1. Introduction
The study of soft-water lakes is essential due to their ecological sensitivity and significance as indicators of environmental change. Characterized by low mineral content and acidic conditions, these lakes support unique ecosystems with specialized plant and animal species. Investigating their water chemistry and biodiversity can reveal valuable information about nutrient cycling, habitat dynamics, and the impacts of anthropogenic factors such as acid rain and pollution. Furthermore, their vulnerability to climate change and human activity highlights the need for conservation efforts to preserve these ecosystems as natural laboratories for understanding broader environmental processes.
The so-called soft-water lakes are the most sensitive to such changes. They are named for their low calcium and magnesium content, resulting in poorly buffered waters that typically exhibit acidic reactions and low nutrient levels. These lakes are often overgrown by specific plant species, isoetids, specially adapted to these nutrient-poor habitats, including Lobelia dortmanna L., Isöetes lacustris L., Littorella uniflora L., and a number of other associated species.
Therefore, such lakes are sometimes called “lobelia lakes”. This term comes from a plant species, the boreal-Atlantic relict
L. dortmanna—consisting of a basal rosette of evergreen elongate leaves typically found in oligotrophic soft-water lakes [
1,
2,
3,
4]. The term is most commonly used in the scientific literature in Poland and Denmark, often in reference to “isoetid lakes” [
5].
Apart from
L. dortmanna, these lakes can become overgrown with other characteristic hydromacrophytes,
L. uniflora and
I. lacustris. Lobelia lakes are most frequent in the boreal and temperate zones of the northern hemisphere as well as at higher elevations of subtropical regions. These lakes are particularly unique within the limnetic ecosystems of Northern and Central Europe and therefore deserve special protection and attention. Investigations of Polish lobelia lakes were initiated in the middle of the twentieth century [
2,
6,
7,
8]. The primary research in this area focused on the physical and chemical composition of water and sediments and analyses of the coverage and characteristics of plant species.
The lakes in this group are mainly acidotrophic lakes. This term was first introduced by A. Thienemann in 1928. This term means low-productivity lakes with pH values below 5.5. Shinkichi Yoshimura (1933) divided acidotrophic lakes into two types: in the first, the acidic reaction of the environment is due to the characteristics of the underlying rocks, and in the second, it is due to biological processes. Later, another type of lake was added to this typification, featuring acidification due to acid rain (anthropogenic causes) [
9].
Significant progress has been made in the study of lobelia lakes in Poland. Lobelia lakes are located mainly in the northern part of the country [
8,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21]. Research has identified about 190 lobelia lakes in Poland, most of which are endorheic water bodies with small areas. Elaboration of the physical, chemical, and biological properties of Polish lobelia lakes allowed Kraska and Piotrowicz [
17] to distinguish four lake subtypes: acidified (oligohumic and polyhumic), balanced, eutrophicated, and degraded. The lobelia lakes in Poland are particularly prone to degradation and the loss of their unique character due to the poorly buffered status of the water, specific catchment physiography and lake basin morphology. The greatest threats to Polish lobelia lakes include acidification from precipitation, alkalization, eutrophication by agricultural activities or recreational use, lowering of water level induced by climate changes, draining of peat bogs’ humic water into the lakes, liming, and fish stocking—all leading to critical changes in the physical and chemical properties of their waters and, consequently, the disappearance of characteristic vegetation [
22].
The study of aquatic vegetation of lakes in Northern Europe, experiencing an anthropogenic load as a result of use in hydropower generation, allowed us to identify important factors causing the deterioration in the ecological condition of the lakes. Water level fluctuations cause shoreline erosion and, depending on the range of fluctuations, affect the species composition of sensitive macrophytes or precipitate their disappearance. For lake sagebrush, winter drawdown should not exceed 3 m [
23]. In natural water bodies of Belarus, commensurable amplitudes of water level fluctuations do not occur, and for acidotrophic lakes in the multiyear section, they do not exceed 50–60 cm.
Research of the relict aquaflora species
I. lacustris and
L. dortmanna, occurring in unique lakes of the Baltic States, Ukraine, and Russia, has primarily focused on geobotanical aspects, including distribution mapping, characterization of growing environments, and species cataloging. The highest concentration of unique lakes is concentrated in the northwestern regions of Russia and in Karelia region, detailed studies on the distribution and growing conditions of rare and protected species remain limited [
24,
25,
26].
An analysis of the structure of lake phytocoenosystems of the marginal glaciation zone of northwestern European Russia with isoetids was carried out by the staff of the Institute of Inland Water Biology of the Russian Academy of Sciences. Their findings suggest that an interpopulation exchange of genetic material in lakes is very difficult, because these water bodies are often located at high hypsometric levels relative to the rest of the hydrosystem. This isolation results in specialized hydrological and hydrochemical regimes skewed towards oligotrophy. Consequently, oligomesotrophic populations of
I. lacustris and
L. dortmanna persist in the hydrophytobiota, ecogenetically linked to pristine oligotrophic waters [
26].
In Belarus, the earliest studies of acidotrophic lakes as habitats of
I. lacustris, L. dortmana, and
Litorella lacustris date back to the early 20th century and have primarily focused on floristic characterization and the distribution of higher aquatic vegetation in Lake Svityaz [
27].
Until the 1970s, four confirmed locations of
I. lacustris growth were known in Belarus [
28]. Since 1973, the BSU initiated a comprehensive study of lakes, which included an investigation of aquatic vegetation. As a result of a large-scale survey in 1976–1977 in the Belarusian Poozerie, seven more places of
I. lacustris growth were identified, and detailed morphological, hydrological, hydrochemical, hydrobiological characteristics of the lakes serving as places of growth of the species were provided [
29].
Botanists of Vitebsk State University have continued floristic studies of lakes serving as habitats of relict-protected species of
I. lacustris and
L. dortmanna [
30]. They have carried out detailed surveys of species composition, growth patterns, physical, and chemical parameters of Lakes Glubokoe, Cherbomyslo, and Bredno, mapping and determining the productivity of their aquatic vegetation [
31,
32,
33]. In Lake Bredno, seven species of macrophytes were identified forming three zones: emergent plants, plants with floating leaves, and submerged macrophytes. This site is the only known location of
L. dortmanna in the Vitebsk region [
31], where it flourishes under optimal conditions. Only seven species of macrophytes were found in the aquatic vegetation of Lake Glubokoe. The weak plant growth in the lake is caused by the narrow littoral, low values of mineralization and content of nutrients, and acidic water conditions. A distinctive feature of the lake is that submerged macrophytes were represented by only two species—
I. lacustris and moss (
Drepanocladus Sendtneri (Schimp.))—and were confined exclusively to the most developed part of the littoral near the northern shore. The
I. lacustris phytocoenosis extended 140 m in length and 30 m in width, covering the gently sloping littoral in the northern slough at depths ranging from 0.7 to 4 m. The calculations indicated that macrophytes in Lake Glubokoe occupied an area of 0.75 ha or 1.8% of the total lake mirror area [
32].
Species diversity, biomass, and the productivity of higher aquatic vegetation in Lake Cherbomyslo 25 years ago were much higher than in other acidotrophic lakes. Fifteen macrophyte species were recorded in the lake. The belt of coastal air–water plants was formed by
P. australis,
Typha angustifolia L,
Carex elata All.,
Equisetum fluviatile L. and
Acorus calamus L. Submerged macrophytes in Lake Cherbomyslo were represented by
I. lacustris. It formed a continuous carpet about 20 m wide, extending to a depth of 3.5 m. In the western part of the lake, the lake bryozoan was abundant in the littoral zone starting at a depth of 40 cm [
33].
Regular field surveys and systematization of the accumulated material allowed the deduction of the main patterns of distribution, peculiarities of growth, environmental interactions, and problems of protection of
Isoëtes lacustris L. populations, as well as developing recommendations for their protection. These recommendations include characteristics of the object of protection, the results of inventory (passporting, distribution) of locations, revision, general conservation measures to preserve the species, a program for monitoring the state of populations, protocol of genetic monitoring of populations, a list of recommendations to optimize growing conditions, how to remove/reduce external anthropogenic threats, etc. [
34].
Building upon this foundation, the distribution of these endangered relict species in 19 unique Belarusian lakes, for which
I. lacustris and
L. dortmanna findings were definitively confirmed by herbarium specimens, was analyzed. The analysis is based on the data of long-term field observations. Investigated parameters included the morphometric, physical, and chemical parameters of lakes, plant species composition, area, and depth of growth, biomass, tiering, occurrence, abundance, and partial projective cover of species. The analysis showed the existence of an inverse dependence of species development and the following environmental parameters: water pH, concentration of total phosphorus, nitrite nitrogen, permanganate and bichromate oxidability. This allowed for the identification of two distinct lake groups. Optimal cenotic and ecological conditions are characteristic of acidotrophic lakes with sandy watersheds covered with forests and bogs, low indicators of water salinity, pH, chromaticity, content of biogenic elements, and high water transparency. Productive thickets and ‘underwater meadows’ are formed in such lakes. As a result of this study, threats to the existence of endangered species were identified and measures to organize the monitoring and creation of natural reserves for species conservation were proposed [
35]. It is very difficult to reverse back the changes in lake ecosystems that have been balanced over thousands of years and became unbalanced during decades of anthropogenic influence.
In recent years, the research has increasingly focused on the genetic diversity and population structure of
I. lacustris confined to oligotrophic lakes, whose distribution in Europe is sharply decreasing. The findings indicate that the decrease in the level of genetic diversity in small, isolated populations in combination with limiting factors (southern limit of distribution and anthropogenic eutrophication and change in lake regime) represent a high risk for the development of populations and distribution of the species in Belarus [
36].
The purpose of this study is the analysis of changes in aquatic vegetation distribution in seven soft-water Belarusian lakes and the identification of the causes of these changes.
3. Results
In recent decades, the chemical composition of the water mass of soft-water lakes in Belarus has changed. Contrary to classic acidotrophic water bodies with a pH below 5.5, most of these lakes exhibit pH values that fluctuate between 4.2 and 7.2. For instance, the water reaction in Lake Beloe (Luninets District) was slightly alkaline.
According to previous and modern studies, water mineralization in acidotrophic lakes was low and varied from 5.4 mg/dm
3 in 2001 in Lake Bredno to 65.5 mg/dm
3 in 2022 in Lake Beloe (Polotsk District). The waters of the lakes belongs to the hydrocarbonate class of calcium and magnesium groups [
39].
Additionally, the concentration of biogenic elements in the water of acidotrophic lakes in Belarus varies significantly. The phosphate ion content in all lakes, except for the hypolimnion of Lake Beloe (Luninets District), was below the sensitivity of the employed method. In Lake Beloe, it was 0.021 mg/dm3. The nitrite ion content did not exceed the sensitivity threshold of the determination method of 0.003 mg/dm3 at any sampling location. Typically, under high dissolved oxygen concentrations, the nitrogen form observed is nitrate, while an oxygen deficiency results in the predominance of the ammonium form. In general, this pattern is observed for the studied water bodies. Thus, nitrate nitrogen predominates in Lakes Cherbomyslo, Beloe (Polotsk District), and Glubokoe. In the other lakes, the ammonium form of nitrogen predominates. In Lake Beloe (Luninets District), the threshold limit value for ammonium nitrogen was exceeded by 1.6 times for domestic and drinking water supply, and by 6.3 times for fish farming. The main reason for the increase in this indicator is the large uncontrolled flow of vacationers, especially on weekends. No excess of the TLV for nitrate nitrogen was detected.
During the summer field studies, the pH in the lakes varied from 4.2 in Lake Glubokoe to 8.4 in Lake Beloe (Luninets District).
The observed changes in the pH values indicate a weakening of the bog nutrition of the studied lakes and the transition of some of them from acidotrophic state to eutrophic one. Over the long term, Lake Beloe displayed the most significant increase in pH, rising from 6.5 in 1989 to 8.4 in 2022, although this dropped to 5.4 in the winter of 2023. Since Lake Beloe is located further south than the other objects of this study, it serves as a valuable model in studying the transformation of soft-water lakes in a changing climate.
An integral indicator for quickly assessing the ecological state of a lake is water transparency. As a rule, light-water acidotrophic lakes are characterized by high transparency. Lakes Glubokoe and Beloe (Polotsk district) are the clearest lakes in Belarus. It shows the high quality of their waters. In the summer of 2022, the transparency of water in our lakes ranged from 2.1 m in Lake Bredno to 6.5 m in Lake Beloe (Polotsk district). The highest transparency during the growing season was recorded in Lake Glubokoe on 17 June 1977 (9.5 m), which is the largest value for Belarusian lakes. In winter, due to low water temperatures and short daylight hours, the phytoplankton biomass usually decreases, which leads to an increase in water transparency. Thus, in Lake Beloe (Polotsk district), on 16 February 2023 it reached 10 m and in Lake Svityaz on 14 February 2023—4.2 m, more than 1.5 times higher than the summer transparency levels.
The most significant decrease in transparency during the observation period occurred in Lake Svityaz (from 7 to 2.5 m). Similar declines were recorded in Lake Glubokoe (from 9.5 to 5.5–6 m) and Lake Bredno (from 4.6 (it was equal to maximum depth) to 2.1 m). An increase in transparency from 4.2 to 6.5 m was noted in Lake Beloe (Polotsk district). Water color, another important indicator of its quality, is influenced by the content of fulvic acids, trivalent iron compounds, and humic acids. In the open part near the surface, the water in seven studied lakes is characterized by very low and low color from 5 to 50°. In the bottom layer, as well as near the shores adjacent to bogs, the water color during periods of intensive bog nutrition may be slightly higher. The increase in water mineralization, ammonium ion concentration, decrease in dissolved oxygen concentration, and transparency indicate a deterioration in the ecological state of acidotrophic lakes in Belarus. The main reason for such changes is human economic activity. The increase in the number of vacationers on all lakes has entailed an additional influx of nutrients. Tourist campsites on Lakes Cherbomyslo, Bolshoe Ostrovito, and Glubokoe are not equipped with wastewater management systems. This inadequate infrastructure, combined with public unawareness, often results in visitors washing dishes and conducting hygiene routines directly in the lakes. These changes in water composition are the reason for the reduction in the biodiversity and area of distribution of aquatic vegetation.
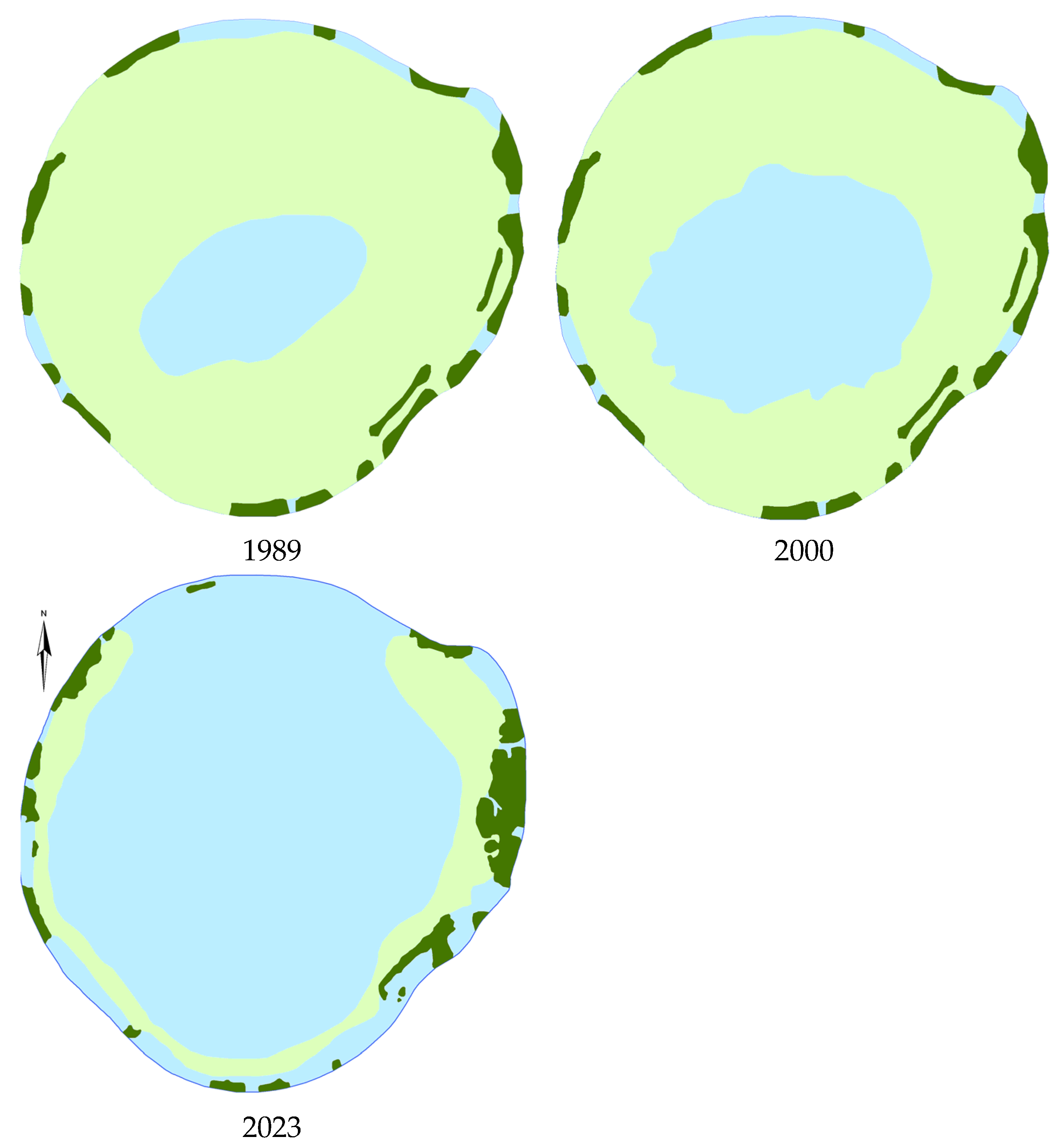
The most pronounced changes in the overgrowth are expressed in Lakes Svityaz and Beloe (Luninets district). In 2001, the higher aquatic vegetation in Lake Beloe was poorly developed. There were only two species of submerged macrophytes and four species of emergent plants with no floating leaves plants with present. Near the water’s edge, in some areas along the western shore,
Carex sp.,
Sagittaria sagittifolia L.,
Alisma plantago-aquatica L., and
A. calamus were found. In the littoral zone, at depths from 0.2 to 0.7 m along the entire shore of the lake, a rare, protected plant species,
L. dortmanna, grew. It formed an almost continuous ring 2–3.5 m wide. The second submerged species,
I. lacustris, grew almost everywhere in the littoral from a depth of 1.0 m and the sublittoral slope to a depth of 5.0 m. Lake Beloe was overgrown by 35%, as shown in
Figure 2. By 2006, the features of lake overgrowing did not change significantly.
A survey of the lake conducted in 2011 revealed a significant alteration in the extent and nature of lake overgrowth compared to previous surveys conducted in 2001 and 2006, with macrophytes now covering only 15% of the lake area. The aquatic vegetation in the lake was poorly developed, represented by two species of submerged hydrophytes and six species of emergent plants. Plants with floating leaves were represented by one species—
Persicaria amphibia (L.) Delarbre. Along the water’s edge down to a depth of 0.3–0.5 m, in some coastal areas, there were
Carex physodes M. Bieb.,
Phragmites australis,
T. angustifolia L.,
A. plantago-aquatica,
Eleocharis acicularis (L.) Roem. & Schult, and
A. calamus. In the littoral zone, at depths from 0.2 to 1.0 m, along the entire shore of the lake,
L. dortmanna grew, forming an almost continuous ring 2.0–3.5 m wide.
I. lacustris grew sporadically at depths of 1.0–3.0 m, with sporadic occurrences of Fontinalis antipyretica Hedw. [
37].
The extent of overgrowing in isoetid lakes is closely related with the transparency, pH, and water mineralization. Consequently, the observed alterations within the lake ecosystem can be linked to a slight, albeit insignificant, increase in mineralization and pH. The appearance in the species composition and distribution of
P. australis,
Typha sp., and
P. amphibia suggests a trend towards shifting the type of lake overgrowth from hydrophytic to helo-hydrophytic [
3,
4]. By 2016, the areas of overgrowth of aerohydrophytes had expanded, with these plants initially observed as single specimens or small groups in the early 2000s. Aquatic plants were represented by four species, among which
C. physodes was dominant. Over 15 years, a belt of aerohydrophytes formed around the entire lake shore in a discontinuous strip with a width of about 2–15 m. They formed mainly pure associations consisting of
Phragmites and
T. angustifolia. These species were identified in 2011. In 2016, they formed thickets with a projective cover of up to 30%. Among the thickets of
T. angustifolia and
P. australis,
S. sagittifolia,
A. plantago-aquatica, and
E. acicularis were noted as an admixture.
Plants with floating leaves were represented by P. amphibia. The species grew as single specimens or small groups (3–5 plants) in the southwestern part of the lake.
Submerged plants were represented by L. dortmanna, I. lacustris, and single specimens of water moss.
L. dortmanna was noted in the littoral zone at depths from 0.3 to 0.8 m along the entire perimeter of the lake. It was absent only in the beach zone. I. lacustris grew at depths of 0.6–2.8 m, indicating a reduction in the area and depth of its distribution.
Changes in the overgrowing of Lake Beloe occurred through the formation of a belt of emergent plants and the spread of new species (Phragmites, T. angustifolia). This led to a decrease in the overgrown area of the lake, which reduced from 30% to 10%, primarily due to submerged plants. Apparently, these changes are associated with the active recreational use of the areas surrounding the lake. There are health camps here, and temporary recreation facilities during the warm season. However, human activities are not the only reason. A decrease in precipitation in the catchment area, especially in the summer, has transformed previously swampy forest into a dry one. This reduction in moisture has resulted in a diminished influx of acidic waters to the lake. Currently, a strip of emergent macrophytes, represented mainly by Phragmites, has finally formed. It interrupts only at the entrances to the water. In the western part of the lake, the emergent plant belt includes Carex and Lythrum salicaria Blush. L. salicaria is noted in the east. I. lacustris grows in the lake as separate oppressed specimens near the western shore at depths of 1.3–1.7 m. L. dortmanna previously formed a continuous strip and is now restricted to the western shore at depths from 0.25 to 1.6 m. P. amphibia was not found. Therefore the strip of plants with floating leaves is absent in the lake.
Thus, the degradation of higher aquatic vegetation is observed in Lake Beloe. It is expressed in a decrease in the overgrowth area from 35% to 3.2% of water surface area [
37]. Submerged macrophytes have almost completely disappeared, and the dominant role belongs to emergent ones.
In Lake Svityaz, a monitoring point has been established to track the dynamics of macrophyte species composition and distribution. According to the results of floristic studies of the lake in 1989, the list of species included species such as
T. angustifolia,
Ranunculus circinatus Sibth.,
Fontinalis, and two species listed in the Red Book of the Republic of Belarus:
Caulinia flexilis and
Hydrilla verticillata (L.f.) Royle. They were not found during the summer survey of the lake in July 2000. The change in the patterns of overgrowing of Lake Svityaz is shown in
Figure 3.
By 2000, the emergent plant communities in Lake Svityaz were represented by four species, including P. australis, Eleocharis palustris (L.) Roem. & Schult., and Schoenoplectus lacustris (L.) Palla and Scirpus sylvaticus L. which was found in the splash zone near the western shore of the lake.
The main coenosis-forming plant strip in the lake is P. australis. It forms a strip, interrupted in places with lagoons, with an average width of 25 m (with a maximum width of up to 200−250 m along the eastern shore).
Along the eastern shore, near the Svorotva River outflow, sparse thickets of
E. palustris were noted at depths of up to 0.5 m. Along the eastern and northwestern shores, together with
L. uniflora,
L. dortmanna is often found in the lower tier [
37].
Emergent plants occupied an area of 0.16 km2 in the lake. This represents about 9% of the lake area.
Plants with floating leaves in Lake Svityaz were represented by two species: Polygonum amphibium L. and Potamogeton natans L. They occupied very small areas. In the northwestern part of the lake, near the shore at depths of 0.2–0.8 m, P. amphibium and P. natans formed a spot with an area of 300 m2. In the southeastern part of the lake, in the Phragmites thickets, single specimens of P. natans were noted.
A clear pattern was observed in the underwater vegetation cover of Lake Svityaz. Depths of 0.3–1.8 m serve the ecological niche for low-growing bottom plants completely submerged in water—the L. uniflora and L. dortmanna. These species are included in the Red Book of the Republic of Belarus. They form a continuous belt along the banks of both pure formations mainly at depths of 1.0–1.5 m and mixed with lobelia at depths of up to 1.0 m, less often with Elodea canadensis Michx., at depths of 1.5–1.8 m. L. dortmanna grew only at depths from 0.3 to 1.0 m near the western and northwestern shores.
In Lake Svityaz, the protected species I. lacustris has been observed to grow in small thickets in the northwestern part of the lake, at depths ranging from 1.7 to 1.8 m. Rare specimens of I. lacustris together with L. dortmanna and L. uniflora were noted along Phragmites thickets at a depth of 0.7 m 100–150 m north of the Novogrudok beach.
The fourth species of protected plants growing in Lake Svityaz was
Nitella gracilis (Smith J.E.) Agardh C.A. It formed a pure formation with a projective cover of 30% at a depth of 1.5 m along the north–north-western shore with an area of no more than 10 m
2. In 2020, the area of this association was only 1 m
2 [
40].
Hydrophytes occupied 11% of the lake area [
37]. The depths from 2.0 m to the maximum distribution of vegetation (4.5 m) were the ecological niche of
Elodea and
Potamogeton.
P. praelongus Wulfen,
P. lucens L.,
Potamogeton compressus L., and
P. crispus L. were rarer. Elodea clearly dominated the thickets, achieving the maximum development in the northern part of the lake at depths of 3.0–4.5 m (average biomass 0.300 kg/m
2, projective cover 70%); in the southern part of the lake, the densest cover of elodea is at depths of 2.5 m, and at depths of 4.5 m the thickets of elodea are sparse, pondweed is more common, the edge of the vegetation is uneven. In total, the thickets of elodea together with pondweed occupied 0.78 km
2. Their total biomass was estimated at 156 tons of air-dry weight. In general, the lake was overgrown by 65%, and the biomass of macrophytes was estimated at 264 tons of air-dry matter, with approximately 85% attributed to underwater plants.
Analysis of the productivity of two autotrophic communities in the lake ecosystem represented by macrophytes (about 40 g/m3) and phytoplankton (less than 10 g/m3) indicates that the main production–destruction function in the ecosystem of the lake belonged to completely submerged plants. The degree of their development is determined by the water quality in Lake Svityaz. At present, due to non-compliance with the regime of protection of the lake from additional eutrophication and pollution from the territory surrounding the lake, this balance is disturbed. A comparison of current findings with data from previous years reveals notable changes, including a decrease in the maximum growth depth of underwater plants from 7 m to just 2 m. L. uniflora is found at depths from 0.2 to 1.5 m, I. lacustris forms a narrow strip along the shores at depths of 1.5–1.8 m; the water moss, which, according to research in the 1980s, covered the bottom at depths of up to 7 m, has disappeared from the underwater vegetation cover. Modern research has not confirmed the growth of three protected plant species in the lake—C. flexilis, N. gracilis and H. verticillata, mentioned during a survey of the lake in the 1990s (information from P.V. Parfenov). A structural reorganization of the species composition of the plant community has occurred. The reduction in the range and disappearance of the I. lacustris and Fontinalis, respectively, an increase in the area under thickets of E. Canadensis, P. natans, as well as the appearance of P. scirpus in the underwater vegetation cover indicate an increase in the trophic level of the lake.
The study of the distribution of aquatic vegetation of Lake Beloe (Polotsk District) was carried out in 1977, 2022, and 2023. In 1977, higher aquatic vegetation occupied about 20% of the lake area. The morphometric features of lake depression (narrow littoral and steep sublittoral) prevented the development of vegetation, but high transparency made it possible for macrophytes (
P. compressus) to penetrate to a greater depth (8.4 m). The depth of distribution of emergent macrophytes reached 1.6 m, and plants with floating leaves up to 2 m. Mosses were found at a depth of 12.7 m [
34].
Carex, Equisetum, and Spargánium sp. were found among the near-shore plants in the lake. Emergent plants were represented mainly by Phragmites. In general, emergent plants did not develop in the lake water area. Overall, emergent plant growth in the lake was sparse, forming only fragmented sections rather than continuous strips, with the widest distribution noted in the northwest (150 m).
Plants with floating leaves were found only in bays. P. amphibia, Nuphar lutea (L.) Sm., and P. natans were noted.
Submerged macrophytes were represented by two species of
Potamogeton (
P. perfoliatus and
P. compressus),
Myriophyllum verticillatum L., and
Elodea [
37]. The limited diversity in macrophyte species and the presence of
I. lacustris define the unique features of the lake’s vegetation. Among the changes that have occurred in the nature of the distribution of higher aquatic vegetation, it is necessary to note the increase in the area of
P. australis growth, and the width of the strip occupied by it. In the north-east of the lake, according to remote sensing data, it reaches 110 m. Plants with floating leaves are distributed mainly in bays, as well as single specimens of
N. lutea.
P. amphibia and
P. natans are present among the
P. australis thickets. The width of the
N. lutea strip in the northern bays reaches 50 m.
Isoetes sp. grows in the northern and north-eastern parts of the lake at depths of 1.3–4 m occasionally forming mixed associations with elodea at depths of approximately 2.5 m.
During initial surveys of Lake Bolshoe Ostrovito,
T. angustifolia,
P. australis,
Comarum palustre L., and
Carex sp. were noted along the shore, forming a band of emergent vegetation up to 10 m wide. Aquatic mosses and
I. lacustris completely covered the lake bottom to a depth of 5 m. In addition to
Isoetes, another rare species was found in the lake—the
Sparganium gramineum Georgi. Its distribution in Belarus requires clarification and further study. The flora of the lake was distinguished by the poor species composition of macrophytes. Emergent plants, as well as plants with floating leaves (
Menyanthes trifoliata L.,
Carex,
P. australis,
T. angustifolia,
N. lutea,
P. amphibia), did not form a continuous strip. They were found as single specimens, forming sparse areas to a depth of 1.0–1.7 m.
Isoetes lacustris grew along the shore to a depth of 2 m, while water moss dominated in the profundal up to 5 m [
37]. The results of the last studies indicate a decrease in the depth of the
I. lacustris to 1.3 m near the northwestern shore and degradation of the water moss thickets. The area of aquatic plants has diminished from 91% of the lake area in the late 1970s to 21% in 2023.
Higher aquatic vegetation in Lake Glubokoe is poorly developed both during the first survey of the lake and now. It is represented by only nine species:
P, australis,
P. amphibia,
Carex elata subsp. omskiana (Meinsh.) Jalas,
I. lacustris, Juncus conglomeratus L.,
Drepanocladus,
N. lutea, Calla palustris L., and
Comarum palustre L. Emergent plants are represented by
P. australis,
Carex elata subsp. Omskiana, and
J. conglomeratus. The only phytocenosis of
Phragmites, 30 m long and 3.5 m wide, was confined to the northern part of the lake, where it occupied sandy soils and depths from the water’s edge to 0.8–1.0 m [
37]. Rare thickets of
C. elata. occupied almost the entire littoral of the lake. Clumps of it were located at a distance of 2–5 m from each other at a depth of up to 0.4–0.5 m. In some places, very rare and low-growing clumps of
P. australis were mixed with
C. elata. Single clumps of it, confined to sandy bottom deposits, were noted near the eastern and southern shores of the open part of the lake. and around the island. Some of its plants reached a height of 50 cm.
The distribution of floating-leaved plants was characterized by pure associations of N. lutea and sporadic single specimens of P. amphibia found in the southwestern bay and near the island. N. lutea formed patches ranging from 50 to 100 m2, predominantly distributed in the northwestern and southwestern bays
Due to the lack of essential biogenic elements in the bottom sediments and water, broadleaf pondweeds were completely absent from the vegetation cover of Lake Glubokoe. Submerged macrophytes were represented by
I. lacustris and water moss. They were confined exclusively to the most gently sloping section of the littoral near the northern shore.
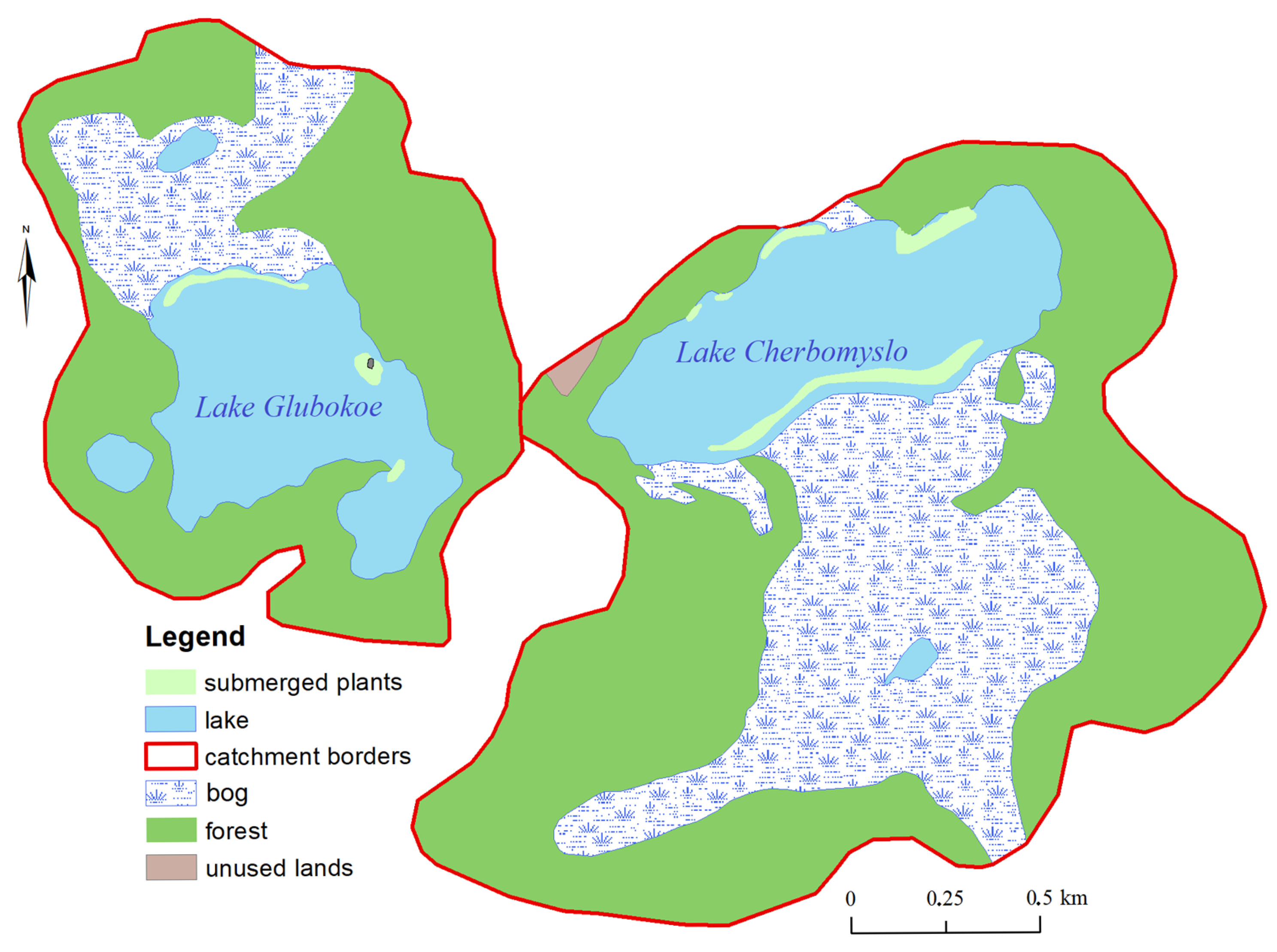
I. lacustris occupied a space measuring 140 m long and 30 m wide. It should be noted that most often the thickets of
I. lacustris are confined to the shores adjacent to bogs. This can be seen in the example of Lakes Glubokoe and Cherbomyslo (
Figure 4).
To the south of the boundary of I. lacustris distribution there was a phytocenosis of Drepanocladus. It was connected with sandy soils and slightly covered with a thin layer of fine-detrital sapropel at a depth of 2 to 7 m (the maximum depth of plant growth).
During the survey of Lake Glubokoe conducted in 2011, all the thickets of
I. lacustris and
Drepanocladus were densely covered with threads of the massively developing alga
Mougeotia. By 2022–2023, the depth of the growth of the
I. lacustris in the north of the lake has decreased [
40]. Previously, it flourished at depths between 0.7 and 4 m; however, in 2023, its growth was limited to depths of 0.7 to 2.2 m. On a positive note, a new area of its distribution was found. It had not been mentioned in any study before. The association frames the island in a semicircle (except for the north and northeast) and grows at a depth by 4 m.
Drepanocladus was not observed deeper than 3.5 m. Among other changes in the lake overgrowth, the reduction in the number of
Carex clumps is noted at a distance of several meters from the shores. Now, it grows only in a narrow strip along the shore. All macrophytes growing in Lake Glubokoe form pure associations. It indicates the initial stage of lake overgrowth.
Only seven species of macrophytes have been documented in Lake Bredno. They formed three strips: air-aquatic plants, plants with floating leaves, and submerged macrophytes. On the border of the littoral and shore, almost along the entire length of the coastline, small but well-developed tussocks of Carex elata subsp. omskiana (Meinsh.) Jalas grew. Some of the tussocks near the eastern cape grew at a distance of up to 15 m from the coastline in the association of Lobelia, but most often it was noted among the thickets of P. australis. E. fluviatile was represented by single plants and practically did not participate in the formation of the vegetation cover. Plants about 80 cm high were noted on sandy deposits at a depth of 0.5 m in the northwestern part of the lake.
The strip of air-aquatic plants was formed by Carex sp., P. australis, and L. dortmanna. Lobelia thickets occupied sandy soils, sometimes with peat layers, at a depth of 30 to 100 cm. They occupied over 1/3 of the littoral area accessible to it. Pure lobelia phytocenoses was distributed on the area of 0.50 ha. The Lobelia population in Lake Bredno was in optimal conditions for its growth.
The floating-leaved plant zone in Lake Bredno was represented by fragmentary pure phytocenoses of
N. lutea and
Nymphaea Alba L. [
31]. Individual clumps of
N. lutea occupied areas ranging from 40 to 1000 m
2 and were confined mainly to the eastern and southeastern parts of the lake. They preferred depths from 70 to 120 cm and sandy soils. Groups of
Nymphaea were typical for the littoral of the western and northwestern parts of the lake. Clumps of
Nuphar occupied an area from 25 to 150 m
2 and were confined to soft sandy-silty soils. The depth of its growth varied from 100 to 180 cm. The conditions for the growth of the Nymphaea in Lake Bredno were close to optimal.
The strip of submerged plants in Lake Bredno was represented by I. lacustris, which occupied the sandy littoral with depths from 60 to 150 cm. Its thickets were absent only in the southwestern part of the lake, where the bottom is covered with silt deposits.
Over the past decade, significant shifts have been observed in the nature of the lake’s overgrowth. A decrease in water transparency coupled with heightened eutrophication processes has led to a decline in the I. lacustris population. Currently, its growth is restricted to southern and eastern parts of the cape at depths of up to 2 m. Deeper than the growth limit of Isoetes, to a depth of 4 m, the bottom is covered with dense thickets of moss, presumably belonging to the species Drepanocladus sendtneri (Schimp. ex H.Müll.) Warnst.
The area and density of lobelia thickets have also decreased. North of the cape, submerged plants are absent, due to gel-like bottom sediments which inhibit the rooting ability of macrophytes.
In Lake Cherbomyslo, P. australis dominates in the layer of emergent macrophytes. Carex, Schoenoplectus lacustris (L.) Palla (not previously noted), T. angustifolia, and E. fluviatile are less common. They form a discontinuous strip varying in width from 1 to 35 m, with the widest sections located in the bays near the northern and northeastern shores of the lake.
Among the plants with floating leaves, there are P. amphibia, N. lutea, P. natans, and Sparganium angustifolium. They are distributed at depths of up to 2 m. The width of the strip is up to 30 m.
Submerged plants grow at depths of 0.3 to 2.2–2.5 m and are represented by I. lacustris. It forms a discontinuous strip up to 80 m wide. Submerged plants are absent on heavily silted areas of the bottom. I. lacustris is most widely distributed near the bogs in the north and south of the lake, the greatest depth of distribution (2.5 m) is noted in the eastern part of the lake.
Changes in the overgrowth of Lake Cherbomyslo are manifested in a reduction in the depth of distribution of submerged macrophytes [
40] from 4 m in 1977 to 2.2–2.5 m in 2023. Due to an error in the reconstruction of the Bolshoe Sitno—Beloe road, the installation of a drainage pipe under the road on the outflow from the lake without taking into account the hypsometric position of the water edge. As a result, backwater conditions developed and led to a rise in the water level. The influx of acidic waters from the bog located to the south of the lake diminished.
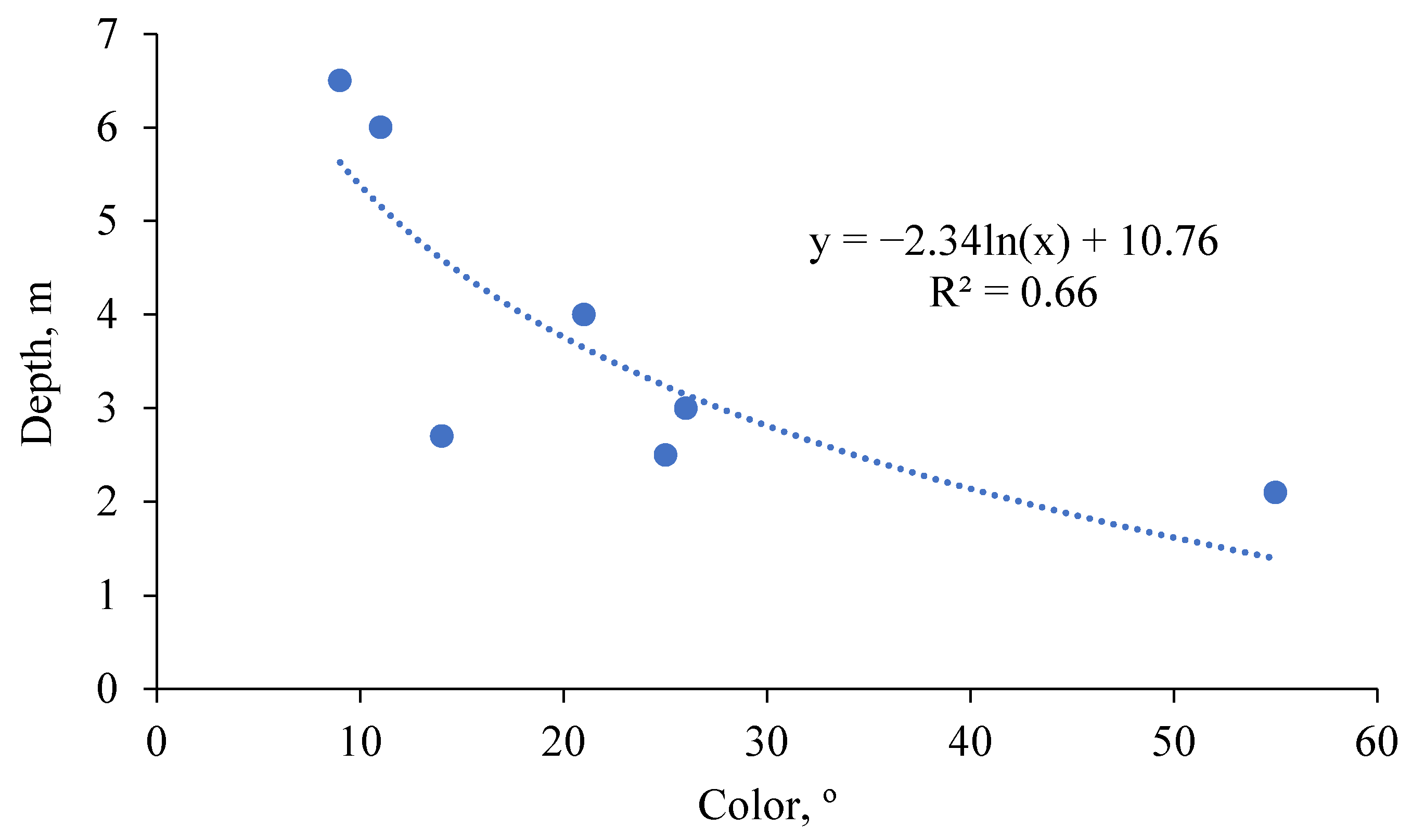
To analyze the connection between the maximum depths of macrophytes distribution we calculated the correlation coefficients between it and different parameters of water chemical composition. Significant coefficient (more than 0.754 with the significance level of 0.05) was found only for water color (Pt-Co scale)—0.81. A graph reflecting the relationship between these parameters is shown in
Figure 5.
A value close to significant (0.72) was also obtained for water transparency. This happens due to some individual characteristics of the lakes. For example, in Lake Beloe (Luninets district), at the bottom, at depths that could be occupied by macrophytes, a suspended detritus was found. It was the dead remains of phytoplankton. Therefore, despite the high transparency of the water in the open part of the water body, the light conditions near the shores and bottom are not very good.
Now, anthropogenic impact leads to the accumulation of biogenic elements. A large amount of nutrients in the water has a beneficial effect on the development of phytoplankton. It causes the increase in pH from acidic to neutral or alkaline means and death of acidophilic plants. Algal blooms were noted in Lakes Bredno and Svityaz in August 2023. Just 20 years ago, such phenomena were not observed on these lakes.
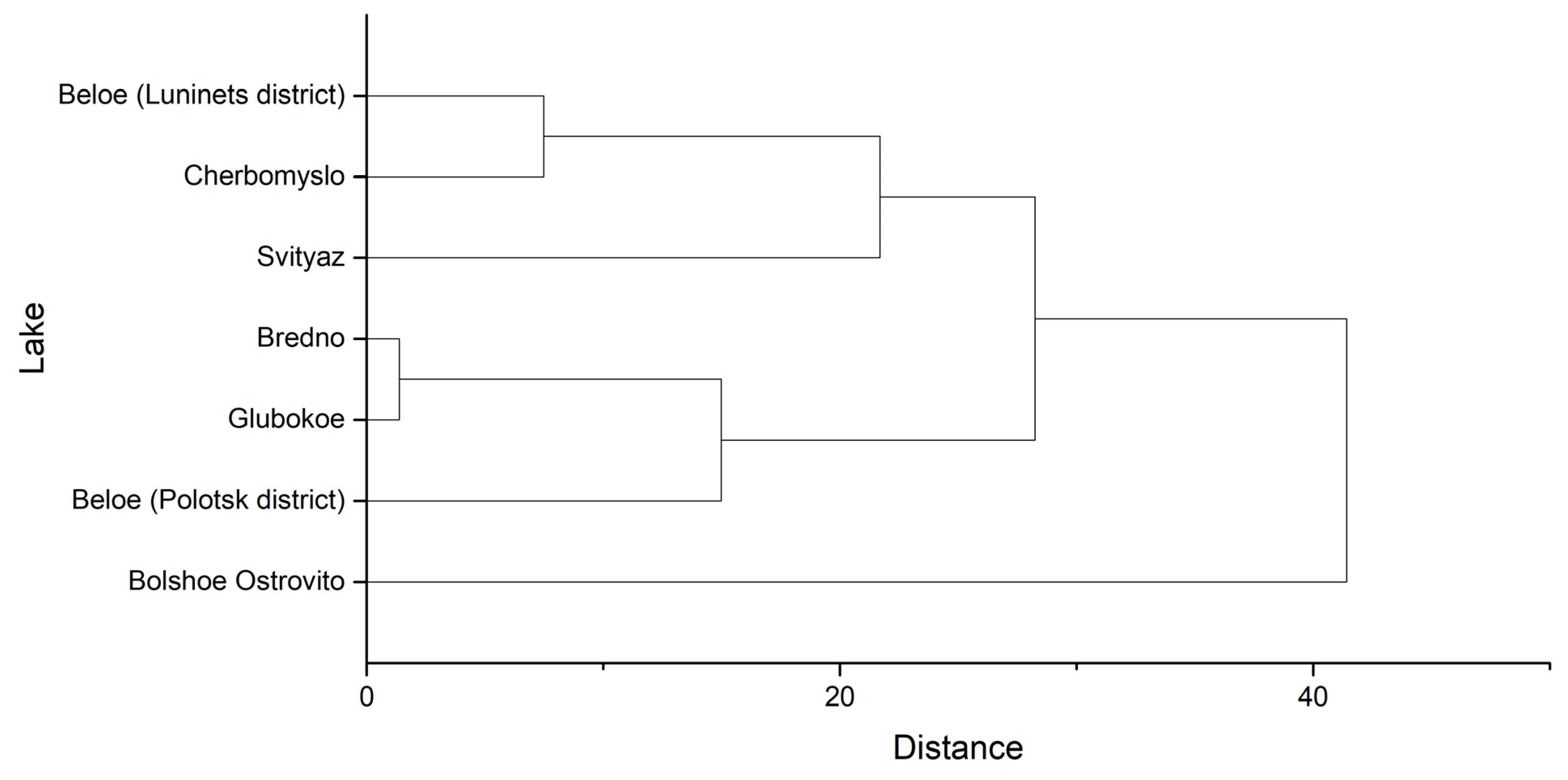
Hierarchical cluster analysis based on comparison of 14 hydrochemical and optical parameters (mineralization components, biogenic elements, pH, transparency, color) allowed us to identify three groups of lakes (
Figure 6).
The first group includes lakes that are subject to the most active anthropogenic impact due to high recreational load in the summer period and are distinguished by the highest degree of degradation of submerged macrophytes. The second group includes lakes with a less pronounced impact of recreation. Lake Bolshoe Ostrovito stands apart from these groups. Despite the relatively low level of anthropogenic impact, the degradation of water plants in it is very pronounced. The reasons for this are yet to be determined.