Environmental Pollution and Biological Invasions Threaten Native Freshwater Infaunal Bivalves in the Guandu River Basin, Southeast Brazil
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Samplings
2.3. Laboratory Procedures
2.4. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ST | sampling station |
References
- Dudgeon, D. Prospects for sustaining freshwater biodiversity in the 21st century: Linking ecosystem structure and function. Curr. Opin. Environ. Sustain. 2010, 2, 422–430. [Google Scholar] [CrossRef]
- Zawal, A.; Lewin, I.; Stępień, E.; Szlauer-Łukaszewska, A.; Buczyńska, E.; Buczyński, P.; Stryjecki, R. The influence of the landscape structure within buffer zones, catchment land use and instream environmental variables on mollusc communities in a medium-sized lowland river. Ecol. Res. 2016, 31, 853–867. [Google Scholar] [CrossRef]
- Leung, B.; Hargreaves, A.L.; Greenberg, D.A.; McGill, B.; Dornelas, M.; Freeman, R. Reply to: Emphasizing declining populations in the Living Planet Report. Nature 2022, 601, E25–E26. [Google Scholar] [CrossRef] [PubMed]
- Arora, N.K.; Mishra, I. United Nations Sustainable Development Goals 2030 and environmental sustainability: Race against time. Environ. Sustain. 2019, 2, 339–342. [Google Scholar] [CrossRef]
- Pinto, G.R.S.; Arbache, J.; Antonaccio, L.; Chiavari, J. Cutting Down the (Hydropower) Plants: How the Amazon Deforestation Is Jeopardizing Electricity Generation in Brazil. Rio de Janeiro. 2024. Available online: https://bit.ly/DeforestationAndHydropower (accessed on 26 March 2025).
- Tubbs Filho, D.; Antunes, J.C.O.; Silva Vettorazzi, J. Bacia Hidrográfica dos Rios Guandu, da Guarda e Guandu-Mirim: Experiências Para a Gestão dos Recursos Hídricos; Instituto Estadual do Meio Ambiente (INEA): Janeiro, Brazil, 2012; p. 339. Available online: https://www.comiteguandu.org.br/conteudo/livroguandu2013.pdf (accessed on 26 March 2025).
- Brito, E.M.S.; Vieira, E.D.R.; Torres, J.P.M.; Malm, O. Persistent organic pollutants in two reservoirs along the Paraíba do Sul-Guandu River system, Rio de Janeiro, Brazil. Quim. Nova 2005, 28, 941–946. [Google Scholar] [CrossRef]
- Lydeard, C.; Cowie, R.H.; Ponder, W.F.; Bogan, A.E.; Bouchet, P.; Clark, S.A.; Thompson, F.G. The global decline of nonmarine mollusks. BioScience 2004, 54, 321–330. [Google Scholar] [CrossRef]
- Strong, E.E.; Gargominy, O.; Ponder, W.F.; Bouchet, P. Global diversity of gastropods (Gastropoda; Mollusca) in freshwater. Hydrobiologia 2008, 595, 149–166. [Google Scholar] [CrossRef]
- Santos, E.B.D. Variação Espaço-Temporal da Qualidade da Água na Bacia do Rio Guandu: Uma Abordagem Visando a Gestão de Recursos Hídricos. Bachelor’s Thesis, Universidade Federal Rural do Rio de Janeiro, Rio de Janeiro, Brazil, 2023; p. 33. Available online: https://rima.ufrrj.br/jspui/handle/20.500.14407/8758 (accessed on 26 March 2025).
- Darrigran, G.; Agudo-Padrón, I.; Baez, P.; Belz, C.; Cardoso, F.; Carranza, A.; Collado, G.; Correoso, M.; Cuezzo, M.G.; Fabres, A.; et al. Non-native mollusks throughout South America: Emergent patterns in an understudied continent. Biol. Invasions 2020, 22, 853–871. [Google Scholar] [CrossRef]
- Vaughn, C.C.; Hakenkamp, C.C. The functional role of burrowing bivalves in freshwater ecosystems. Freshw. Biol. 2001, 46, 1431–1446. [Google Scholar] [CrossRef]
- Yeager, M.M.; Cherry, D.S.; Neves, R.J. Feeding and burrowing behaviors of juvenile rainbow mussels, Villosa iris (Bivalvia: Unionidae). J. N. Am. Benthol. Soc. 1994, 13, 217–222. [Google Scholar] [CrossRef]
- Williams, J.D.; Warren, M.L.; Cummings, K.S., Jr.; Harris, J.L.; Neves, R.J. Conservation status of freshwater mussels of the United States and Canada. Fisheries 1993, 18, 6–22. [Google Scholar] [CrossRef]
- Strayer, D.L.; Caraco, N.F.; Cole, J.J.; Findlay, S.; Pace, M.L. Transformation of Freshwater Ecosystems by Bivalves. BioScience 1999, 49, 19–27. [Google Scholar] [CrossRef]
- McMahon, R.F. Evolutionary and physiological adaptations of aquatic invasive animals: R Selection versus Resistance. Can. J. Fish. Aquat. Sci. 2002, 59, 1235–1244. [Google Scholar] [CrossRef]
- Sousa, R.; Antunes, C.; Guilhermino, L. Species composition and monthly variation of the Mollusca fauna in the freshwater subtidal area of the River Minho Estuary. Estuar. Coast. Shelf Sci. 2007, 75, 75–100. [Google Scholar] [CrossRef]
- Miyahira, I.C.; Carneiro, J.B.; Gonçalves, I.C.B.; Lacerda, L.E.M.D.; Oliveira, J.L.D.; Vasconcelos, M.C.D.; Santos, S.B.D. Freshwater mollusks and environmental assessment of Guandu River, Rio de Janeiro, Brazil. Biota Neotrop. 2017, 17, e20170342. [Google Scholar] [CrossRef]
- Ferreira, A.P. Environmental investigation of psychiatric pharmaceuticals: Guandu River, Rio De Janeiro State, Southeast Brazil. J. Chem. Health Risks 2014, 4, 25–32. [Google Scholar]
- Malm, O.; Pfeiffer, W.C.; Fiszman, M.; Azcue, J.M.P. Heavy metal concentrations and availability in the bottom sediments of the Paraiba do Sul-Guandu River system, RJ, Brazil. Environ. Sci. Technol. Lett. 1989, 10, 675–680. [Google Scholar] [CrossRef]
- Terlizzi, A.; Fraschetti, S.; Guidetti, P.; Boero, F. The effects of sewage discharge on shallow hard substrate sessile assemblages. Mar. Pollut. Bul. 2005, 44, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Cope, W.G.; Bergeron, C.M.; Archambault, J.M.; Jones, J.W.; Beaty, B.; Lazaro, P.R.; Damian, S.; Callihan, J.S.; Rogers, J.J. Understanding the influence of multiple pollutant stressors on the decline of freshwater mussels in a biodiversity hotspot. Sci. Total Environ. 2021, 773, 144757. [Google Scholar] [CrossRef]
- Bespalaya, Y.V.; Aksenova, O.V.; Sokolova, S.E.; Shevchenko, A.R.; Tomilova, A.A.; Zubrii, N.A. Biodiversity and distributions of freshwater mollusks in relation to chemical and physical factors in the thermokarst lakes of the Gydan Peninsula, Russia. Hydrobiologia 2021, 848, 3031–3044. [Google Scholar] [CrossRef]
- Pérez-Quintero, J.C. Distribution patterns of freshwater molluscs along environmental gradients in the southern Guadiana River basin (SW Iberian Peninsula). Hydrobiologia 2011, 678, 65–76. [Google Scholar] [CrossRef]
- Schuler, M.S.; Hintz, W.D.; Jones, D.K.; Mattes, B.M.; Stoler, A.B.; Relyea, R.A. The effects of nutrient enrichment and invasive mollusks in freshwater environments. Ecosphere 2020, 11, e03196. [Google Scholar] [CrossRef]
- Jiang, X.; Li, Z.; Shu, F.; Chen, J. Effects of river-lake disconnection and eutrophication on freshwater mollusc assemblages in floodplain lakes: Loss of congeneric species leads to changes in both assemblage composition and taxonomic relatedness. Environ. Pollut. 2022, 292, 118330. [Google Scholar] [CrossRef] [PubMed]
- McMahon, T.A.; Halstead, N.T.; Johnson, S.; Raffel, T.R.; Romansic, J.M.; Crumrine, P.W.; Rohr, J.R. Fungicide-induced declines of freshwater biodiversity modify ecosystem functions and services. Ecol. Lett. 2020, 15, 714–722. [Google Scholar] [CrossRef]
- Miyahira, I.C.; Clavijo, C.; Callil, C.T.; Cuezzo, M.G.; Darrigran, G.; Gomes, S.R.; Lasso, C.A.; Mansur, M.C.D.; Pena, M.S.; Ramírez, R.; et al. The conservation of non-marine molluscs in South America: Where we are and how to move forward. Biodivers. Conserv. 2022, 31, 2543–2574. [Google Scholar] [CrossRef]
- Miyahira, I.C.; Mansur, M.C.D.; Lacerda, L.E.M.; Gonçalves, I.C.B.; Sant’Anna, G.G.; Santos, S.B. Protected areas and native freshwater bivalves are not in the same place in south-east Brazil. Aquat. Conserv. 2023, 33, 102–114. [Google Scholar] [CrossRef]
- Miyahira, I.C.; Neves, R.A.F.; Rocha, R.J.S.; Castelo-Branco, C.W.; Santos, L.N. A new basin, a new river, a new home-the introduction of Limnoperna fortunei (Dunker, 1857) in Paraíba do Sul basin and its potential consequences to the most important basin for water supply in Southeast Brazil. BioInvasions Rec. 2024, 13, 799–814. [Google Scholar] [CrossRef]
- Instituto Estadual do Meio Ambiente. Relatório da Qualidade da Água do Estado do Rio de Janeiro—Rio de Janeiro. 2013. Available online: https://www.inea.rj.gov.br/wp-content/uploads/2019/01/Consolidado-2013-RH-II.pdf (accessed on 26 March 2025).
- Serber, J.B. Diagnóstico Ambiental das Atividades do Pólo Industrial de Queimados Como Subsídio ao Termo de Ajustamento de Conduta na Gestão Sustentável da Bacia Hidrográfica do Rio Guandu, RJ. Master’s Thesis, Universidade do Estado do Rio de Janeiro, Rio de Janeiro, Brazil, 2005. Available online: https://comiteguandu.org.br/wp-content/uploads/2021/11/DIAGNOSTICO-AMBIENTAL-DAS-ATIVIDADES-DO-POLO-INDUSTRIAL-DE.pdf (accessed on 26 March 2025).
- Resolução CONAMA n° 357/2005, de 17 de Março de 2005. Dispõe Sobre a Classificação dos Corpos de Água e Diretrizes Ambientais Para o Seu Enquadramento, bem Como Estabelece as Condições e Padrões de Lançamento de Efluentes, e dá Outras Providências. Available online: https://conama.mma.gov.br/?option=com_sisconama&task=arquivo.download&id=450 (accessed on 26 March 2025).
- Instituto Estadual do Ambiente. Portal Geo INEA: Base de Dados Espaciais. Available online: https://geoportal.inea.rj.gov.br/portal/apps/experiencebuilder/experience/?id=811a0feace564581afae2f9149b8031d (accessed on 26 March 2025).
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association (APHA): Washington, DC, USA, 2001; p. 1325. [Google Scholar]
- ASTM. Standard test methods of ammonia nitrogen in water. In Manual of Water and Environmental Technology, D1426; ASTM: West Conshohocken, PA, USA, 2021; Available online: https://standards.iteh.ai/catalog/standards/astm/4e86e02a-fa1b-49c4-b263-1f0d37181970/astm-d1426-08#:~:text=SCOPE,any%2C%20associated%20with%20its%20use. (accessed on 15 January 2025).
- Bott, E.D.; Riley, E.A.; Kahr, B.; Reid, P.J. Unraveling the dispersed kinetics of dichlorofluorescein in potassium hydrogen phthalate crystals. J. Phys. Chem. A 2010, 114, 7331–7337. [Google Scholar] [CrossRef]
- Wentworth, C.K. A scale of grade and class terms for clastic sediments. J. Geol. 1922, 30, 377–392. [Google Scholar] [CrossRef]
- Shepard, F.P. Nomenclature based on sand-silt-clay ratios. J. Sediment. Res. 1954, 24, 151–158. [Google Scholar]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater, 18th ed.; American Public Health Association (APHA): Washington, DC, USA, 1992. [Google Scholar]
- Simpson, S.L.; Batley, G.E.; Chariton, A.A.; Stauber, J.L.; King, C.K.; Chapman, J.C.; Hyne, R.V.; Gale, S.A.; Roach, A.C.; Maher, W.A. Handbook for Sediment Quality Assessment; EMCR: Bangor, NSW, Australia, 2005; p. 126. [Google Scholar]
- Suguio, K. Introdução a Sedimentologia; Edgard Blucher & Editora da Universidade de São Paulo: São Paulo, Brazil, 1973; p. 317. [Google Scholar]
- Wendlandt, W.W.M. Thermal Analysis; John Wiley: New York, NY, USA, 1986; p. 460. [Google Scholar]
- Protazio, L.T.S.M.C.N.; Tanaka, S.M.C.N.; Cavalcante, P.R.S. Avaliação de procedimentos de extração sequencial de fósforo em sedimento. Analytica 2004, 8, 35–41. [Google Scholar]
- Legendre, P.; Gallagher, E.D. Ecologically meaningful transformations for ordination of species data. Oecologia 2001, 129, 271–280. [Google Scholar] [CrossRef]
- Bacha, L.; Ventura, R.; Barrios, M.; Seabra, J.; Tschoeke, D.; Garcia, G.; Masi, B.; Macedo, L.; Godoy, J.M.O.; Cosenza, C.; et al. Risk of collapse in water quality in the Guandu River (Rio de Janeiro, Brazil). Microb. Ecol. 2022, 84, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Bianco, K.; Albano, R.M.; Oliveira, S.S.A.; Alves Nascimento, A.P.; dos Santos, T.; Clementino, M.M. Possible health impacts due to animal and human fecal pollution in water intended for drinking water supply of Rio de Janeiro, Brazil. J. Water Supply Res. Technol. 2020, 69, 70–84. [Google Scholar] [CrossRef]
- Cooper, N.L.; Bidwell, J.R.; Cherry, D.S. Potential effects of Asian clam (Corbicula fluminea) die-offs on native freshwater mussels (Unionidae) II: Porewater ammonia. J. N. Am. Bethol. Soc. 2005, 24, 381–394. [Google Scholar] [CrossRef]
- Pereira, D. Bivalves límnicos na América do Sul: Subsídios Para a Conservação de Espécies Nativas e Para o Controle do Bivalve Invasor Limnoperna fortunei (DUNKER, 1857). PhD. Thesis, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil, 2014. Available online: http://hdl.handle.net/10183/206392 (accessed on 26 March 2025).
- Ferreira, T.P.; Bauerfeldt, G.F.; Castro, R.N.; Magalhães, V.S.; Alves, M.C.; Scott, F.B.; Cid, Y.P. Determination of fipronil and fipronil-sulfone in surface waters of the Guandu River Basin by high-performance liquid chromatography with mass spectrometry. Bullet. Environ. Contam. Toxicol. 2022, 108, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.V.; Teixeira, J.T.D.S.; Lima, V.M.D.; Borba, H.R. Induction of cytotoxic and genotoxic effects of Guandu River waters in the Allium cepa system. Rev. Ambiente Água 2015, 10, 48–58. [Google Scholar] [CrossRef]
- Lauritsen, D.D. Filter-feeding in Corbicula fluminea and its effect on seston removal. J. N. Am. Benthol. Soc. 1986, 5, 165–172. [Google Scholar] [CrossRef]
- Neves, R.A.F.; Naveira, C.; Miyahira, I.C.; Portugal, S.G.M.; Krepsky, N.; Santos, L.N. Are invasive species always negative to aquatic ecosystem services? the role of dark false mussel for water quality improvement in a multi-impacted urban coastal lagoon. Water Res. 2020, 184, 116108. [Google Scholar] [CrossRef]
- Beaver, J.R.; Crisman, T.L.; Brock, R.J. Grazing effects of an exotic bivalve (Corbicula fluminea) on hypereutrophic lake water. Lake Reserv. Manag. 1991, 7, 45–51. [Google Scholar] [CrossRef]
- Yamamuro, M.; Koike, I. Nitrogen metabolism of the filter-feeding bivalve Corbicula japonica and its significance in primary production of a brackish lake in Japan. Limnol. Oceanogr. 1993, 38, 997–1007. [Google Scholar] [CrossRef]
- Nakamura, Y.; Kerciku, F. Effects of filter-feeding bivalves on the distribution of water quality and nutrient cycling in a eutrophic coastal lagoon. J. Mar. Syst. 2000, 26, 209–221. [Google Scholar] [CrossRef]
- Hakenkamp, C.C.; Palmer, M.A. Introduced bivalves in freshwater ecosystems: The impact of Corbicula on organic matter dynamics in a sandy stream. Oecologia 1999, 119, 445–451. [Google Scholar] [CrossRef]
- Modesto, V.; Ilarri, M.; Labecka, A.M.; Ferreira-Rodríguez, N.; Coughlan, N.E.; Liu, X.; Sousa, R. What we know and do not know about the invasive Asian clam Corbicula fluminea. Hydrobiologia 2025, 852, 1183–1214. [Google Scholar] [CrossRef]
- Philipp, E.E.; Abele, D. Masters of longevity: Lessons from long-lived bivalves—A mini-review. Gerontology 2010, 56, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Albentosa, M.; Sánchez-Hernández, M.; Campillo, J.A.; Moyano, F.J. Relationship between physiological measurements (SFG-scope for growth-) and the functionality of the digestive gland in Mytilus galloprovincialis. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2012, 163, 286–295. [Google Scholar] [CrossRef]
- Sokolova, I.M.; Frederich, M.; Bagwe, R.; Lannig, G.; Sukhotin, A.A. Energy homeostasis as an integrative tool for assessing limits of environmental stress tolerance in aquatic invertebrates. Mar. Environ. Res. 2012, 79, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sousa, R.; Antunes, C.; Guilhermino, L.E.D.P.S. Ecology of the invasive Asian clam Corbicula fluminea (Müller, 1774) in aquatic ecosystems: An overview. Int. J. Limnol. 2008, 44, 85–94. [Google Scholar] [CrossRef]
- Modesto, V.; Castro, P.; Lopes-Lima, M.; Antunes, C.; Ilarri, M.; Sousa, R. Potential impacts of the invasive species Corbicula fluminea on the survival of glochidia. Sci. Total Environ. 2019, 673, 157–164. [Google Scholar] [CrossRef]
- Vannote, R.L.; Minshall, G.W.; Cummins, K.; Sedell, J.R.; Cushing, C.E. The river continuum concept. Can. J. Fish. Aquat. Sci. 1980, 37, 130–137. [Google Scholar] [CrossRef]
- Cristofaro, C.D.S.; Castelo-Branco, C.W.; Rocha, M.I.D.A.; Portugal, S.G.M. Assessing glyphosate concentrations in six reservoirs of Paraíba do Sul and Guandu River Basins in southeast Brazil. Rev. Ambiente Água 2021, 16, e2615. [Google Scholar] [CrossRef]
- Marmontel, C.V.F.; Rodrigues, V.A. Parâmetros Indicativos para Qualidade da Água em Nascentes com Diferentes Coberturas de Terra e Conservação da Vegetação Ciliar. Floresta E Ambiente 2015, 22, 171–181. [Google Scholar] [CrossRef]
- Derisio, J.C. Introdução ao Controle de Poluição Ambiental, 2nd ed.; Signus Editora: São Paulo, Brazil, 2000. [Google Scholar]
- Barbosa, R.G.; Sleutels, T.; Verstraete, W.; Boon, N. Hydrogen oxidizing bacteria are capable of removing orthophosphate to ultra-low concentrations in a fed batch reactor configuration. Bioresour. Technol. 2020, 311, 123494. [Google Scholar] [CrossRef] [PubMed]
- Castelo-Grande, T.; Augusto, P.A.; Rico, J.; Marcos, J.; Iglesias, R.H.L.; Barbosa, D. Magnetic water treatment in a wastewater treatment plant: Part I—Sorption and magnetic particles. J. Environ. Manag. 2021, 281, 111872. [Google Scholar] [CrossRef]
- Rocha, E.G.; Feitosa, P.H.C.; Amorim, C.M.; Barbosa, D.L. Temporal and spatial trends of a floating islands system’s efficiency. J. Environ. Manag. 2021, 277, 111367. [Google Scholar] [CrossRef]

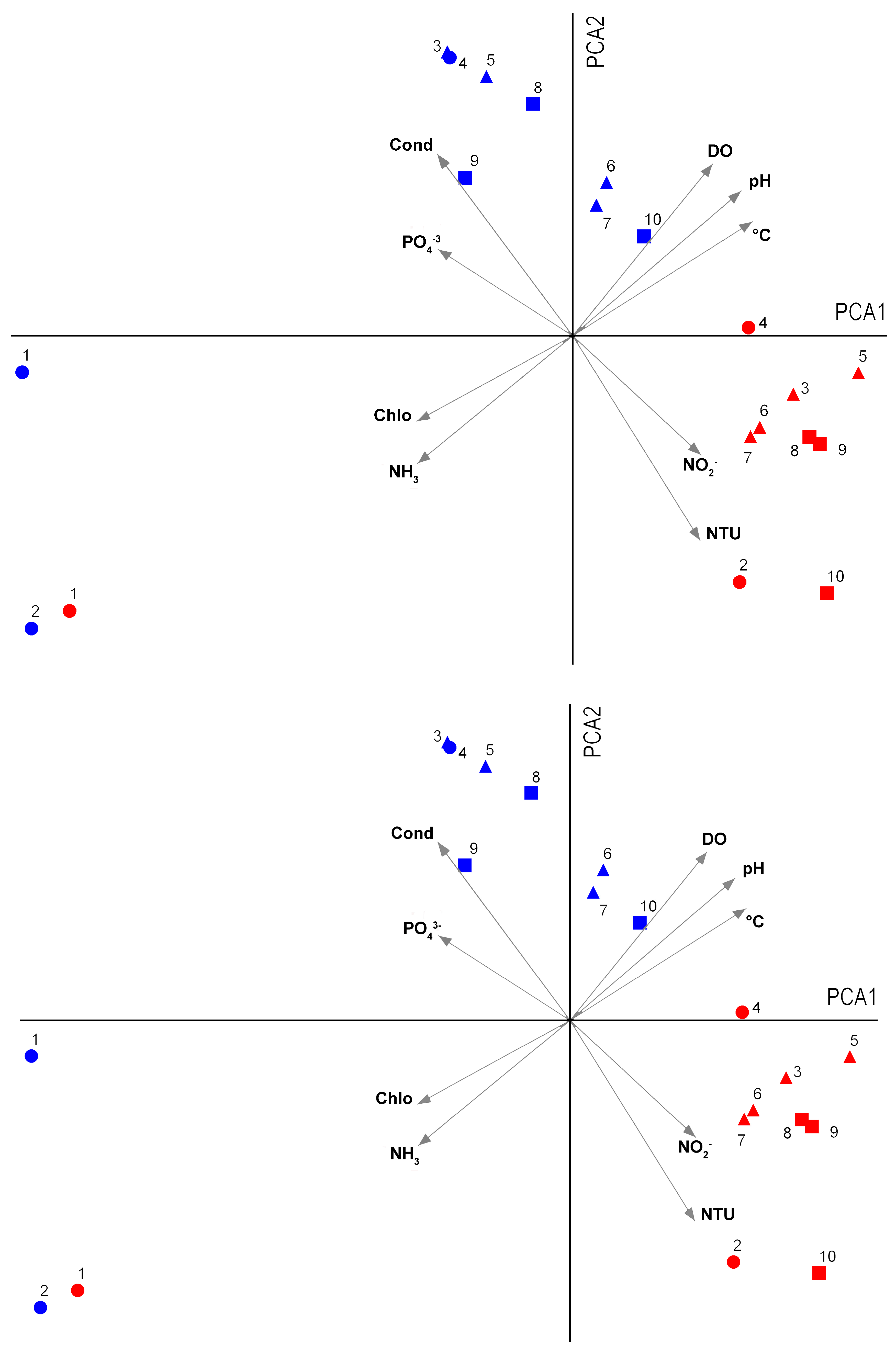
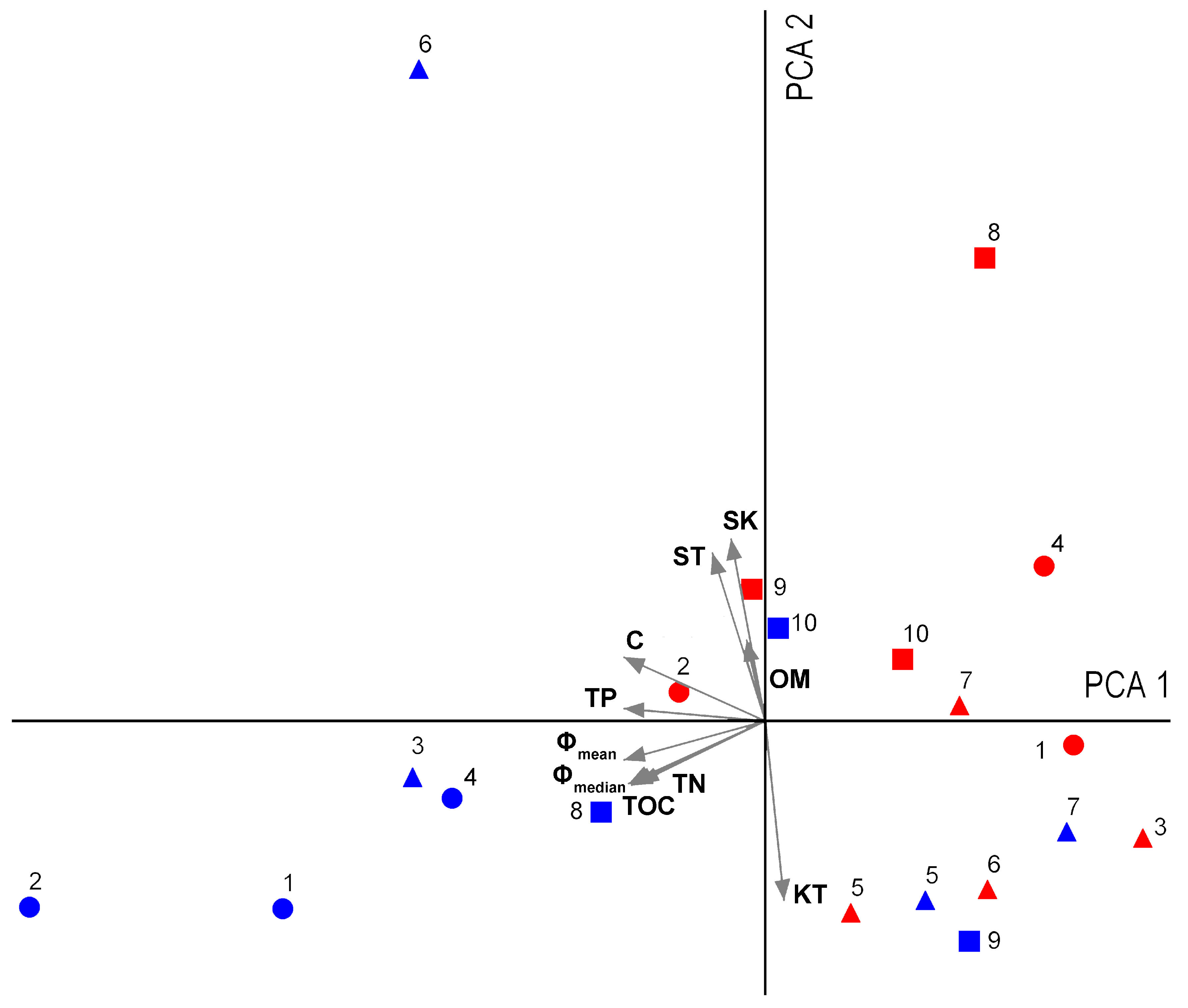
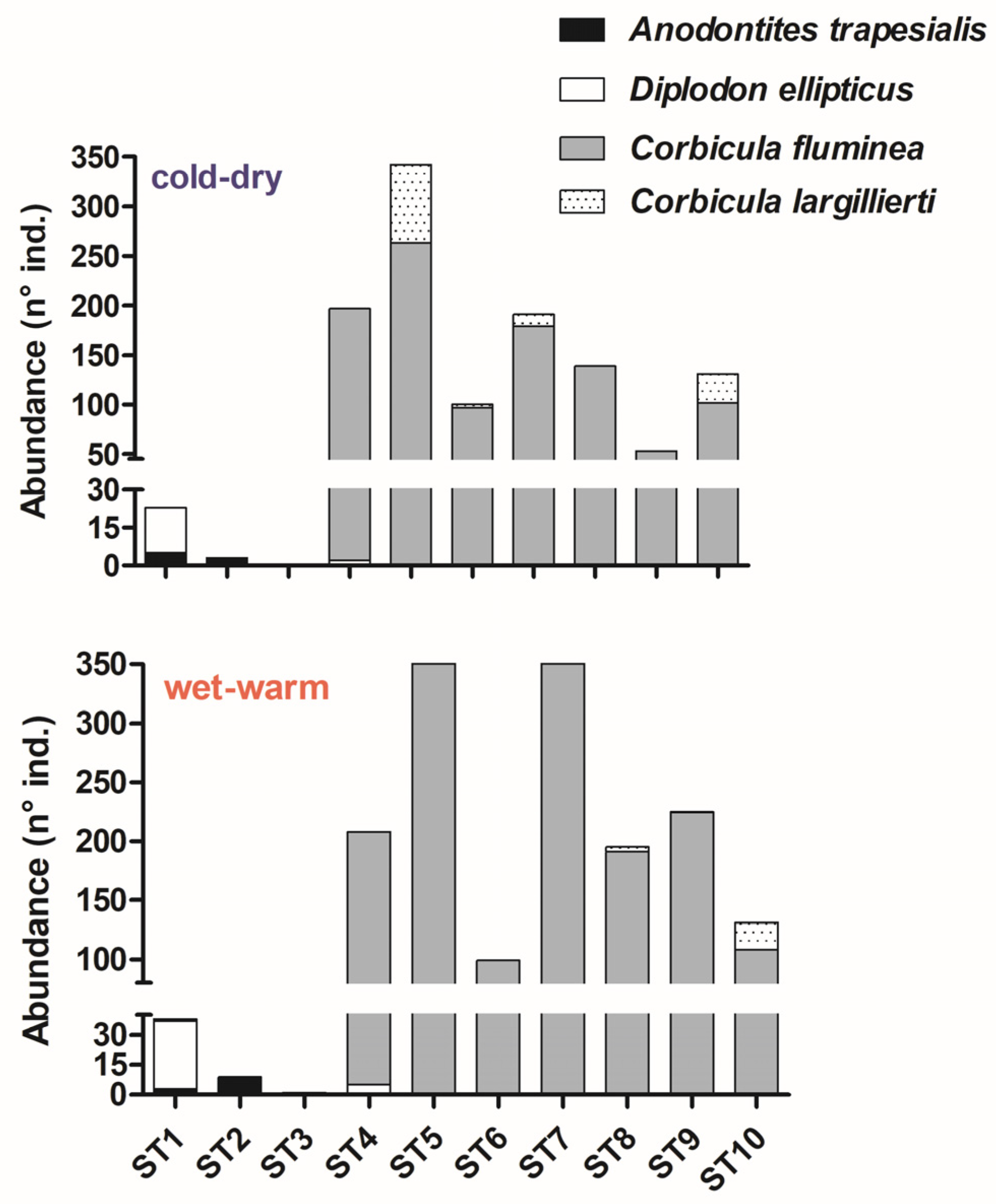
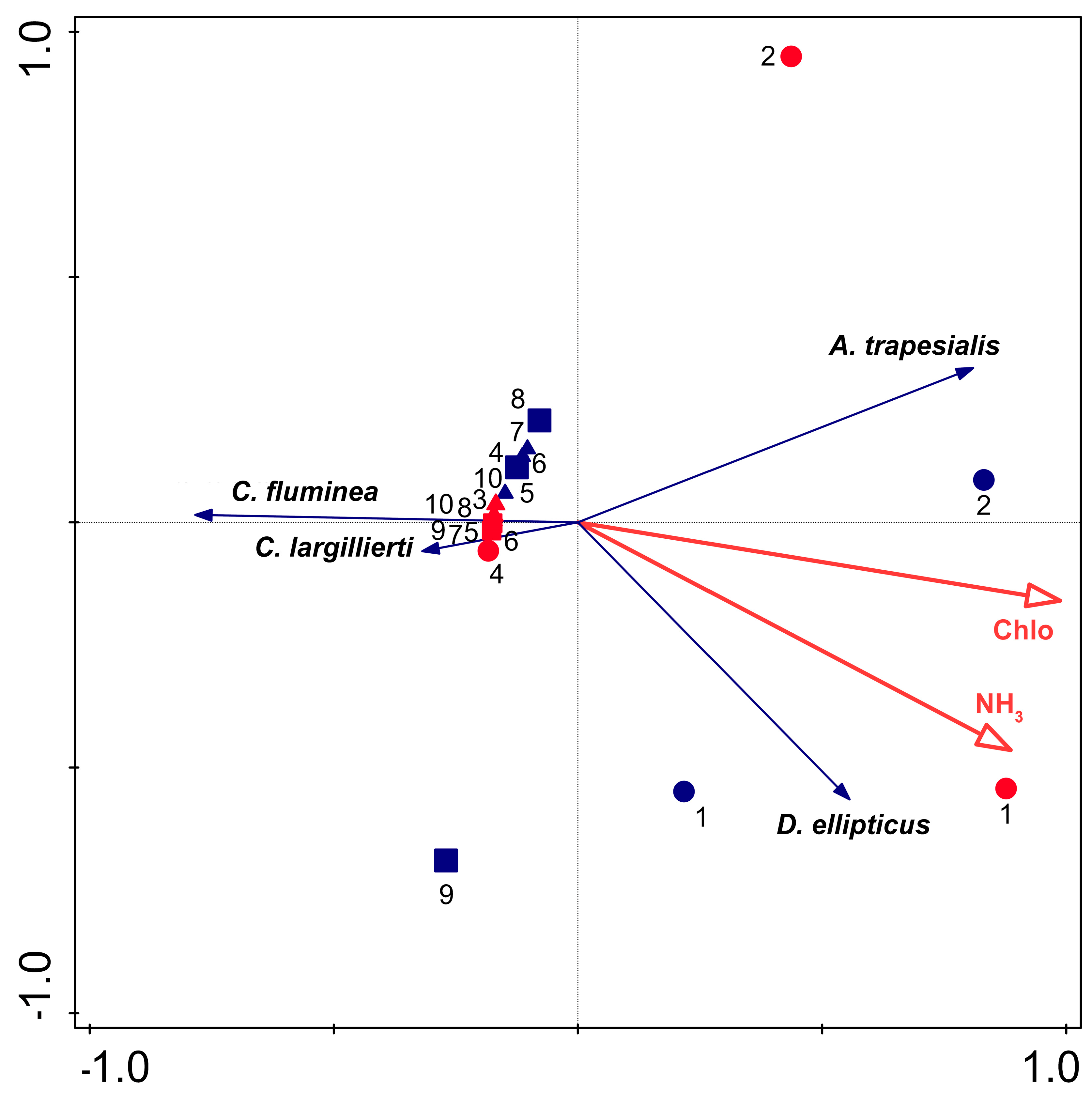
| Variables | ST1 | ST2 | ST3 | ST4 | ST5 | ST6 | ST7 | ST8 | ST9 | ST10 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cold–dry | °C | 22.66 | 23.0 | 22.03 | 23.85 | 22.15 | 21.89 | 21.49 | 22.72 | 22.34 | 21.86 |
| Cond (μS/cm) | 180.0 | 264.0 | 89.50 | 88.00 | 89.00 | 90.00 | 89.50 | 89.00 | 86.00 | 97.50 | |
| DO (mg/L) | 3.67 | 1.70 | 8.36 | 6.73 | 9.03 | 9.58 | 9.00 | 7.90 | 7.63 | 8.63 | |
| DO (%) | 42.0 | 16.20 | 95.75 | 79.20 | 103.05 | 709.65 | 107.05 | 94.70 | 90.30 | 107.15 | |
| pH | 6.11 | 6.93 | 6.90 | 6.28 | 6.68 | 6.62 | 6.35 | 6.52 | 6.48 | 7.34 | |
| NTU | 1.77 | 30.68 | 1.10 | 0.35 | 2.71 | 3.07 | 3.21 | 0.43 | 1.08 | 3.75 | |
| Chlo (µg/L) | 31.0 | 64.0 | 1.00 | 2.00 | 1.00 | 2.50 | 3.00 | 4.00 | 1.00 | 2.00 | |
| PO43− (mg/L) | 2.200 | 1.550 | 1.500 | 1.150 | 1.250 | 0.060 | 0.070 | 0.050 | 0.065 | 0.050 | |
| NH3 (mg/L) | 4.531 | 6.372 | 0.140 | 0.018 | 0.049 | 0.023 | 0.036 | 0.00 | 0.182 | 0.024 | |
| NO2− (mg/L) | 0.000 | 0.007 | 0.000 | 0.000 | 0.000 | 0.033 | 0.049 | 0.000 | 0.000 | 0.168 | |
| Wet–warm | °C | 27.96 | 28.22 | 24.54 | 25.51 | 24.94 | 25.51 | 25.46 | 25.45 | 25.49 | 24.53 |
| Cond (μS/cm) | 258.0 | 82.0 | 75.5 | 76.5 | 74.0 | 88.0 | 86.5 | 73.0 | 75.5 | 80.5 | |
| DO (mg/L) | 3.85 | 12.48 | 7.45 | 5.23 | 8.35 | 7.09 | 6.57 | 6.45 | 6.27 | 4.24 | |
| DO (%) | 49.10 | 159.9 | 88.95 | 64.75 | 100.8 | 88.00 | 82.45 | 80.60 | 79.10 | 55.35 | |
| pH | 7.05 | 8.65 | 6.49 | 6.39 | 6.74 | 6.40 | 6.37 | 6.13 | 6.29 | 7.33 | |
| NTU | 13.46 | 17.63 | 20.63 | 9.10 | 18.67 | 23.98 | 24.19 | 21.39 | 21.81 | 40.78 | |
| Chlo (µg/L) | 73.5 | 29.5 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| PO43− (mg/L) | 1.310 | 0.300 | 0.125 | 0.000 | 0.055 | 0.035 | 0.05 | 0.045 | 0.000 | 0.065 | |
| NH3 (mg/L) | 8.826 | 0.827 | 0.079 | 0.225 | 0.000 | 0.055 | 0.109 | 0.127 | 0.091 | 0.091 | |
| NO2− (mg/L) | 0.069 | 0.049 | 0.035 | 0.059 | 0.058 | 0.066 | 0.058 | 0.041 | 0.046 | 0.064 |
| Variables | ST1 | ST2 | ST3 | ST4 | ST5 | ST6 | ST7 | ST8 | ST9 | ST10 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cold–dry | Grain size parameters | ||||||||||
| Φmean | 3.71 | 4.73 | 4.11 | 5.21 | 2.65 | 3.14 | 1.24 | 2.87 | 1.98 | 2.68 | |
| Φmedian | 3.51 | 4.53 | 3.93 | 4.90 | 2.58 | 3.14 | 1.29 | 3.05 | 1.90 | 2.84 | |
| Sorting (Φ) | 2.10 | 2.09 | 1.92 | 2.05 | 1.26 | 3.45 | 1.57 | 2.51 | 1.84 | 2.95 | |
| Skewness | 0.19 | 0.17 | 0.18 | 0.24 | 0.23 | 1.73 | −0.01 | −0.07 | 0.12 | 0.03 | |
| Kurtosis | 1.15 | 1.09 | 1.15 | 1.03 | 1.30 | 0.32 | 1.20 | 1.18 | 1.85 | 0.77 | |
| Sediment classification * | |||||||||||
| Mean | VFS | CS | CS | MS | FS | VFS | MS | FS | MS | FS | |
| Sorting | VPS | VPS | PS | VPS | PS | PS | PS | VPS | PS | VPS | |
| Asymmetry | PA | PA | PA | PA | PA | VPA | AS | AS | PA | AS | |
| Kurtosis | L | M | L | M | L | L | L | L | VL | P | |
| Folk’s classification | MS | SM | MS | SM | SM | SGS | SGMS | SGMS | SGMS | SGMS | |
| Sediment content | |||||||||||
| OM (mg/g) | 0.630 | 0.370 | 0.890 | 0.650 | 0.330 | 0.390 | 0.460 | 0.660 | 0.400 | 0.620 | |
| Carbonates (%) | 3.470 | 5.020 | 3.760 | 4.290 | 0.960 | 6.640 | 0.700 | 2.760 | 1.210 | 1.100 | |
| C (mg/g) | 59.94 | 48.38 | 25.68 | 15.10 | 1.560 | 0.660 | 1.320 | 36.53 | 2.290 | 11.45 | |
| N (mg/g) | 5.000 | 4.850 | 2.150 | 1.580 | 0.240 | 0.060 | 0.170 | 1.820 | 0.270 | 1.000 | |
| P (mg/g) | 0.896 | 2.394 | 0.922 | 0.732 | 0.353 | 1.460 | 0.171 | 0.481 | 0.316 | 0.320 | |
| Wet–warm | Grain size parameters | ||||||||||
| Φmean | 0.89 | 1.81 | 0.32 | 0.78 | 2.55 | 2.09 | 1.61 | 1.37 | 1.83 | 2.30 | |
| Φmedian | 0.71 | 2.03 | 0.23 | 0.83 | 2.56 | 2.12 | 1.90 | −0.36 | 2.28 | 1.91 | |
| Sorting (Φ) | 1.93 | 1.99 | 1.93 | 1.38 | 1.83 | 0.78 | 2.24 | 2.88 | 2.68 | 2.64 | |
| Skewness | 0.19 | −0.05 | 0.37 | −0.07 | −0.03 | 0.04 | 0.01 | 0.90 | −0.04 | 0.27 | |
| Kurtosis | 1.20 | 0.77 | 0.75 | 0.93 | 1.60 | 0.91 | 1.13 | 0.55 | 0.64 | 0.96 | |
| Sediment classification * | |||||||||||
| Mean | CS | MS | CS | CS | FS | FS | MS | MS | MS | FS | |
| Sorting | PS | PS | PS | PS | PS | MS | VPS | VPS | VPS | VPS | |
| Asymmetry | PA | AS | VPA | AS | AS | AS | AS | VPA | AS | PA | |
| Kurtosis | L | P | P | M | VL | M | L | VP | VP | M | |
| Folk’s classification | SGS | SGMS | SGS | SGS | SGMS | S | SGMS | SGMS | SGMS | SGMS | |
| Sediment content | |||||||||||
| OM (mg/g) | 0.420 | 0.940 | 0.760 | 0.090 | 0.670 | 0.210 | 0.840 | 1.160 | 0.810 | 0.500 | |
| Carbonates (%) | 0.930 | 2.390 | 1.150 | 0.560 | 1.470 | 1.530 | 1.060 | 1.110 | 2.530 | 0.420 | |
| C (mg/g) | 2.610 | 26.54 | 9.140 | 0.550 | 8.800 | 0.440 | 3.610 | 3.660 | 19.93 | 5.540 | |
| N (mg/g) | 0.290 | 1.790 | 0.590 | 0.000 | 0.650 | 0.000 | 0.290 | 0.460 | 1.170 | 1.210 | |
| P (mg/g) | 0.169 | 1.050 | 0.274 | 0.096 | 0.425 | 0.287 | 0.207 | 0.187 | 0.396 | 0.096 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, N.; Miyahira, I.C.; Rodrigues, A.J.S.; Santos, L.N.; Neves, R.A.F. Environmental Pollution and Biological Invasions Threaten Native Freshwater Infaunal Bivalves in the Guandu River Basin, Southeast Brazil. Limnol. Rev. 2025, 25, 24. https://doi.org/10.3390/limnolrev25020024
Rodrigues N, Miyahira IC, Rodrigues AJS, Santos LN, Neves RAF. Environmental Pollution and Biological Invasions Threaten Native Freshwater Infaunal Bivalves in the Guandu River Basin, Southeast Brazil. Limnological Review. 2025; 25(2):24. https://doi.org/10.3390/limnolrev25020024
Chicago/Turabian StyleRodrigues, Nathália, Igor C. Miyahira, Antonio J. S. Rodrigues, Luciano N. Santos, and Raquel A. F. Neves. 2025. "Environmental Pollution and Biological Invasions Threaten Native Freshwater Infaunal Bivalves in the Guandu River Basin, Southeast Brazil" Limnological Review 25, no. 2: 24. https://doi.org/10.3390/limnolrev25020024
APA StyleRodrigues, N., Miyahira, I. C., Rodrigues, A. J. S., Santos, L. N., & Neves, R. A. F. (2025). Environmental Pollution and Biological Invasions Threaten Native Freshwater Infaunal Bivalves in the Guandu River Basin, Southeast Brazil. Limnological Review, 25(2), 24. https://doi.org/10.3390/limnolrev25020024








