False Positive Results of Phosphatidylethanol (PEth) Quantitation in Dried Blood Spots (DBS): The Influence of Alcohol Vapors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Blood Sampling
2.3. Sample Preparation
2.4. Instrumentation for Qualification Phosphatidyl Derivatives
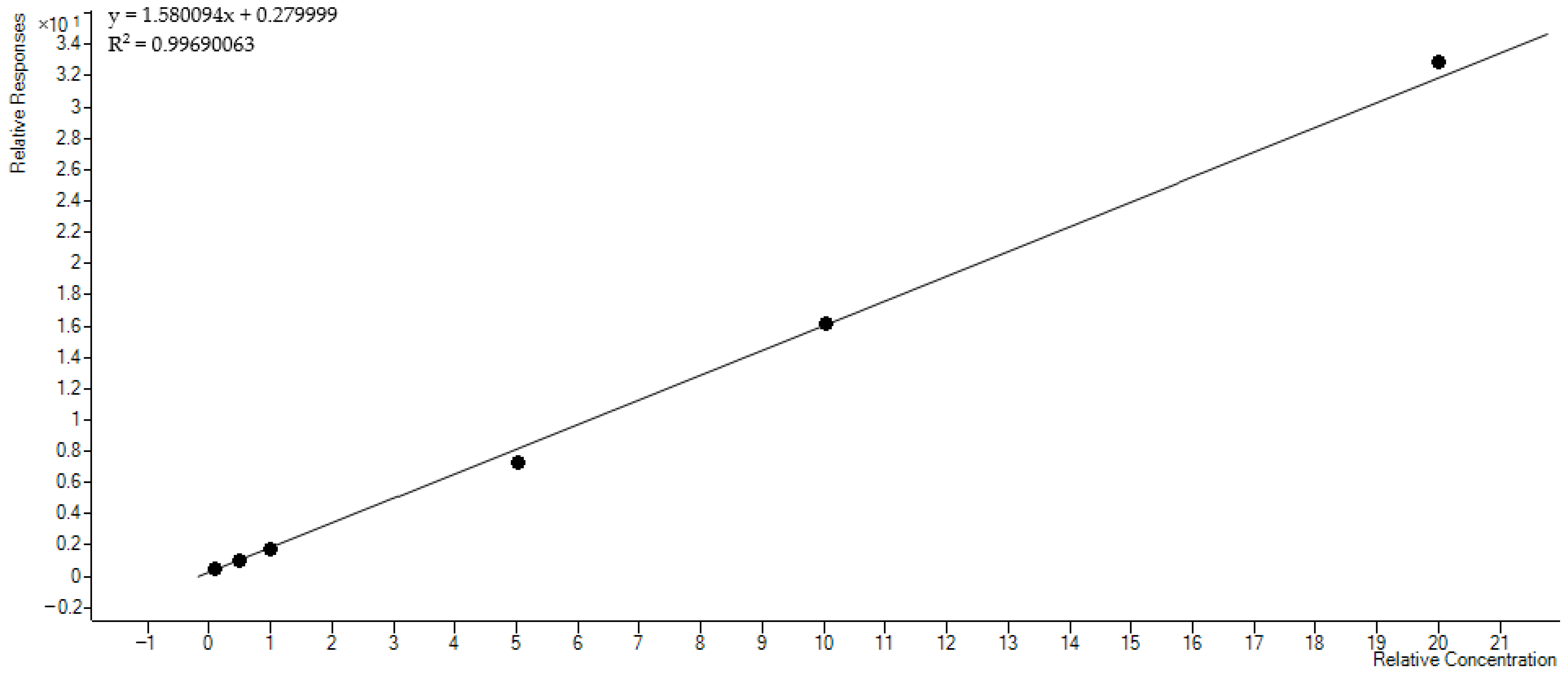
2.5. Instrumentation and Validation Procedure for Quantification PEth in DBS
3. Results
3.1. Synthesis of Phosphatidyl Derivatives in Alcohol Vapors
3.2. Quantification of PEth
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gustavsson, L. Phosphatidylethanol Formation: Specific Effects of Ethanol Mediated via Phospholipase D. Alcohol Alcohol. 1995, 30, 391–406. [Google Scholar] [PubMed]
- Gnann, H.; Engelmann, C.; Skopp, G.; Winkler, M.; Auwärter, V.; Dresen, S.; Ferreirós, N.; Wurst, F.M.; Weinmann, W. Identification of 48 Homologues of Phosphatidylethanol in Blood by LC-ESI-MS/MS. Anal. Bioanal. Chem. 2010, 396, 2415–2423. [Google Scholar] [CrossRef] [PubMed]
- Viel, G.; Boscolo-Berto, R.; Cecchetto, G.; Fais, P.; Nalesso, A.; Ferrara, S.D. Phosphatidylethanol in Blood as a Marker of Chronic Alcohol Use: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2012, 13, 14788–14812. [Google Scholar] [CrossRef]
- Ulwelling, W.; Smith, K. The PEth Blood Test in the Security Environment: What It Is; Why It Is Important; and Interpretative Guidelines. J. Forensic Sci. 2018, 63, 1634–1640. [Google Scholar] [CrossRef] [PubMed]
- Luginbühl, M.; Stöth, F.; Schröck, A.; Gaugler, S.; Weinmann, W. Quantitative Determination of Phosphatidylethanol in Dried Blood Spots for Monitoring Alcohol Abstinence. Nat. Protoc. 2021, 16, 283–308. [Google Scholar] [CrossRef]
- Isaksson, A.; Walther, L.; Hansson, T.; Andersson, A.; Alling, C. Phosphatidylethanol in Blood (B-PEth): A Marker for Alcohol Use and Abuse. Drug Test. Anal. 2011, 3, 195–200. [Google Scholar] [CrossRef]
- Oppolzer, D.; Barroso, M.; Gallardo, E. Bioanalytical Procedures and Developments in the Determination of Alcohol Biomarkers in Biological Specimens. Bioanalysis 2016, 8, 229–251. [Google Scholar] [CrossRef] [PubMed]
- Aboutara, N.; Jungen, H.; Szewczyk, A.; Sterneck, M.; Müller, A.; Iwersen-Bergmann, S. Analysis of Six Different Homologues of Phosphatidylethanol from Dried Blood Spots Using Liquid Chromatography–Tandem Mass Spectrometry. Drug Test. Anal. 2021, 13, 140–147. [Google Scholar] [CrossRef]
- Zheng, Y.; Beck, O.; Helander, A. Method Development for Routine Liquid Chromatography–Mass Spectrometry Measurement of the Alcohol Biomarker Phosphatidylethanol (PEth) in Blood. Clin. Chim. Acta 2011, 412, 1428–1435. [Google Scholar] [CrossRef]
- Luginbühl, M.; Weinmann, W.; Butzke, I.; Pfeifer, P. Monitoring of Direct Alcohol Markers in Alcohol Use Disorder Patients during Withdrawal Treatment and Successive Rehabilitation. Drug Test. Anal. 2019, 11, 859–869. [Google Scholar] [CrossRef]
- Schröck, A.; Hernández Redondo, A.; Martin Fabritius, M.; König, S.; Weinmann, W. Phosphatidylethanol (PEth) in Blood Samples from “Driving under the Influence” Cases as Indicator for Prolonged Excessive Alcohol Consumption. Int. J. Leg. Med. 2016, 130, 393–400. [Google Scholar] [CrossRef]
- Andresen-Streichert, H.; Beres, Y.; Weinmann, W.; Schröck, A.; Müller, A.; Skopp, G.; Pischke, S.; Vettorazzi, E.; Lohse, A.; Nashan, B. Improved Detection of Alcohol Consumption Using the Novel Marker Phosphatidylethanol in the Transplant Setting: Results of a Prospective Study. Transpl. Int. 2017, 30, 611–620. [Google Scholar] [CrossRef]
- Fleming, M.F.; Smith, M.J.; Oslakovic, E.; Lucey, M.R.; Vue, J.X.; Al-Saden, P.; Levitsky, J. Phosphatidylethanol Detects Moderate-to-heavy Alcohol Use in Liver Transplant Recipients. Alcohol. Clin. Exp. Res. 2017, 41, 857–862. [Google Scholar] [CrossRef]
- Helander, A.; Hansson, T. National Harmonization of the Alcohol Biomarker PEth. Lakartidningen 2013, 110, 1747–1748. [Google Scholar]
- Tanna, S.; Lawson, G. Self-Sampling and Quantitative Analysis of DBS: Can it Shift the Balance in over-Burdened Healthcare Systems? Bioanalysis 2015, 7, 1963–1966. [Google Scholar] [CrossRef]
- Faller, A.; Richter, B.; Kluge, M.; Koenig, P.; Seitz, H.K.; Skopp, G. Stability of Phosphatidylethanol Species in Spiked and Authentic Whole Blood and Matching Dried Blood Spots. Int. J. Leg. Med. 2013, 127, 603–610. [Google Scholar] [CrossRef]
- Bakhireva, L.N.; Shrestha, S.; Gutierrez, H.L.; Berry, M.; Schmitt, C.; Sarangarm, D. Stability of Phosphatidylethanol in Dry Blood Spot Cards. Alcohol Alcohol. 2016, 51, 275–280. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Decontamination and Reprocessing of Medical Devices for Health-Care Facilities; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Briko, N.I.; Kagramanyan, I.N.; Nikiforov, V.V.; Suranova, T.G.; Chernyavskaya, O.P.; Polezhaeva, N.A. COVID-19 pandemic. Measures to Combat Its Spread in the Russian Federation. Epidemiol. Vaccine Prev. 2020, 19, 4–12–12. [Google Scholar]
- Bush, K.; Kivlahan, D.R.; McDonell, M.B.; Fihn, S.D.; Bradley, K.A.; Ambulatory Care Quality Improvement Project (ACQUIP). The AUDIT Alcohol Consumption Questions (AUDIT-C): An Effective Brief Screening Test for Problem Drinking. Arch. Intern. Med. 1998, 158, 1789–1795. [Google Scholar] [CrossRef]
- FDA. Bioanalytical Method Validation Guidance for Industry; Food and Drug Administration: Rockville, MD, USA, 2018.
- Varga, A.; Hansson, P.; Johnson, G.; Alling, C. Normalization Rate and Cellular Localization of Phosphatidylethanol in Whole Blood from Chronic Alcoholics. Clin. Chim. Acta 2000, 299, 141–150. [Google Scholar] [CrossRef]
- Jones, J.; Jones, M.; Plate, C.; Lewis, D. The Detection of 1-Palmitoyl-2-Oleoyl-Sn-Glycero-3-Phosphoethanol in Human Dried Blood Spots. Anal. Methods 2011, 3, 1101–1106. [Google Scholar] [CrossRef]
- Kummer, N.; Ingels, A.-S.; Wille, S.M.; Hanak, C.; Verbanck, P.; Lambert, W.E.; Samyn, N.; Stove, C.P. Quantification of Phosphatidylethanol 16:0/18:1, 18:1/18:1, and 16:0/16:0 in Venous Blood and Venous and Capillary Dried Blood Spots from Patients in Alcohol Withdrawal and Control Volunteers. Anal. Bioanal. Chem. 2016, 408, 825–838. [Google Scholar] [CrossRef] [Green Version]
- Luginbühl, M.; Gaugler, S.; Weinmann, W. Fully Automated Determination of Phosphatidylethanol 16: 0/18: 1 and 16: 0/18: 2 in Dried Blood Spots. J. Anal. Toxicol. 2019, 43, 489–496. [Google Scholar] [CrossRef]
- Beck, O.; Mellring, M.; Löwbeer, C.; Seferaj, S.; Helander, A. Measurement of the Alcohol Biomarker Phosphatidylethanol (PEth) in Dried Blood Spots and Venous Blood—Importance of Inhibition of Post-Sampling Formation from Ethanol. Anal. Bioanal. Chem. 2021, 413, 5601–5606. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bashilov, A.; Osipenko, S.; Ikonnikova, K.; Kovaleva, O.; Izotov, B.; Nikolaev, E.; Kostyukevich, Y. False Positive Results of Phosphatidylethanol (PEth) Quantitation in Dried Blood Spots (DBS): The Influence of Alcohol Vapors. Separations 2022, 9, 250. https://doi.org/10.3390/separations9090250
Bashilov A, Osipenko S, Ikonnikova K, Kovaleva O, Izotov B, Nikolaev E, Kostyukevich Y. False Positive Results of Phosphatidylethanol (PEth) Quantitation in Dried Blood Spots (DBS): The Influence of Alcohol Vapors. Separations. 2022; 9(9):250. https://doi.org/10.3390/separations9090250
Chicago/Turabian StyleBashilov, Anton, Sergey Osipenko, Karolina Ikonnikova, Oxana Kovaleva, Boris Izotov, Evgeny Nikolaev, and Yury Kostyukevich. 2022. "False Positive Results of Phosphatidylethanol (PEth) Quantitation in Dried Blood Spots (DBS): The Influence of Alcohol Vapors" Separations 9, no. 9: 250. https://doi.org/10.3390/separations9090250
APA StyleBashilov, A., Osipenko, S., Ikonnikova, K., Kovaleva, O., Izotov, B., Nikolaev, E., & Kostyukevich, Y. (2022). False Positive Results of Phosphatidylethanol (PEth) Quantitation in Dried Blood Spots (DBS): The Influence of Alcohol Vapors. Separations, 9(9), 250. https://doi.org/10.3390/separations9090250







