Nitrogen-Doped Nickel Graphene Core Shell Synthesis: Structural, Morphological, and Chemical Composition for Planar Hybrid Solar Cells Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Nitrogen-Doped Ni-Graphene Core-Shell Nanoparticles Preparation
2.2. Characterization of Nitrogen-Doped Ni-Graphene CSNPs
2.3. The Electron Transfer Layer Preparation for Planar Hybrid Solar Cells Application
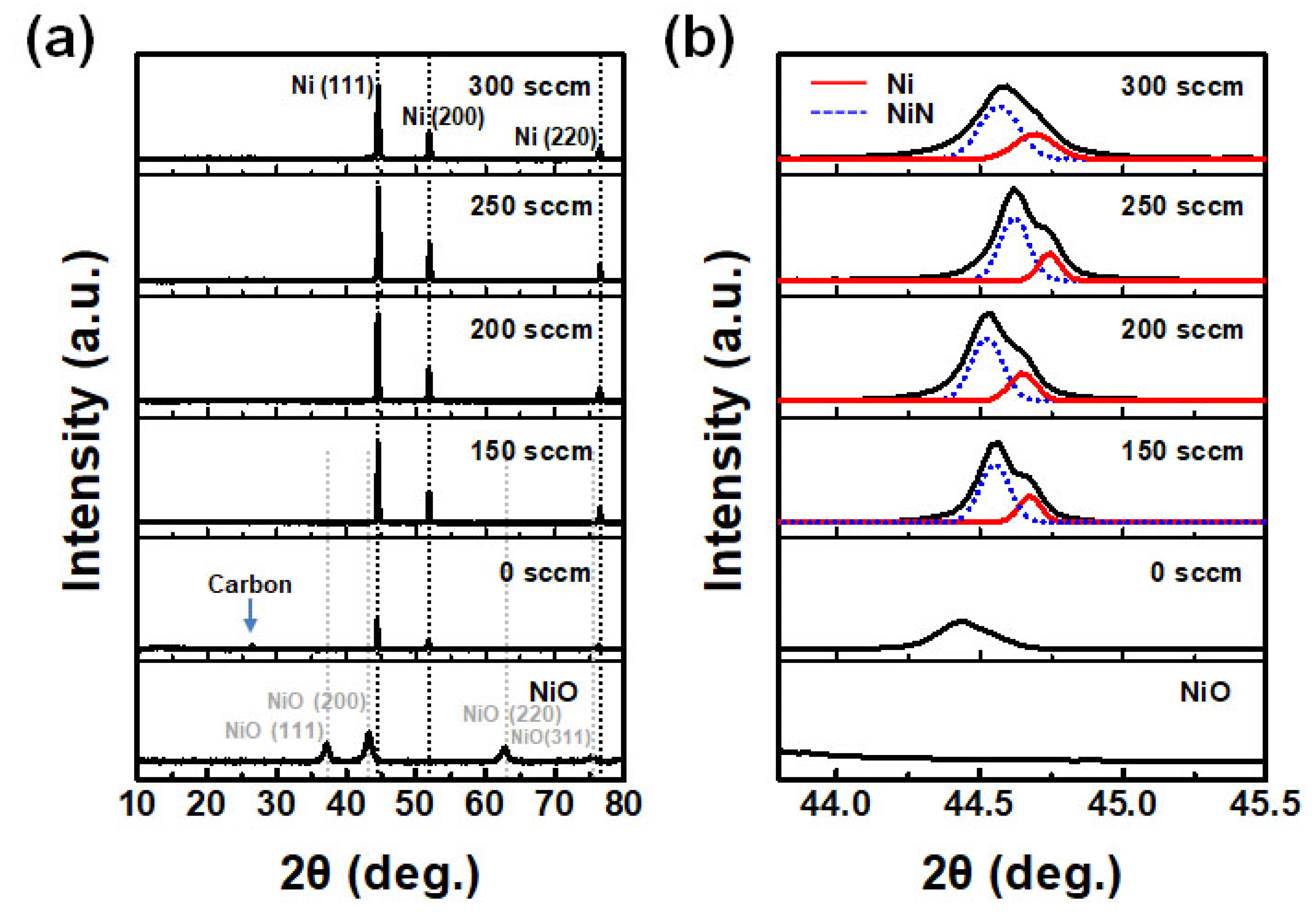
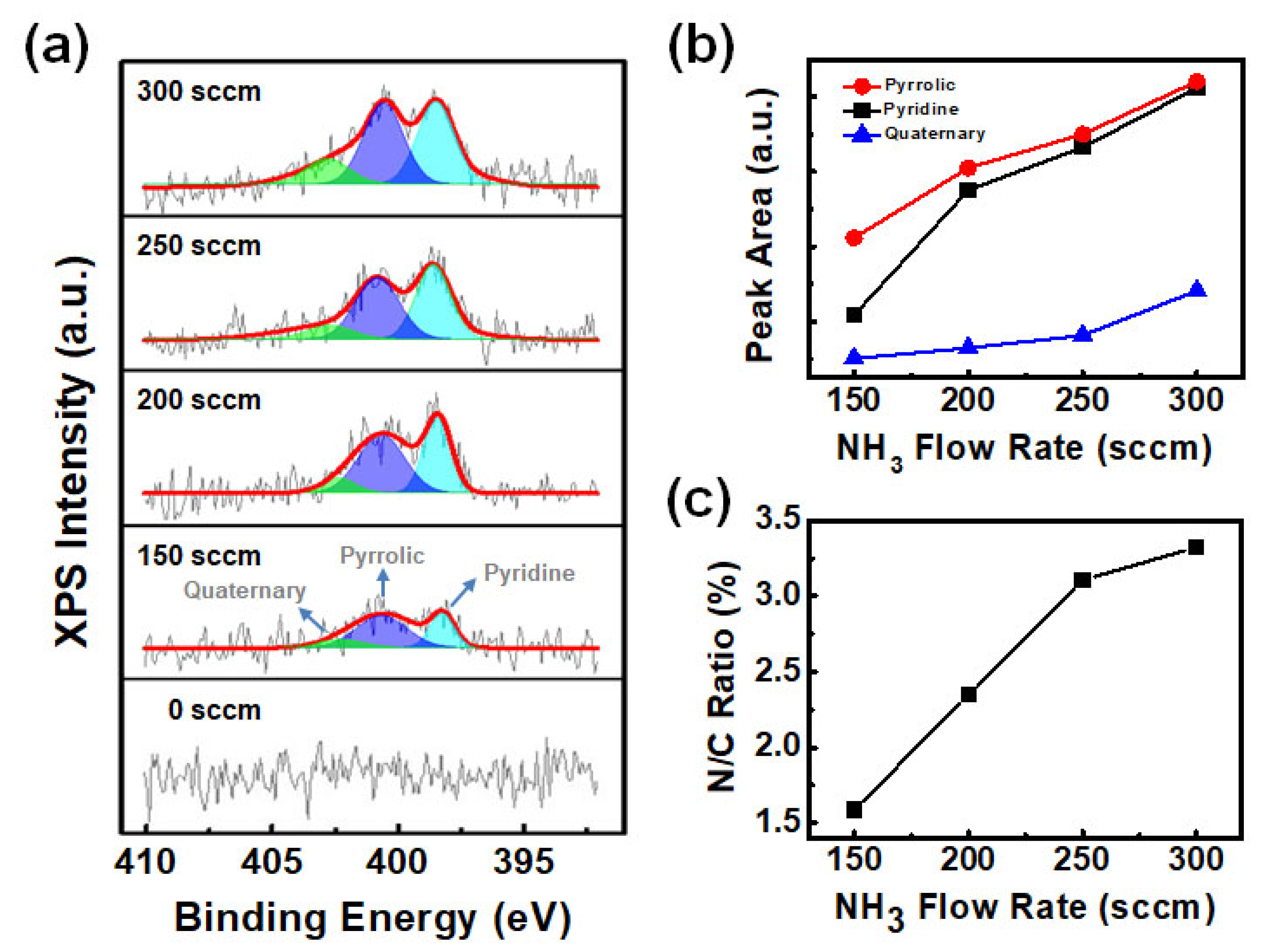
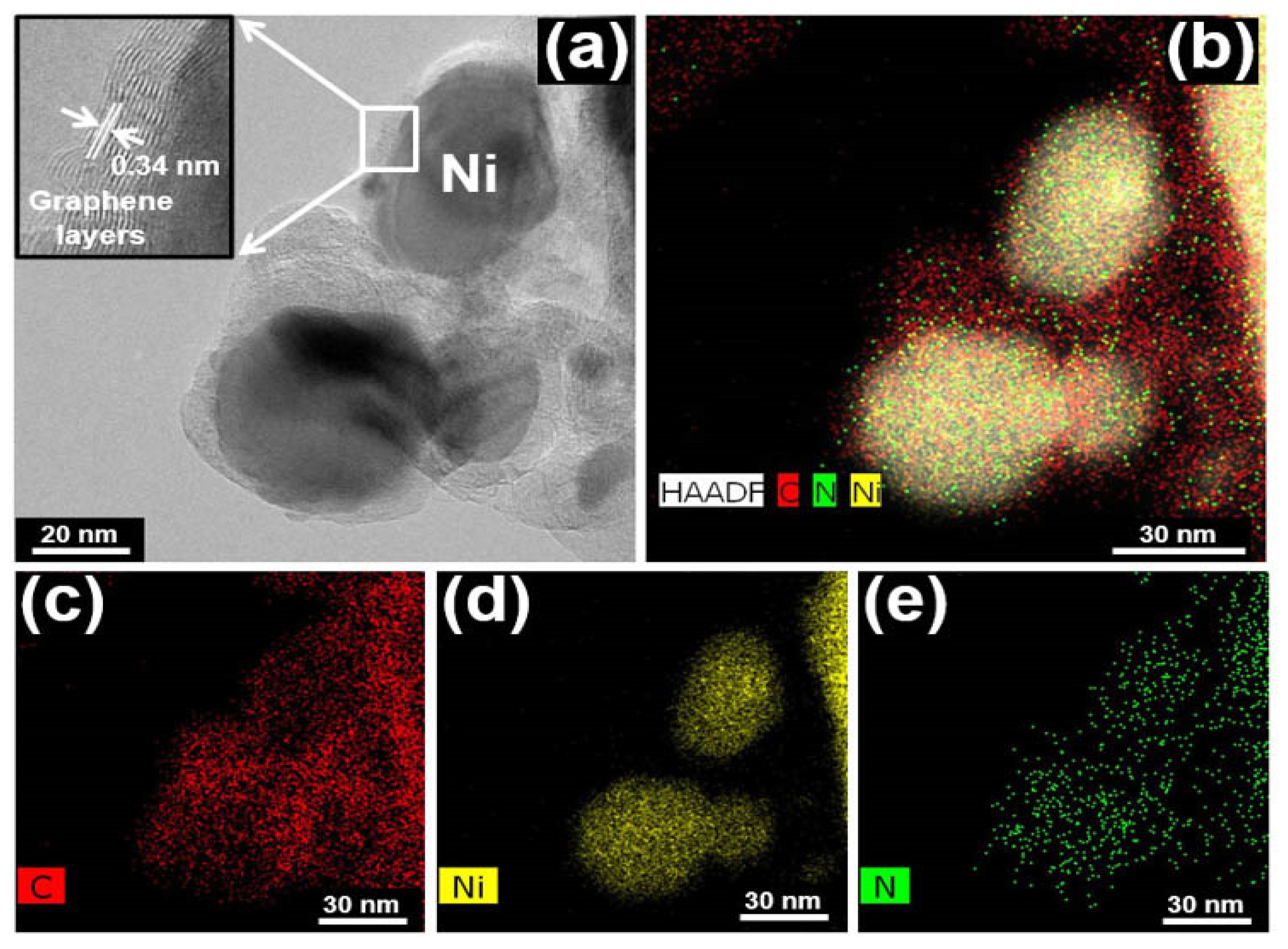
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aldakov, D.; Reiss, P. Safer-by-Design Fluorescent Nanocrystals: Metal Halide Perovskites vs Semiconductor Quantum Dots. J. Phys. Chem. C 2019, 123, 12527–12541. [Google Scholar] [CrossRef]
- Huynh, W.U.; Dittmer, J.J.; Alivisatos, A.P. Hybrid Nanorod Polymer Solar Cells. Science 2002, 295, 2425–2427. [Google Scholar] [CrossRef] [PubMed]
- Chunyan, Y.; Yingying, S.; Xinjie, L.; Cheng, L.; Junfeng, T.; Jianfeng, L.; Peng, Z.; Yangjun, X. In Situ Growth of Metal Sulfide Nanocrystals in Poly(3-hexylthiophene): [6,6]-Phenyl C61-Butyric Acid Methyl Ester Films for Inverted Hybrid Solar Cells with Enhanced Photocurrent. Nanoscale Res. Lett. 2018, 13, 1–9. [Google Scholar]
- Tan, F.; Qu, S.; Wang, L.; Jiang, Q.; Zhang, W.; Wang, Z. Core/shell-shaped CdSe/PbS nanotetrapods for efficient organic–inorganic hybrid solar cells. J. Mater. Chem. 2014, 2, 14502–14510. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, J.; Church, C.P.; Miller, E.M.; Luther, J.M.; Klimov, V.I.; Beard, M.C. PbSe quantum dot solar cells with more than 6% efficiency fabricated in ambient atmosphere. Nano Lett. 2014, 14, 6010–6015. [Google Scholar] [CrossRef]
- Ding, D.; Rath, T.; Lanzetta, L.; Manuel Marin-Beloqui, J.; Haque, S.A. Efficient hybrid solar cells based on solution processed mesoporous TiO2/Tin (II) sulfide heterojunctions. ACS Appl. Energy Mater. 2018, 1, 3042–3047. [Google Scholar] [CrossRef]
- Tan, F.; Qu, S.; Wu, J.; Liu, K.; Zhou, S.; Wang, Z. Preparation of SnS2 colloidal quantum dots and their application in organic/inorganic hybrid solar cells. Nanoscale Res. Lett. 2011, 6, 1–8. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, X.; Lei, H.; Tan, Z.; Chen, J. Characterization of evaporated tin sulfide and its application for hybrid solar cell. Mater. Lett. 2019, 251, 234–237. [Google Scholar] [CrossRef]
- Gao, Y.; Yip, H.L.; Hau, S.K.; O’Malley, K.M.; Cho, N.C.; Chen, H.; Jen, A.K.Y. Anode modification of inverted polymer solar cells using graphene oxide. Appl. Phys. Lett. 2010, 97, 251. [Google Scholar] [CrossRef]
- Negash, A.; Demeku, A.M.; Molloro, L.H. Application of reduced graphene oxide as the hole transport layer in organic solar cells synthesized from waste dry cells using the electrochemical exfoliation method. New J. Chem. 2022, 46, 13001–13009. [Google Scholar] [CrossRef]
- Jun, G.H.; Jin, S.H.; Lee, B.; Kim, B.H.; Chae, W.S.; Hong, S.H.; Jeon, S. Enhanced conduction and charge-selectivity by N-doped graphene flakes in the active layer of bulk-heterojunction organic solar cells. Energy Environ. Sci. 2013, 6, 3000–3006. [Google Scholar] [CrossRef]
- Chang, D.W.; Choi, H.J.; Filer, A.; Baek, J.B. Graphene in photovoltaic applications: Organic photovoltaic cells (OPVs) and dye-sensitized solar cells (DSSCs). J. Mater. Chem. 2014, 2, 12136–12149. [Google Scholar] [CrossRef]
- Truong, N.T.N.; Lam, N.H.; Reddy, V.R.M.; Tamboli, M.S.; Kim, C.D.; Park, C. Core-shell nickel-graphene nanoparticles for efficient tin sulfide/polymer bulk hetero-junction solar cells. J. Mater. Sci. Mater. Electron. 2021, 32, 24575–24583. [Google Scholar] [CrossRef]
- Kim, C.D.; Truong, N.T.N.; Pham, V.T.H.; Jo, Y.; Lee, H.R.; Park, C. Conductive electrodes based on Ni–graphite core–shell nanoparticles for heterojunction solar cells. Mater. Chem. Phys. 2019, 223, 557–563. [Google Scholar] [CrossRef]
- Manukyan, K.V.; Avetisyan, A.G.; Shuck, C.E.; Chatilyan, H.A.; Rouvimov, S.; Kharatyan, S.L.; Mukasyan, A.S. Nickel oxide reduction by hydrogen: Kinetics and structural transformations. J. Phys. Chem. C. 2015, 119, 16131–16138. [Google Scholar] [CrossRef]
- Zhao, X.; Ando, Y. Raman spectra and X-ray diffraction patterns of carbon nanotubes prepared by hydrogen arc discharge. Jpn. J. Appl. Phys. 1998, 37, 4846–4849. [Google Scholar] [CrossRef]
- Morimoto, N.; Kubo, T.; Nishina, Y. Tailoring the oxygen content of graphite and reduced graphene oxide for specific applications. Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef]
- Shen, J.; Zheng, X.; Peng, L.; Waterhouse, G.I.; Tan, L.; Yang, J.; Li, L.; Wei, Z. Heteroatom Modification of Nanoporous Nickel Surfaces for Electrocatalytic Water Splitting. ACS Appl. Nano Mater. 2020, 3, 11298–11306. [Google Scholar] [CrossRef]
- Kim, C.D.; Lee, H.R.; Kim, H.T. Effect of NH3 gas ratio on the formation of nitrogen-doped carbon nanotubes using thermal chemical vapor deposition. Mater. Chem. Phys. 2016, 183, 315–319. [Google Scholar] [CrossRef]
- Wang, H.; Maiyalagan, T.; Wang, X. Review on recent progress in nitrogen-doped graphene: Synthesis, characterization, and its potential applications. ACS Catal. 2012, 2, 781–794. [Google Scholar] [CrossRef]
- Zainal Ariffin, N.H.; Mohammad Haniff, M.A.S.; Syono, M.I.; Ambri Mohamed, M.; Hamzah, A.A.; Hashim, A.M. Low-Temperature Nitrogen Doping of Nanocrystalline Graphene Films with Tunable Pyridinic-N and Pyrrolic-N by Cold-Wall Plasma-Assisted Chemical Vapor Deposition. ACS Omega 2021, 6, 23710–23722. [Google Scholar] [CrossRef] [PubMed]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef]
- Kumar, R.; Mehta, B.R.; Bhatnagar, M.; Mahapatra, S.; Salkalachen, S.; Jhawar, P. Graphene as a transparent conducting and surface field layer in planar Si solar cells. Nanoscale Res. Lett. 2014, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Le, H.D.; Ngo, T.T.T.; Le, D.Q.; Nguyen, X.N.; Phan, N.M. Synthesis of multi-layer graphene films on copper tape by atmospheric pressure chemical vapor deposition method. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 4, 035012. [Google Scholar]
- Ju, Z.; Zhang, S.; Xing, Z.; Zhuang, Q.; Qiang, Y.; Qian, Y. Direct synthesis of few-layer F-doped graphene foam and its lithium/potassium storage properties. ACS Appl. Mater. Interfaces 2016, 8, 20682–20690. [Google Scholar] [CrossRef]
- Wang, Y.; Kurunthu, D.; Scott, G.W.; Bardeen, C.J. Fluorescence quenching in conjugated polymers blended with reduced graphitic oxide. J. Phys. Chem. C 2010, 114, 4153–4159. [Google Scholar] [CrossRef]
- Yang, N.; Zhai, J.; Wang, D.; Chen, Y.; Jiang, L. Two-dimensional graphene bridges enhanced photoinduced charge transport in dye-sensitized solar cells. ACS Nano 2010, 4, 887–894. [Google Scholar] [CrossRef]
- Liscio, A.; Veronese, G.P.; Treossi, E.; Suriano, F.; Rossella, F.; Bellani, V.; Rita, R.; Paolo, S.; Palermo, V. Charge transport in graphene–polythiophene blends as studied by Kelvin Probe Force Microscopy and transistor characterization. J. Mater. Chem. 2011, 21, 2924–2931. [Google Scholar] [CrossRef]

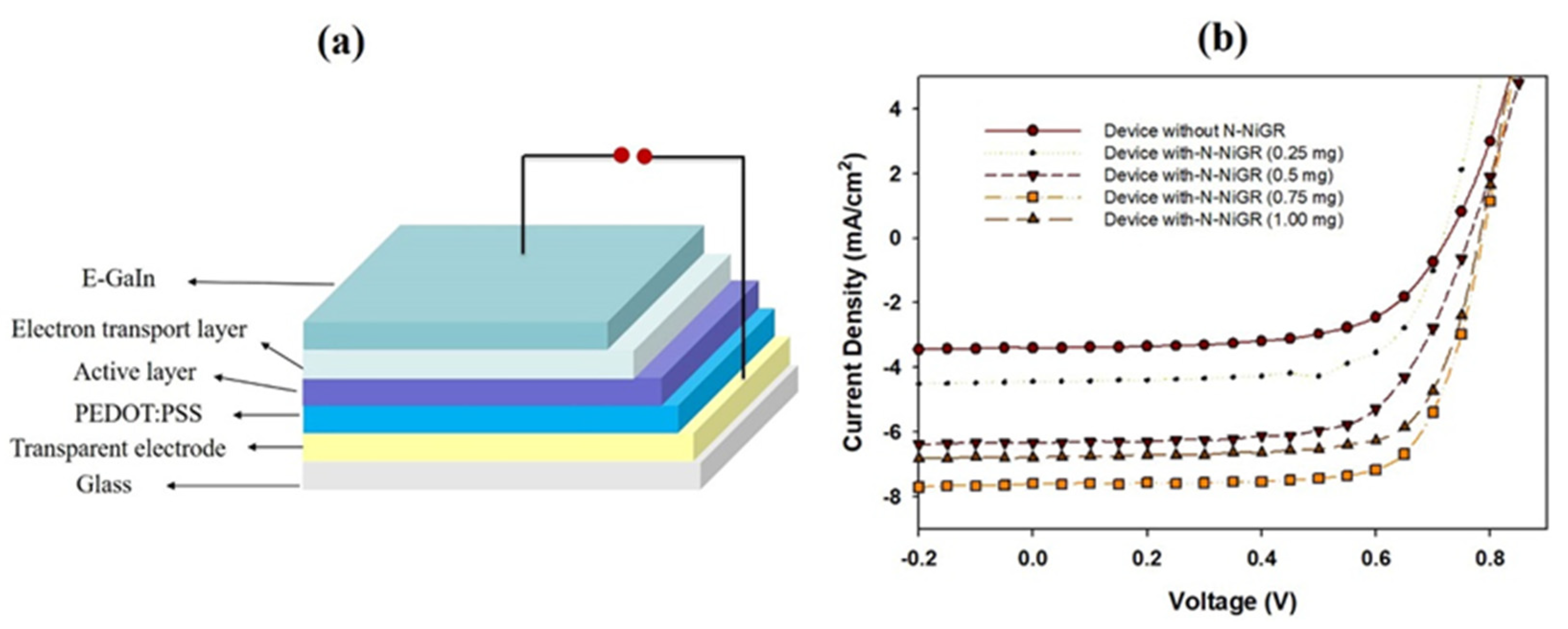
| Device Structure | Jsc (mA/cm2) | Voc (V) | FF (%) | Eff (%) |
|---|---|---|---|---|
| Without N-NiGR | 3.41 | 0.73 | 61.70 | 1.53 |
| With N-NiGR (0.25 mg) | 4.42 | 0.73 | 67.0 | 2.12 |
| With N-NiGR (0.5 mg) | 6.80 | 0.77 | 71.2 | 3.78 |
| With N-NiGR (0.75 mg) | 7.61 | 0.77 | 72.1 | 4.35 |
| With N-NiGR (1.00 mg) | 6.32 | 0.77 | 66.4 | 3.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, S.B.; Jo, Y.; Lam, N.H.; Truong, N.T.N.; Jung, J.H.; Kim, C.-D. Nitrogen-Doped Nickel Graphene Core Shell Synthesis: Structural, Morphological, and Chemical Composition for Planar Hybrid Solar Cells Application. Photonics 2023, 10, 18. https://doi.org/10.3390/photonics10010018
Kang SB, Jo Y, Lam NH, Truong NTN, Jung JH, Kim C-D. Nitrogen-Doped Nickel Graphene Core Shell Synthesis: Structural, Morphological, and Chemical Composition for Planar Hybrid Solar Cells Application. Photonics. 2023; 10(1):18. https://doi.org/10.3390/photonics10010018
Chicago/Turabian StyleKang, Seung Beom, Younjung Jo, Nguyen Hoang Lam, Nguyen Tam Nguyen Truong, Jae Hak Jung, and Chang-Duk Kim. 2023. "Nitrogen-Doped Nickel Graphene Core Shell Synthesis: Structural, Morphological, and Chemical Composition for Planar Hybrid Solar Cells Application" Photonics 10, no. 1: 18. https://doi.org/10.3390/photonics10010018
APA StyleKang, S. B., Jo, Y., Lam, N. H., Truong, N. T. N., Jung, J. H., & Kim, C.-D. (2023). Nitrogen-Doped Nickel Graphene Core Shell Synthesis: Structural, Morphological, and Chemical Composition for Planar Hybrid Solar Cells Application. Photonics, 10(1), 18. https://doi.org/10.3390/photonics10010018




