Preliminary Design and Evaluation of a B-Scan OCT-Guided Needle
Abstract
:1. Introduction
2. Experimental Section (Development and Testing of a B-scan OCT-guided 36-Gauge Needle)
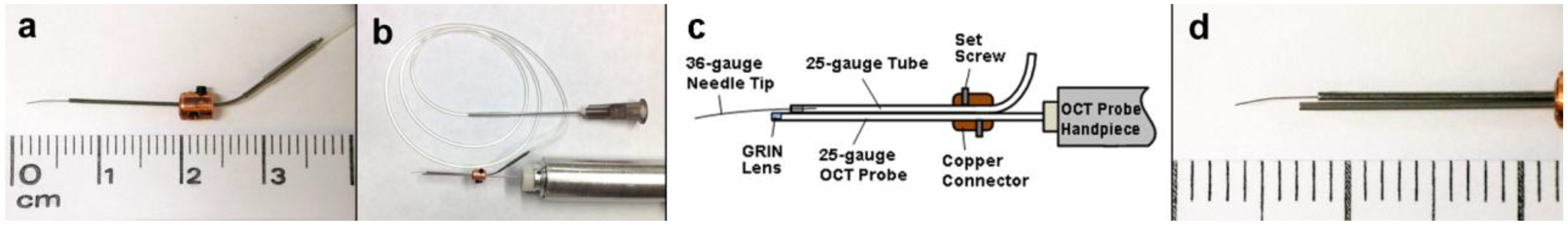
3. Results and Discussion
3.1. Design of the Combined OCT Probe and Needle
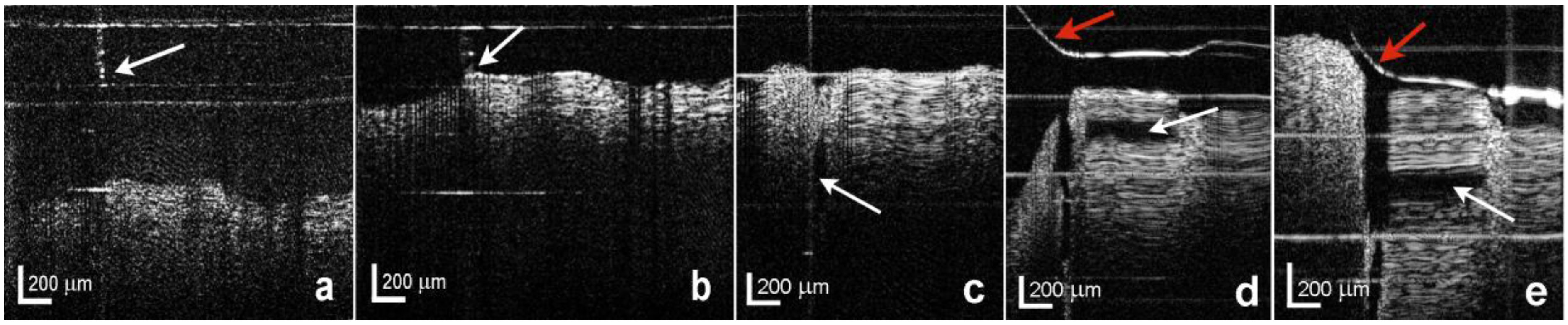
3.2. Evaluation of the Combined OCT Probe and Needle
4. Conclusions
Acknowledgments
Author Contributions
Conflict of Interest
References
- Binder, S.; Falkner-Radler, C.I.; Hauger, C.; Matz, H.; Glittenberg, C. Feasibility of intrasurgical spectral-domain optical coherence tomography. Retina 2011, 31, 1332–1336. [Google Scholar]
- Hahn, P.; Migacz, J.; O’Donnell, R.; Day, S.; Lee, A.; Lin, P.; Vann, R.; Kuo, A.; Fekrat, S.; Mruthyunjaya; et al. Preclinical evaluation and intraoperative human retinal imaging with a high-resolution microscope-integrated spectral domain optical coherence tomography device. Retina 2013, 33, 1328–1337. [Google Scholar]
- Ehlers, J.P.; Xu, D.; Kaiser, P.K.; Singh, R.P.; Srivastava, S.K. Intrasurgical dynamics of macular hole surgery: An assessment of surgery-induced ultrastructural alterations with intraoperative optical coherence tomography. Retina 2014, 34, 213–221. [Google Scholar]
- Henry, C.R.; Berrocal, A.M.; Hess, D.J.; Murray, T.G. Intraoperative spectral-domain optical coherence tomography in coats’ disease. Ophthalmic Surg. Laser. Imag. 2012, 43, e80–e84. [Google Scholar]
- Dayani, P.N.; Maldonado, R.; Farsiu, S.; Toth, C.A. Intraoperative use of handheld spectral domain optical coherence tomography imaging in macular surgery. Retina 2009, 29, 1457–1468. [Google Scholar]
- Lee, L.B.; Srivastava, S.K. Intraoperative spectral-domain optical coherence tomography during complex retinal detachment repair. Ophthalmic Surg. Laser. Imag. 2011, 42, e71–e74. [Google Scholar]
- Almony, A.; Nudleman, E.; Shah, G.K.; Blinder, K.J.; Eliott, D.B.; Mittra, R.A.; Tewari, A. Techniques, rationale, and outcomes of internal limiting membrane peeling. Retina 2012, 32, 877–891. [Google Scholar] [CrossRef]
- Carpentier, C.; Zanolli, M.; Wu, L.; Sepulveda, G.; Berrocal, M.H.; Saravia; Diaz-Llopis, M.; Gallego-Pinazo, R.; Filsecker, L.; Verdaguer-Diaz, J.I.; Milan-Navarro, R.; et al. Residual internal limiting membrane after epiretinal membrane peeling: results of the Pan-American Collaborative Retina Study Group. Retina 2013, 33, 2026–2031. [Google Scholar]
- Rothman, A.L.; Folgar, F.A.; Tong, A.Y.; Toth, C.A. Spectral domain optical coherence tomography characterization of pediatric epiretinal membranes. Retina 2014, 34, 1323–1334. [Google Scholar] [CrossRef]
- Kang, J.U.; Huang, Y.; Zhang, K.; Ibrahim, Z.; Cha, J.; Lee, W.P.A.; Brandacher, G.; Gehlbach, P.L. Real-time three-dimensional Fourier-domain optical coherence tomography video image guided microsurgeries. J. Biomed. Optic. 2012, 17, 1–6. [Google Scholar]
- Nguyen, P.; Chopra, V. Applications of optical coherence tomography in cataract surgery. Current Opin. Ophthalmol. 2013, 24, 47–52. [Google Scholar] [CrossRef]
- Abell, R.G.; Kerr, N.M.; Vote, B.J. Femtosecond laser-assisted cataract surgery compared with conventional cataract surgery. Clinical Experim. Ophthalmol. 2013, 41, 455–462. [Google Scholar] [CrossRef]
- Lee, C.; Kim, K.; Han, S.; Kim, S.; Lee, J.H.; Kim, H.K.; Kim, C.; Jung, W.; Kim, J. Stimulated penetrating keratoplasty using real-time virtual intraoperative surgical optical coherence tomography. J. Biomed. Optic. 2014, 19, 1–3. [Google Scholar]
- Juthani, V.V.; Goshe, J.M.; Srivastava, S.K.; Ehlers, J.P. Association between transient interface fluid on intraoperative OCT and textural interface opacity after DSAEK surgery in the PIONEER study. Cornea 2014, 33, 887–892. [Google Scholar] [CrossRef]
- Matz, H.; Binder, S.; Glittenberg, C.; Scharioth, G.; Findl, O.; Hirnschall, N.; Hauger, C. Intraoperative applications of OCT in ophthalmic surgery. Biomedizinische Technik (Berlin) 2012, 57, 297. [Google Scholar]
- Ehlers, J.P.; McNutt, S.A.; Kaiser, P.K.; Srivastava, S.K. Contrast-enhanced intraoperative optical coherence tomography. British J. Ophthalmol. 2013, 97, 1384–1386. [Google Scholar] [CrossRef]
- Tao, Y.K.; Srivastava, S.K.; Ehlers, J.P. Microscope-integrated intraoperative OCT with electrically tunable focus and heads-up display for imaging of ophthalmic surgical maneuvers. Biomed. Optic. Exp. 2014, 5, 1877–1885. [Google Scholar] [CrossRef]
- Ehlers, J.P.; Tam, T.; Kaiser, P.K.; Martin, D.F.; Smith, G.M.; Srivastava, S.K. Utility of intraoperative optical coherence tomography during vitrectomy surgery for vitreomacular traction syndrome. Retina 2014, 34, 1341–1346. [Google Scholar] [CrossRef]
- Hahn, P.; Migacz, J.; O’Connell, R.; Maldonado, R.S.; Izatt, J.A.; Toth, C.A. The use of optical coherence tomography in intraoperative ophthalmic imaging. Ophthal. Surg. Laser. Imag. 2011, 42, S85–S94. [Google Scholar] [CrossRef]
- Ehlers, J.P.; Tao, Y.K.; Farsiu, S.; Maldonado, R.; Izatt, J.A.; Toth, C.A. Integration of a spectral domain optical coherence tomography system into a surgical microscope for intraoperative imaging. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3153–3159. [Google Scholar] [CrossRef]
- Hahn, P.; Migacz, J.; O’Connell, R.; Izatt, J.A.; Toth, C.A. Unprocessed real-time imaging of vitreoretinal surgical maneuvers using a microscope-integrated spectral-domain optical coherence tomography system. Graefe’s Arch. Clinical Experim. Ophthalmol. 2013, 251, 213–220. [Google Scholar]
- Ehlers, J.P.; Tao, Y.K.; Farsiu, S.; Maldonado, R.; Izatt, J.A.; Toth, C.A. Visualization of real-time intraoperative maneuvers with a microscope-mounted spectral domain optical coherence tomography system. Retina 2013, 33, 232–236. [Google Scholar] [CrossRef]
- Tao, Y.K.; Srivastava, S.K.; Ehlers, J.P. Microscope-integrated intraoperative OCT with electrically tunable focus and heads-up display for imaging of ophthalmic surgical maneuvers. Biomed. Optic. Exp. 2014, 5, 1877–1885. [Google Scholar] [CrossRef]
- Joos, K.M.; Shen, J.H. Miniature real-time intraoperative forward-imaging optical coherence tomography probe. Biomed. Optic. Exp. 2013, 4, 1342–1350. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, X.; Song, C.; Kang, J.U. Motion compensated hand-held common-path Fourier-domain optical coherence tomography probe for image-guided intervention. Biomed. Optic. Exp. 2012, 3, 3105–3108. [Google Scholar] [CrossRef]
- Song, C.; Gehlbach, P.L.; Kang, J.U. Ball lens fiber optic sensor based smart handheld microsurgical instrument. Proc SPIE 2013, 8576, 1–6. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Joos, K.M.; Shen, J.-H. Preliminary Design and Evaluation of a B-Scan OCT-Guided Needle. Photonics 2014, 1, 260-266. https://doi.org/10.3390/photonics1030260
Joos KM, Shen J-H. Preliminary Design and Evaluation of a B-Scan OCT-Guided Needle. Photonics. 2014; 1(3):260-266. https://doi.org/10.3390/photonics1030260
Chicago/Turabian StyleJoos, Karen M., and Jin-Hui Shen. 2014. "Preliminary Design and Evaluation of a B-Scan OCT-Guided Needle" Photonics 1, no. 3: 260-266. https://doi.org/10.3390/photonics1030260
APA StyleJoos, K. M., & Shen, J.-H. (2014). Preliminary Design and Evaluation of a B-Scan OCT-Guided Needle. Photonics, 1(3), 260-266. https://doi.org/10.3390/photonics1030260




