M.; Rogowska, A.M.; Szyguła, R.; Asanova, B.; Nowicka, D. Efficacy of hydrogen purification and cosmetic acids in the treatment of acne vulgaris: A preliminary report. J. Clin. Med. 2022, 11, 6269. [Google Scholar] [CrossRef]
- Landry, M.R. Thermoporometry by differential scanning calorimetry: Experimental considerations and applications. Thermochim. Acta 2005, 433, 27–50. [Google Scholar] [CrossRef]
- Riikonen, J.; Salonen, J.; Lehto, V.-P. Utilising Thermoporometry to Obtain New Insights into Nanostructured Materials—Review Part; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Lin, S.-J.; Wu, R.-J.; Tan, H.-Y.; Lo, W.; Lin, W.-C.; Young, T.-H.; Hsu, C.-J.; Chen, J.-S.; Jee, S.-H.; Dong, C.-Y. Evaluating cutaneous photoaging by use of multiphoton fluorescence and second-harmonic generation microscopy. Opt. Lett. 2005, 30, 2275–2277. [Google Scholar] [CrossRef]
- Calzavara-Pinton, P.; Longo, C.; Venturini, M.; Sala, R.; Pellacani, G. Reflectance confocal microscopy for in vivo skin imaging. Photochem. Photobiol. 2008, 84, 1421–1430. [Google Scholar] [CrossRef]
- Brown, E.; McKee, T.; DiTomaso, E.; Pluen, A.; Seed, B.; Boucher, Y.; Jain, R.K. Dynamic imaging of collagen and its modulation in tumors in vivo using second-harmonic generation. Nat. Med. 2003, 9, 796–800. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A. Optical coherence tomography. Science 1991, 254, 1178–1181. [Google Scholar] [CrossRef] [PubMed]
- Fercher, A.F. Optical coherence tomography. J. Biomed. Opt. 1996, 1, 157–173. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, M.; Morsy, H.A.; Thrane, L.; Jemec, G.B. Morphology and epidermal thickness of normal skin imaged by optical coherence tomography. Dermatology 2008, 217, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Drexler, W.; Morgner, U.; Ghanta, R.K.; Kärtner, F.X.; Schuman, J.S.; Fujimoto, J.G. Ultrahigh-resolution ophthalmic optical coherence tomography. Nat. Med. 2001, 7, 502–507. [Google Scholar] [CrossRef]
- Tearney, G.; Brezinski, M.; Southern, J.; Bouma, B.; Boppart, S.; Fujimoto, J. Optical biopsy in human gastrointestinal tissue using optical coherence tomography. Am. J. Gastroenterol. 1997, 92, 1800–1804. [Google Scholar]
- Lee, J.; Saleah, S.A.; Jeon, B.; Wijesinghe, R.E.; Lee, D.-E.; Jeon, M.; Kim, J. Assessment of the Inner Surface Roughness of 3D Printed Dental Crowns via Optical Coherence Tomography Using a Roughness Quantification Algorithm. IEEE Access 2020, 8, 133854–133864. [Google Scholar] [CrossRef]
- Shakhov, A.V.; Terentjeva, A.B.; Kamensky, V.A.; Snopova, L.B.; Gelikonov, V.M.; Feldchtein, F.I.; Sergeev, A.M. Optical coherence tomography monitoring for laser surgery of laryngeal carcinoma. J. Surg. Oncol. 2001, 77, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, K.; Wijesinghe, R.E.; Jeon, D.; Lee, S.H.; Jeon, M.; Jang, J.H. Decalcification using ethylenediaminetetraacetic acid for clear microstructure imaging of cochlea through optical coherence tomography. J. Biomed. Opt. 2016, 21, 081204. [Google Scholar] [CrossRef]
- Seong, D.; Lee, C.; Jeon, M.; Kim, J. Doppler Optical Coherence Tomography for Otology Applications: From Phantom Simulation to In Vivo Experiment. Appl. Sci. 2021, 11, 5711. [Google Scholar] [CrossRef]
- Vakoc, B.J.; Fukumura, D.; Jain, R.K.; Bouma, B.E. Cancer imaging by optical coherence tomography: Preclinical progress and clinical potential. Nat. Rev. Cancer 2012, 12, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Seong, D.; Jeon, D.; Wijesinghe, R.E.; Park, K.; Kim, H.; Lee, E.; Jeon, M.; Kim, J. Ultrahigh-Speed Spectral-Domain Optical Coherence Tomography up to 1-MHz A-Scan Rate Using Space–Time-Division Multiplexing. IEEE Trans. Instrum. Meas. 2021, 70, 1–8. [Google Scholar] [CrossRef]
- Lee, J.; Lee, S.-Y.; Wijesinghe, R.E.; Ravichandran, N.K.; Han, S.; Kim, P.; Jeon, M.; Jung, H.-Y.; Kim, J. On-field in situ inspection for marssonina coronaria infected apple blotch based on non-invasive bio-photonic imaging module. IEEE Access 2019, 7, 148684–148691. [Google Scholar] [CrossRef]
- Saleah, S.A.; Wijesinghe, R.E.; Lee, S.-Y.; Ravichandran, N.K.; Seong, D.; Jung, H.-Y.; Jeon, M.; Kim, J. On-field optical imaging data for the pre-identification and estimation of leaf deformities. Sci. Data 2022, 9, 698. [Google Scholar] [CrossRef] [PubMed]
- Boppart, S.A.; Bouma, B.E.; Brezinski, M.E.; Tearney, G.J.; Fujimoto, J.G. Imaging developing neural morphology using optical coherence tomography. J. Neurosci. Methods 1996, 70, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.S.; Wijesinghe, R.E.; Lee, C.; Lee, S.Y.; Jung, H.Y.; Jeon, M.; Kim, J. In vivo observation of metamorphosis of Plodia interpunctella Hübner using three-dimensional optical coherence tomography. Entomol. Res. 2017, 47, 256–262. [Google Scholar] [CrossRef]
- Tripathi, S.R.; Miyata, E.; Ishai, P.B.; Kawase, K. Morphology of human sweat ducts observed by optical coherence tomography and their frequency of resonance in the terahertz frequency region. Sci. Rep. 2015, 5, 9071. [Google Scholar] [CrossRef]
- Liu, M.; Buma, T. Biometric mapping of fingertip eccrine glands with optical coherence tomography. IEEE Photonics Technol. Lett. 2010, 22, 1677–1679. [Google Scholar] [CrossRef]
- Potlov, A.Y.; Frolov, S.; Proskurin, S. Visualization of anatomical structures of biological tissues by optical coherence tomography with digital processing of morphological data. Biomed. Eng. 2020, 54, 9–13. [Google Scholar] [CrossRef]
- Knuettel, A.R.; Boehlau-Godau, M. Spatially confined and temporally resolved refractive index and scattering evaluation in human skin performed with optical coherence tomography. J. Biomed. Opt. 2000, 5, 83–92. [Google Scholar] [CrossRef]
- Ding, H.; Lu, J.Q.; Wooden, W.A.; Kragel, P.J.; Hu, X.-H. Refractive indices of human skin tissues at eight wavelengths and estimated dispersion relations between 300 and 1600 nm. Phys. Med. Biol. 2006, 51, 1479. [Google Scholar] [CrossRef] [PubMed]
- Uhoda, E.; Piérard-Franchimont, C.; Petit, L.; Piérard, G.E. The conundrum of skin pores in dermocosmetology. Dermatology 2005, 210, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Ash, C.; Dubec, M.; Donne, K.; Bashford, T. Effect of wavelength and beam width on penetration in light-tissue interaction using computational methods. Lasers Med. Sci. 2017, 32, 1909–1918. [Google Scholar] [CrossRef]
- Kuck, M.; Strese, H.; Alawi, S.A.; Meinke, M.C.; Fluhr, J.W.; Burbach, G.J.; Krah, M.; Sterry, W.; Lademann, J. Evaluation of optical coherence tomography as a non-invasive diagnostic tool in cutaneous wound healing. Ski. Res. Technol. 2014, 20, 1–7. [Google Scholar] [CrossRef]
- Ghosh, B.; Mandal, M.; Mitra, P.; Chatterjee, J. Attenuation corrected-optical coherence tomography for quantitative assessment of skin wound healing and scar morphology. J. Biophotonics 2021, 14, e202000357. [Google Scholar] [CrossRef]
- Lee, S.J.; Seok, J.; Jeong, S.Y.; Park, K.Y.; Li, K.; Seo, S.J. Facial pores: Definition, causes, and treatment options. Dermatol. Surg. 2016, 42, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.Y.; Yu, D.S.; Oh, C.H. Quantitative research on skin pore widening using a stereoimage optical topometer and Sebutape®. Ski. Res. Technol. 2007, 13, 162–168. [Google Scholar] [CrossRef]
- Sugata, K.; Nishijima, T.; Kitahara, T.; Takema, Y. Confocal laser microscopic imaging of conspicuous facial pores in vivo: Relation between the appearance and the internal structure of skin. Ski. Res. Technol. 2008, 14, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.Y.; Chung, T.-S.; Gryta, M. Hydrophobic PVDF hollow fiber membranes with narrow pore size distribution and ultra-thin skin for the fresh water production through membrane distillation. Chem. Eng. Sci. 2008, 63, 2587–2594. [Google Scholar] [CrossRef]
- Varghese, J.S.; Chellappa, N.; Fathima, N.N. Gelatin–carrageenan hydrogels: Role of pore size distribution on drug delivery process. Colloids Surf. B Biointerfaces 2014, 113, 346–351. [Google Scholar] [CrossRef]

| Skin Pore Region | Total Area | Total B-Scan | Total Volume | Total Volume | STD± |
|---|---|---|---|---|---|
| um2 | number | um3 | mm3 | mm3 | |
| Before sebum extraction | 1.801 × 107 | 126 | 6.289 × 109 | 6.28 | 0.03 |
| After sebum extraction | 2.188 × 107 | 160 | 9.699 × 109 | 9.61 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luna, J.A.; Saleah, S.A.; Kim, H.; Kang, D.; Seong, D.; Kim, Y.; Kim, H.; Wijesinghe, R.E.; Kim, J.; Jeon, M. A Case Report on Skin Sebum Extraction Using High Lateral Resolution Spectral-Domain Optical Coherence Tomography. Photonics 2023, 10, 30. https://doi.org/10.3390/photonics10010030
Luna JA, Saleah SA, Kim H, Kang D, Seong D, Kim Y, Kim H, Wijesinghe RE, Kim J, Jeon M. A Case Report on Skin Sebum Extraction Using High Lateral Resolution Spectral-Domain Optical Coherence Tomography. Photonics. 2023; 10(1):30. https://doi.org/10.3390/photonics10010030
Chicago/Turabian StyleLuna, Jannat Amrin, Sm Abu Saleah, Hyunmo Kim, Dongwan Kang, Daewoon Seong, Yoonseok Kim, Hayoung Kim, Ruchire Eranga Wijesinghe, Jeehyun Kim, and Mansik Jeon. 2023. "A Case Report on Skin Sebum Extraction Using High Lateral Resolution Spectral-Domain Optical Coherence Tomography" Photonics 10, no. 1: 30. https://doi.org/10.3390/photonics10010030
APA StyleLuna, J. A., Saleah, S. A., Kim, H., Kang, D., Seong, D., Kim, Y., Kim, H., Wijesinghe, R. E., Kim, J., & Jeon, M. (2023). A Case Report on Skin Sebum Extraction Using High Lateral Resolution Spectral-Domain Optical Coherence Tomography. Photonics, 10(1), 30. https://doi.org/10.3390/photonics10010030







