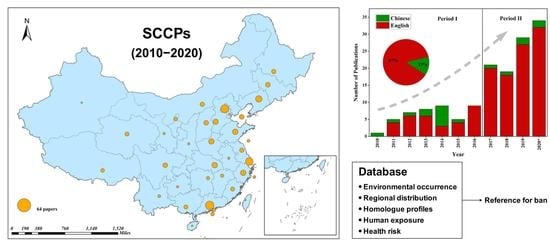
Occurrence, Distribution and Health Risk of Short-Chain Chlorinated Paraffins (SCCPs) in China: A Critical Review
Abstract
:1. Introduction
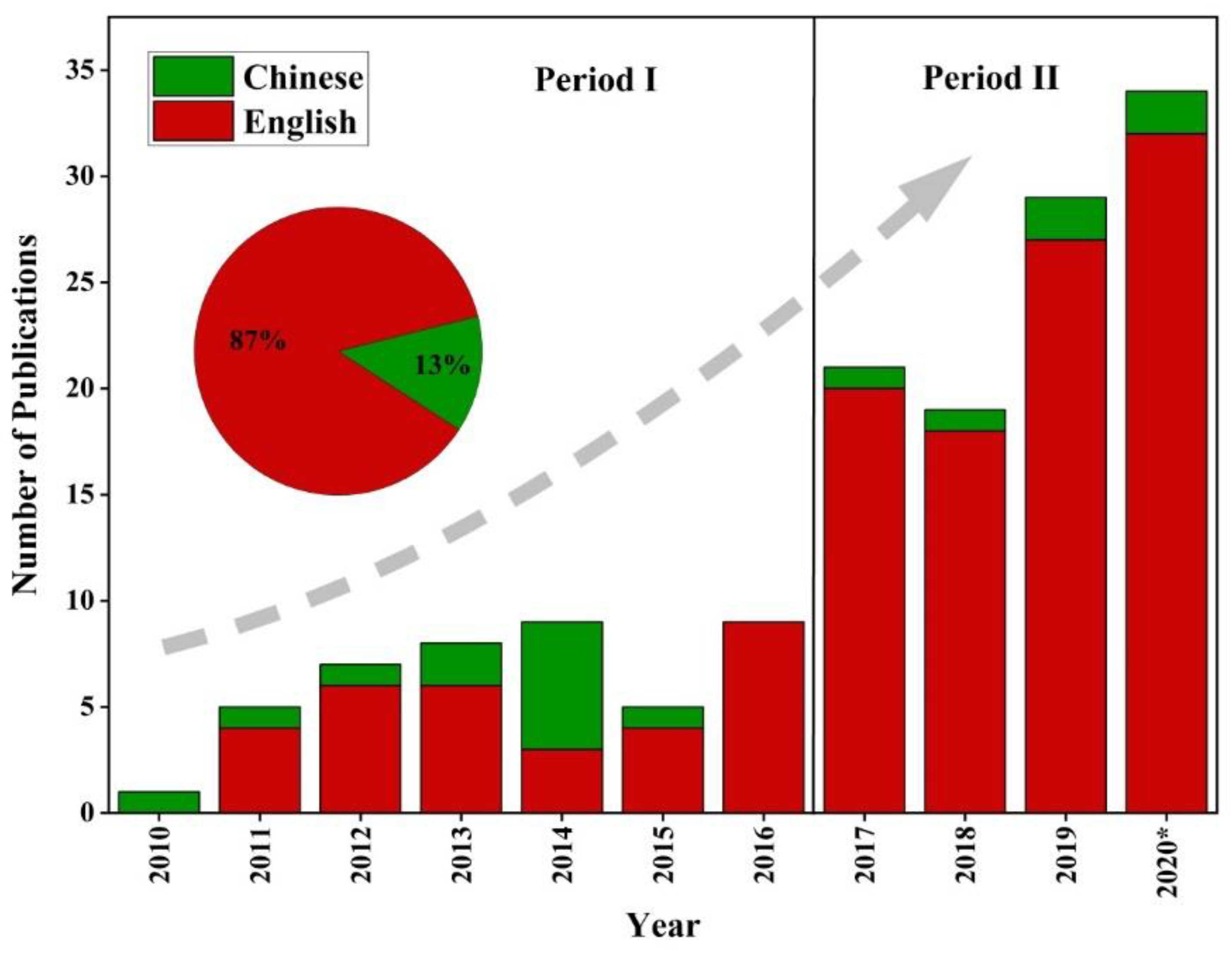
2. Literature Survey and Analysis
3. Levels and Distribution of SCCPs in Environmental Matrices
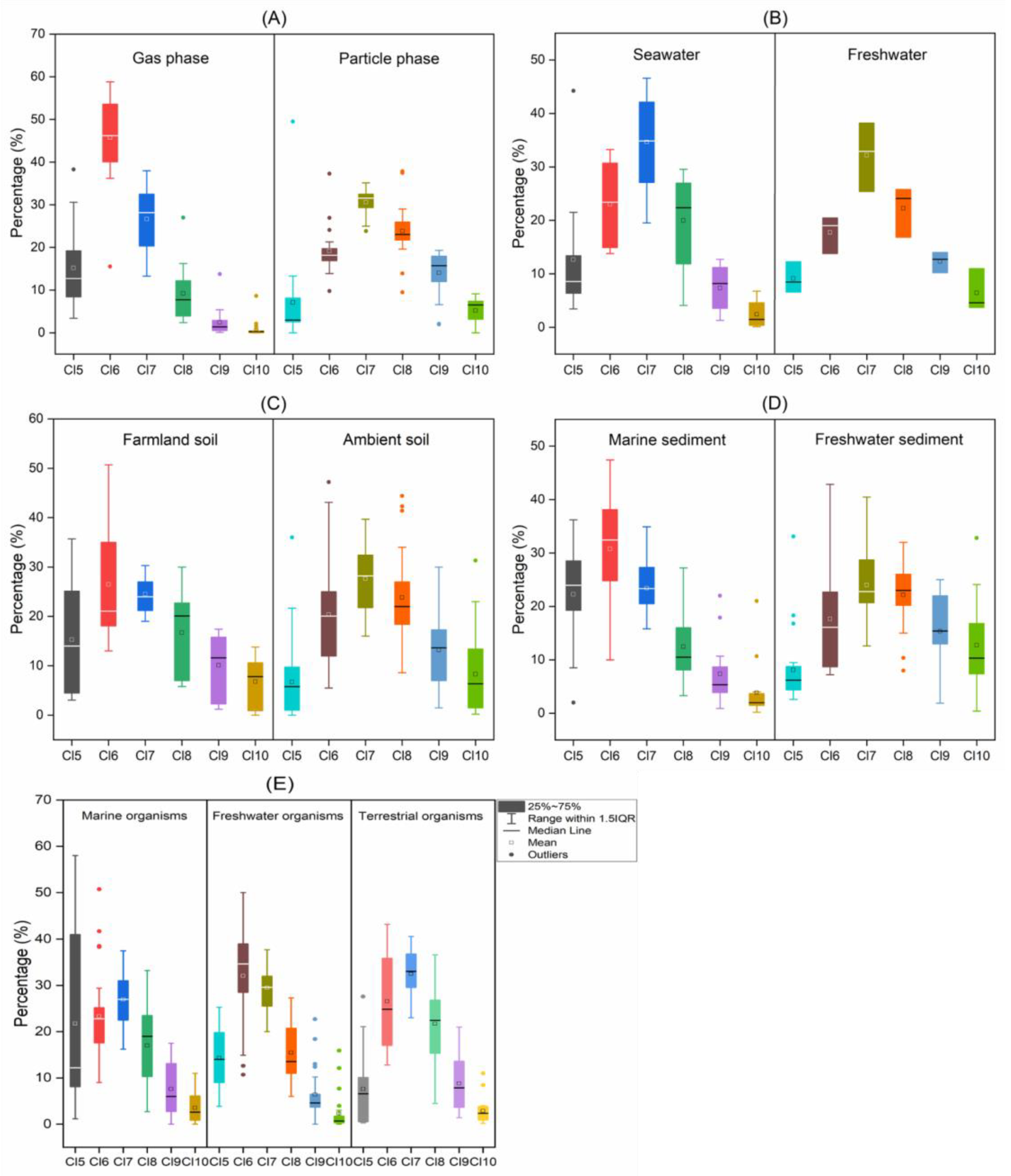
3.1. Air
3.2. Water
3.3. Soil
3.4. Sediment
3.5. Biota
3.6. Regional Characteristics
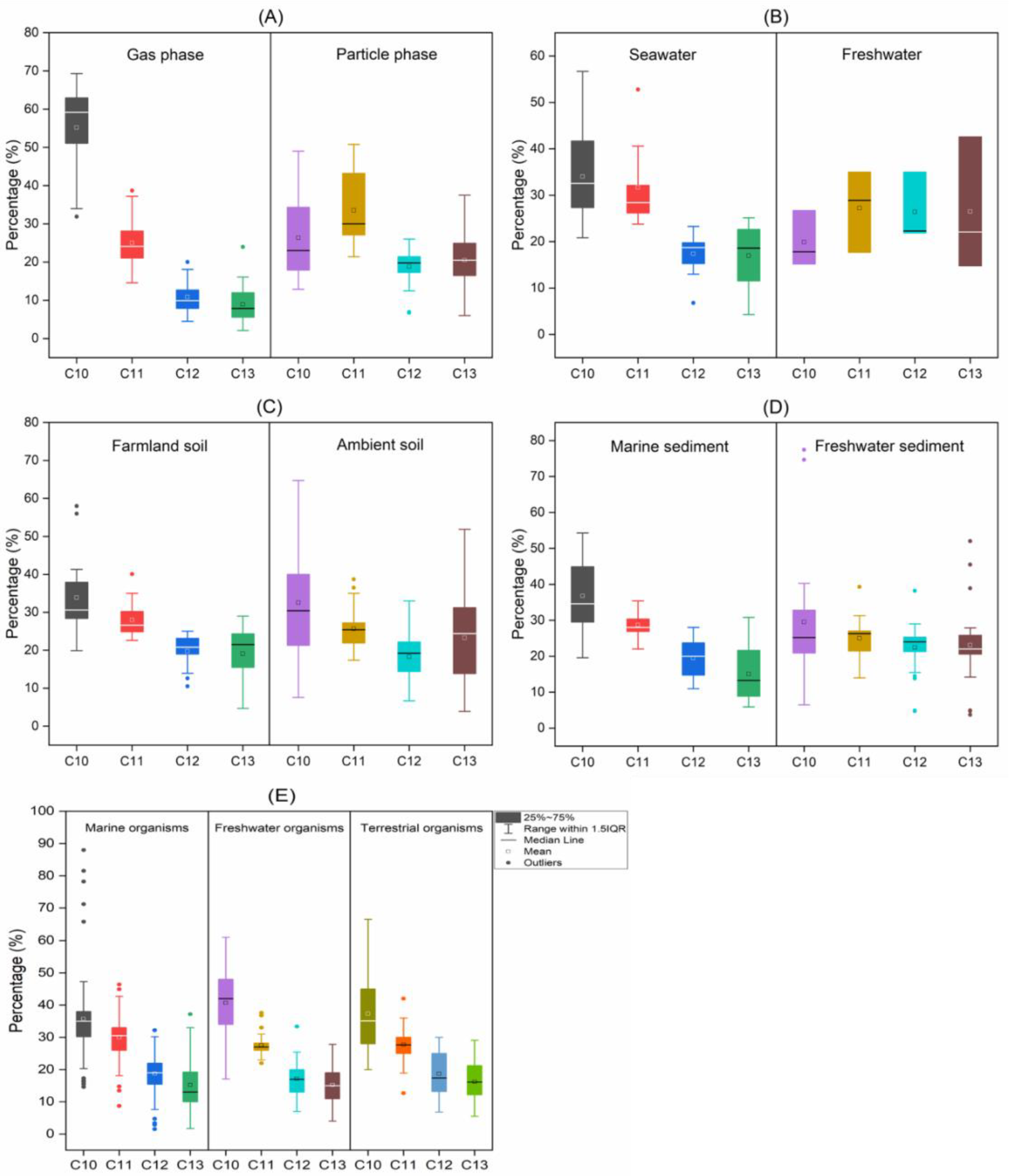
4. SCCP Homologue Profiles
4.1. SCCP Profiles
4.2. Principal Component Analysis (PCA) by Carbon and Chlorine Profiles
4.3. Environmental Implications of SCCP Profiles
5. Daily Intake and Human Health Risk of SCCPs
5.1. Levels and Profiles of SCCPs in Food and Human Tissue
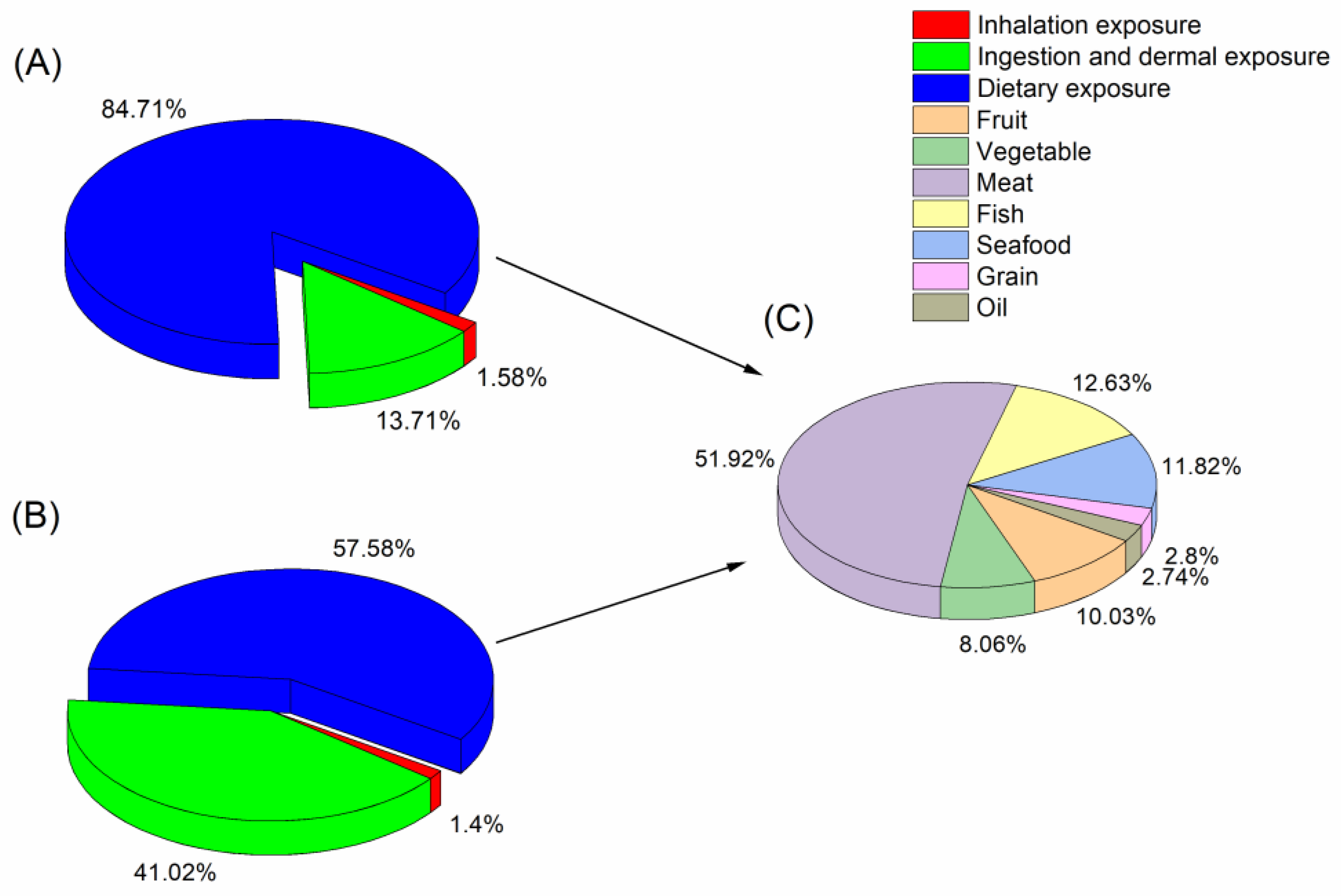
5.2. Daily Intakes of SCCPs via Inhalation, Ingestion and Dermal, and Dietary Exposure
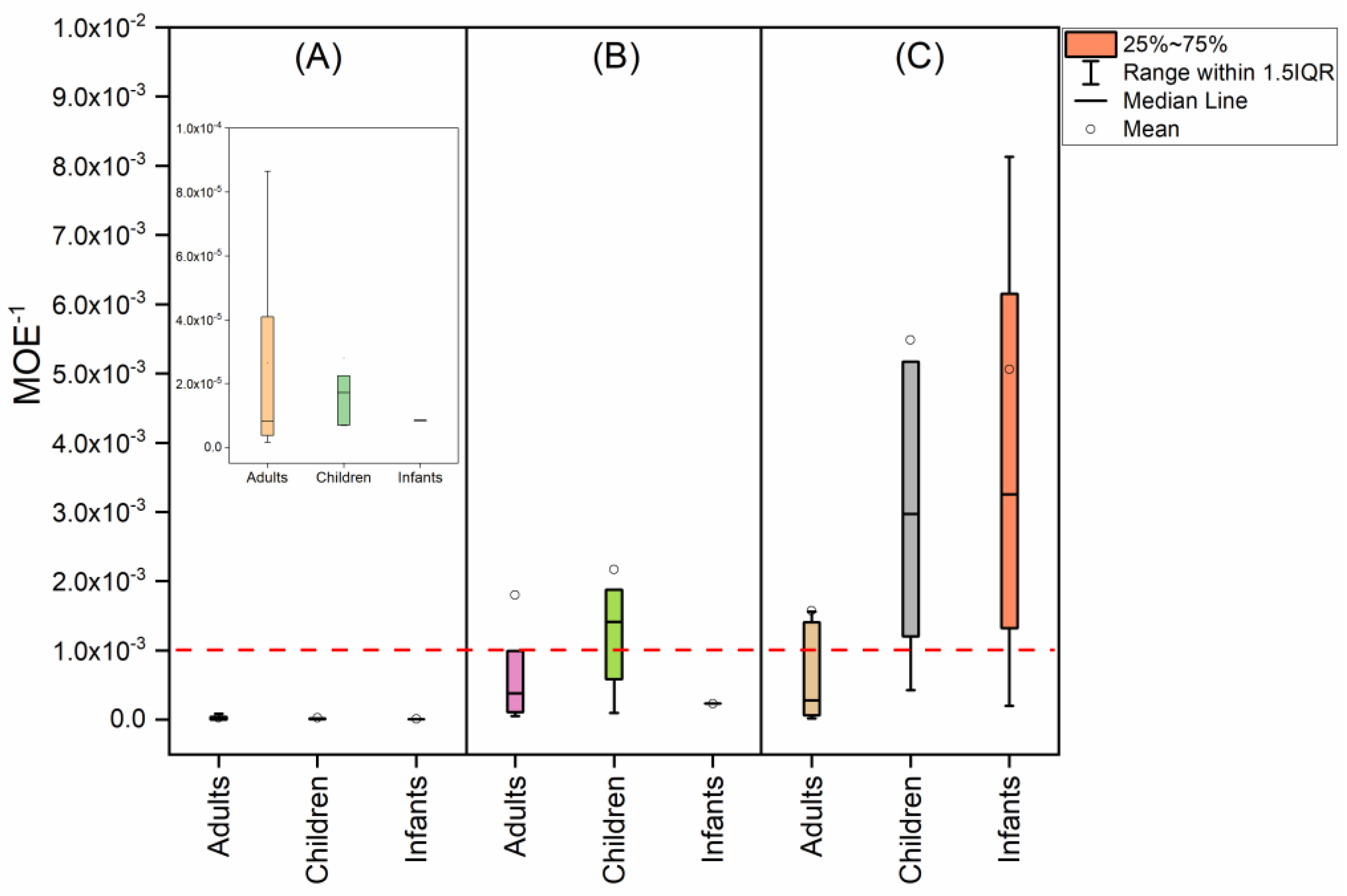
5.3. Human Health Risk of SCCPs
6. Conclusions and Prospects
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bayen, S.; Obbard, J.P.; Thomas, G.O. Chlorinated paraffins: A review of analysis and environmental occurrence. Environ. Int. 2006, 32, 915–929. [Google Scholar] [CrossRef] [PubMed]
- Stejnarova, P.; Coelhan, M.; Kostrhounova, R.; Parlar, H.; Holoubek, I. Analysis of short chain chlorinated paraffins in sediment samples from the Czech Republic by short-column GC/ECNI-MS. Chemosphere 2005, 58, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Gluge, J.; Wang, Z.; Bogdal, C.; Scheringer, M.; Hungerbuhler, K. Global production, use, and emission volumes of short-chain chlorinated paraffins—A minimum scenario. Sci. Total Environ. 2016, 573, 1132–1146. [Google Scholar] [CrossRef] [PubMed]
- Feo, M.L.; Eljarrat, E.; Barcelo, D. Occurrence, fate and analysis of polychlorinated n-alkanes in the environment. Trend. Anal. Chem. 2009, 28, 778–791. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, J.; Xue, Z.; Jin, X.; Jin, Y.; Fu, Z. The environmental distribution and toxicity of short-chain chlorinated paraffins and underlying mechanisms: Implications for further toxicological investigation. Sci. Total Environ. 2019, 695, 133834. [Google Scholar] [CrossRef]
- UNEP/POPS/COP. Recommendation by the Persistent Organic Pollutants Review Committee to list short-chain chlorinated paraffins in Annex A to the Convention and draft text of the proposed amendment. In Proceedings of the Parties to the Stockholm Convention on Persistent Organic Pollutants, Eighth Meeting, Geneva, Switzerland, 24 April–5 May 2017. [Google Scholar]
- Stiehl, T.; Pfordt, J.; Ende, M. Globale destillation. J. Verbrauch. Lebensm. 2008, 3, 61–81. [Google Scholar] [CrossRef]
- POPRC. Short-Chained Chlorinated Paraffins: Risk Profile. In United Nations Environmental Programme Stockholm Convention on Persistent Organic Pollutants; Geneva, Switzerland. 2015. Available online: https://chm.pops.int/Portals/0/download.aspx?d=UNEP-POPS-POPRC.11-10-Add.2.English.pdf (accessed on 5 July 2020).
- WCC. International Chlorinated Alkanes Industry Association (ICAIA) Newsletter. World Chlorine Council. 2014. Available online: https://www.eurochlor.org/media/88258/20140908_icaia_newsletter_03_final.pdf (accessed on 5 July 2020).
- SSCTC. The Market Research Report of CPs. Shanghai Shuoxun Chemical Technology Co., Ltd. 2012. Available online: https://shuoxunreport.cn.makepolo.com (accessed on 5 July 2020).
- Chen, C.; Chen, A.; Li, L.; Peng, W.; Weber, R.; Liu, J. Distribution and emission estimation of short-and medium-chain chlorinated paraffins in Chinese products through detection-based mass balancing. Environ. Sci. Technol. 2021, 55, 7335–7343. [Google Scholar] [CrossRef]
- Van Mourik, L.M.; Leonards, P.E.G.; Gaus, C.; de Boer, J. Recent developments in capabilities for analysing chlorinated paraffins in environmental matrices: A review. Chemosphere 2015, 136, 259–272. [Google Scholar] [CrossRef]
- Yuan, B.; Wang, Y.; Fu, J.; Jiang, G. Evaluation of the pollution levels of short chain chlorinated paraffins in soil collected from an e-waste dismantling area in China. Organohalogen Compd. 2009, 71, 3106–3109. [Google Scholar]
- Li, Q.; Li, J.; Wang, Y.; Xu, Y.; Pan, X.; Zhang, G.; Luo, C.; Kobara, Y.; Nam, J.-J.; Jones, K.C. Atmospheric short-chain chlorinated paraffins in China, Japan, and South Korea. Environ. Sci. Technol. 2012, 46, 11948–11954. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Chen, R.; Zou, Y.; Dong, L.; Hai, R.; Huang, Y. Spatial distribution and profile of atmospheric short-chain chlorinated paraffins in the Yangtze River Delta. Environ. Pollut. 2020, 259, 113958. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, M.; Ma, S.; Li, G.; Yu, Y.; An, T. Chlorinated paraffins in the indoor and outdoor atmospheric particles from the Pearl River Delta: Characteristics, sources, and human exposure risks. Sci. Total Environ. 2019, 650, 1041–1049. [Google Scholar] [CrossRef]
- Cao, D.; Gao, W.; Wu, J.; Lv, K.; Xin, S.; Wang, Y.; Jiang, G. Occurrence and human exposure assessment of short- and medium-chain chlorinated paraffins in dusts from plastic sports courts and synthetic turf in Beijing, China. Environ. Sci. Technol. 2019, 53, 443–451. [Google Scholar] [CrossRef]
- Liu, L.-H.; Ma, W.-L.; Liu, L.-Y.; Huo, C.-Y.; Li, W.-L.; Gao, C.-J.; Li, H.-L.; Li, Y.-F.; Chan, H.M. Occurrence, sources and human exposure assessment of SCCPs in indoor dust of northeast China. Environ. Pollut. 2017, 225, 232–243. [Google Scholar] [CrossRef]
- Shi, L.; Gao, Y.; Zhang, H.; Geng, N.; Xu, J.; Zhan, F.; Ni, Y.; Hou, X.; Chen, J. Concentrations of short- and medium-chain chlorinated paraffins in indoor dusts from malls in China: Implications for human exposure. Chemosphere 2017, 172, 103–110. [Google Scholar] [CrossRef]
- Ma, X.; Wang, Y.; Gao, W.; Wang, Y.; Wang, Z.; Yao, Z.; Jiang, G. Air-seawater gas exchange and dry deposition of chlorinated paraffins in a typical inner sea (Liaodong Bay), North China. Environ. Sci. Technol. 2018, 52, 7729–7735. [Google Scholar] [CrossRef]
- Wang, X.-T.; Jia, H.-H.; Hu, B.-P.; Cheng, H.-X.; Zhou, Y.; Fu, R. Occurrence, sources, partitioning and ecological risk of short- and medium-chain chlorinated paraffins in river water and sediments in Shanghai. Sci. Total Environ. 2019, 653, 475–484. [Google Scholar] [CrossRef]
- Zeng, L.; Wang, T.; Wang, P.; Liu, Q.; Han, S.; Yuan, B.; Zhu, N.; Wang, Y.; Jiang, G. Distribution and trophic transfer of short-chain chlorinated paraffins in an aquatic ecosystem receiving effluents from a sewage treatment plant. Environ. Sci. Technol. 2011, 45, 5529–5535. [Google Scholar] [CrossRef]
- Zeng, L.; Li, H.; Wang, T.; Gao, Y.; Xiao, K.; Du, Y.; Wang, Y.; Jiang, G. Behavior, fate, and mass loading of short chain chlorinated paraffins in an advanced municipal sewage treatment plant. Environ. Sci. Technol. 2013, 47, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Wang, T.; Ruan, T.; Liu, Q.; Wang, Y.; Jiang, G. Levels and distribution patterns of short chain chlorinated paraffins in sewage sludge of wastewater treatment plants in China. Environ. Pollut. 2012, 160, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Aamir, M.; Yin, S.; Zhou, Y.; Xu, C.; Liu, K.; Liu, W. Congener-specific C10–C13 and C14–C17 chlorinated paraffins in Chinese agricultural soils: Spatio-vertical distribution, homologue pattern and environmental behavior. Environ. Pollut. 2019, 245, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-T.; Xu, S.-Y.; Wang, X.-K.; Hu, B.-P.; Jia, H.-H. Occurrence, homologue patterns and source apportionment of short- and medium-chain chlorinated paraffins in suburban soils of Shanghai, China. Chemosphere 2017, 180, 302–311. [Google Scholar] [CrossRef]
- Wang, X.-T.; Zhang, Y.; Miao, Y.; Ma, L.-L.; Li, Y.-C.; Chang, Y.-Y.; Wu, M.-H. Short-chain chlorinated paraffins (SCCPs) in surface soil from a background area in China: Occurrence, distribution, and congener profiles. Environ. Sci. Pollut. Res. 2013, 20, 4742–4749. [Google Scholar] [CrossRef]
- Chen, M.; Lu, F.; Chen, J.; Luo, X.; Mai, B. Temporal distributions of chlorinated paraffins in sediments core from the Pearl River Delta. Environ. Chem. 2014, 33, 832–836. (In Chinese) [Google Scholar]
- Ma, X.; Chen, C.; Zhang, H.; Gao, Y.; Wang, Z.; Yao, Z.; Chen, J.; Chen, J. Congener-specific distribution and bioaccumulation of short-chain chlorinated paraffins in sediments and bivalves of the Bohai Sea, China. Mar. Pollut. Bull. 2014, 79, 299–304. [Google Scholar] [CrossRef]
- Zhang, C.; Chang, H.; Wang, H.; Zhu, Y.; Zhao, X.; He, Y.; Sun, F.; Wu, F. Spatial and temporal distributions of short-, medium-, and long-chain chlorinated paraffins in sediment cores from nine lakes in China. Environ. Sci. Technol. 2019, 53, 9462–9471. [Google Scholar] [CrossRef]
- Du, X.; Yuan, B.; Zhou, Y.; Benskin, J.P.; Qiu, Y.; Yin, G.; Zhao, J. Short-, medium-, and long-chain chlorinated paraffins in wildlife from paddy fields in the Yangtze River Delta. Environ. Sci. Technol. 2018, 52, 1072–1080. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Chen, L.; Jiang, G.; He, Q.; Ren, L.; Gao, B.; Cai, L. Bioaccumulation and biomagnification of short-chain chlorinated paraffins in marine organisms from the Pearl River Estuary, South China. Sci. Total Environ. 2019, 671, 262–269. [Google Scholar] [CrossRef]
- Wang, C.; Gao, Y.; Zhang, H.; Fan, J.; Chen, J. Bioaccumulation characteristics of short-chain chlorinated paraffinsin Liaodong Bay, Northeast China. Environ. Chem. 2011, 30, 44–49. (In Chinese) [Google Scholar]
- Qiao, L.; Gao, L.; Zheng, M.; Xia, D.; Li, J.; Zhang, L.; Wu, Y.; Wang, R.; Cui, L.; Xu, C. Mass fractions, congener group patterns, and placental transfer of short- and medium-chain chlorinated paraffins in paired maternal and cord serum. Environ. Sci. Technol. 2018, 52, 10097–10103. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, W.; Wang, Y.; Jiang, G. Distribution and pattern profiles of chlorinated paraffins in human placenta of Henan Province, China. Environ. Sci. Technol. Lett. 2018, 5, 9–13. [Google Scholar] [CrossRef]
- Xia, D.; Gao, L.-R.; Zheng, M.-H.; Li, J.-G.; Zhang, L.; Wu, Y.-N.; Qiao, L.; Tian, Q.-C.; Huang, H.-T.; Liu, W.-B.; et al. Health risks posed to infants in rural China by exposure to short- and medium-chain chlorinated paraffins in breast milk. Environ. Int. 2017, 103, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Liang, X.; Li, D.; Zhuo, M.; Zhang, S.; Huang, Q.; Liao, Y.; Xie, Z.; Guo, T.; Yuan, Z. Occurrence, fate and ecological risk of chlorinated paraffins in Asia: A review. Environ. Int. 2016, 92, 373–387. [Google Scholar] [CrossRef]
- Terry, B.; Alaee, M.; Stern, G. New persistent chemicals in the Arctic environment. In Synopsis of Research Conducted under the 1999–2000 Northern Contaminants Program; 2001; pp. 93–104. [Google Scholar]
- Tomy, G.T.; Fisk, A.T.; Westmore, J.B.; Muir, D.C. Environmental chemistry and toxicology of polychlorinated n-alkanes. Rev. Environ. Contam. Toxicol. 1998, 158, 53–128. [Google Scholar]
- Barber, J.L.; Sweetman, A.J.; Thomas, G.O.; Braekevelt, E.; Stern, G.A.; Jones, K.C. Spatial and temporal variability in air concentrations of short-chain (C10–C13) and medium-chain (C14–C17) chlorinated n-alkanes measured in the UK atmosphere. Environ. Sci. Technol. 2005, 39, 4407–4415. [Google Scholar] [CrossRef]
- Borgen, A.; Schlabach, M. Polychlorinated alkanes in ambient air from Bear Island. Organohalogen 2002, 59, 303–306. [Google Scholar]
- Borgen, A.R.; Schlabach, M.; Gundersen, H. Polychlorinated alkanes in Arctic air. Organohalogen Compd. 2000, 47, 272–275. [Google Scholar]
- Diefenbacher, P.S.; Bogdal, C.; Gerecke, A.C.; Gluege, J.; Schmid, P.; Scheringer, M.; Hungerbuehler, K. Short-chain chlorinated paraffins in Zurich, Switzerland-Atmospheric concentrations and emissions. Environ. Sci. Technol. 2015, 49, 9778–9786. [Google Scholar] [CrossRef]
- Chaemfa, C.; Xu, Y.; Li, J.; Chakraborty, P.; Syed, J.H.; Malik, R.N.; Wang, Y.; Tian, C.; Zhang, G.; Jones, K.C. Screening of atmospheric short- and medium-chain chlorinated paraffins in India and Pakistan using polyurethane foam based passive air sampler. Environ. Sci. Technol. 2014, 48, 4799–4808. [Google Scholar] [CrossRef] [PubMed]
- Van Mourik, L.M.; Wang, X.; Paxman, C.; Leonards, P.E.G.; Wania, F.; de Boer, J.; Mueller, J.F. Spatial variation of short- and medium-chain chlorinated paraffins in ambient air across Australia. Environ. Pollut. 2020, 261, 114141. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Zhu, X.; Gao, Y.; Chen, J.; Wang, L.; Yuan, H.; Li, X.; Wang, W.; Dong, X. Gas-particle partitioning behavior of short-chain chlorinated paraffins in urban air of Dalian. J. Dalian Jiaotong Univ. 2017, 38, 78–84. (In Chinese) [Google Scholar]
- Gao, W.; Wu, J.; Wang, Y.; Jiang, G. Distribution and congener profiles of short-chain chlorinated paraffins in indoor/outdoor glass window surface films and their film-air partitioning in Beijing, China. Chemosphere 2016, 144, 1327–1333. [Google Scholar] [CrossRef]
- Huang, H.; Gao, L.; Xia, D.; Qiao, L.; Wang, R.; Su, G.; Liu, W.; Liu, G.; Zheng, M. Characterization of short- and medium-chain chlorinated paraffins in outdoor/indoor PM10/PM2.5/PM1.0 in Beijing, China. Environ. Pollut. 2017, 225, 674–680. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, N.; Cui, Y.; Jiang, W.; Wang, L.; Wang, Z.; Chen, X.; Jiang, L.; Ding, L. Short-chain chlorinated paraffin (SCCP) pollution from a CP production plant in China: Dispersion, congener patterns and health risk assessment. Chemosphere 2018, 211, 456–464. [Google Scholar] [CrossRef]
- Wang, T.; Han, S.; Yuan, B.; Zeng, L.; Li, Y.; Wang, Y.; Jiang, G. Summer-winter concentrations and gas-particle partitioning of short chain chlorinated paraffins in the atmosphere of an urban setting. Environ. Pollut. 2012, 171, 38–45. [Google Scholar] [CrossRef]
- Zhu, X.; Bai, H.; Gao, Y.; Chen, J.; Yuan, H.; Wang, L.; Wang, W.; Dong, X.; Li, X. Concentrations and inhalation risk assessment of short-chain polychlorinated paraffins in the urban air of Dalian, China. Environ. Sci. Pollut. Res. 2017, 24, 21203–21212. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Cheng, Z.; Li, Q.; Pan, X.; Zhang, R.; Liu, D.; Luo, C.; Liu, X.; Katsoyiannis, A.; et al. Short- and medium-chain chlorinated paraffins in air and soil of subtropical terrestrial environment in the Pearl River Delta, South China: Distribution, composition, atmospheric deposition fluxes, and environmental fate. Environ. Sci. Technol. 2013, 47, 2679–2687. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Li, H.; Yu, H.; Yang, L.; Chen, X.; Cai, Z. Seasonal variations and inhalation risk assessment of short-chain chlorinated paraffins in PM2.5 of Jinan, China. Environ. Pollut. 2019, 245, 325–330. [Google Scholar] [CrossRef]
- Eisenreich, S.J.; Hornbuckle, K.; Jones, K.C. The global legacy of POPs: Special issue. Environ. Sci. Technol. 2021, 55, 9397–9399. [Google Scholar] [CrossRef] [PubMed]
- Zhan, F.; Zhang, H.; Wang, J.; Xu, J.; Yuan, H.; Gao, Y.; Su, F.; Chen, J. Release and gas-particle partitioning behaviors of short-chain chlorinated paraffins (SCCPs) during the thermal treatment of polyvinyl chloride flooring. Environ. Sci. Technol. 2017, 51, 9005–9012. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Lam, J.C.W.; Zhu, M.; Wang, F.; Zhou, W.; Du, B.; Zeng, L.; Zeng, E.Y. Combined effects of dust and dietary exposure of occupational workers and local residents to short- and medium-chain chlorinated paraffins in a mega e-waste recycling industrial park in South China. Environ. Sci. Technol. 2018, 52, 11510–11519. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.-H.; Tang, B.; Luo, X.-J.; Zheng, X.-B.; Peng, P.-A.; Mai, B.-X. Organohalogen pollutants in surface particulates from workshop floors of four major e-waste recycling sites in China and implications for emission lists. Sci. Total Environ. 2016, 569, 982–989. [Google Scholar] [CrossRef] [Green Version]
- Wong, F.; Suzuki, G.; Michinaka, C.; Yuan, B.; Takigami, H.; de Wit, C.A. Dioxin-like activities, halogenated flame retardants, organophosphate esters and chlorinated paraffins in dust from Australia, the United Kingdom, Canada, Sweden and China. Chemosphere 2017, 168, 1248–1256. [Google Scholar] [CrossRef]
- Hilger, B.; Fromme, H.; Voelkel, W.; Coelhan, M. Effects of chain length, chlorination degree, and structure on the octanol-water partition coefficients of polychlorinated n-alkanes. Environ. Sci. Technol. 2011, 45, 2842–2849. [Google Scholar]
- ECB. European Union Risk Assessment Report: Alkanes, C10–13, Chloro. 1st Priority List. vol. 4. European Chemicals Bureau, Joint Research Centre. 2000. Available online: https://echa.europa.eu/documents/10162/6434698/orats_final_rar_alkanes_c10-13_chloro_en.pdf (accessed on 9 July 2020).
- ECB. European Union Risk Assessment Report: Alkanes, C14–17, Chloro. Part 1—Environment. 3rd Priority List. vol. 58. European Chemicals Bureau, Joint Research Centre. 2005. Available online: https://echa.europa.eu/documents/10162/6434698/orats_final_rar_alkanes_c14-17_chloro_en.pdf (accessed on 9 July 2020).
- Houde, M.; Muir, D.C.G.; Tomy, G.T.; Whittle, D.M.; Teixeira, C.; Moore, S. Bioaccumulation and trophic magnification of short- and medium-chain chlorinated paraffins in food webs from Lake Ontario and Lake Michigan. Environ. Sci. Technol. 2008, 42, 3893–3899. [Google Scholar] [CrossRef]
- Gandolfi, F.; Malleret, L.; Sergent, M.; Doumenq, P. Parameters optimization using experimental design for headspace solid phase micro-extraction analysis of short-chain chlorinated paraffins in waters under the European water framework directive. J. Chromatogr. A 2015, 1406, 59–67. [Google Scholar] [CrossRef]
- Iino, F.; Takasuga, T.; Senthilkumar, K.; Nakamura, N.; Nakanishi, J. Risk assessment of short-chain chlorinated paraffins in Japan based on the first market basket study and species sensitivity distributions. Environ. Sci. Technol. 2005, 39, 859–866. [Google Scholar] [CrossRef]
- Nicholls, C.R.; Allchin, C.R.; Law, R.J. Levels of short and medium chain length polychlorinated n-alkanes in environmental samples from selected industrial areas in England and Wales. Environ. Pollut. 2001, 114, 415–430. [Google Scholar] [CrossRef]
- Zhao, N.; Cui, Y.; Wang, P.; Li, S.; Jiang, W.; Luo, N.; Wang, Z.; Chen, X.; Ding, L. Short-chain chlorinated paraffins in soil, sediment, and seawater in the intertidal zone of Shandong Peninsula, China: Distribution and composition. Chemosphere 2019, 220, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhang, H.; Wang, Z.; Yao, Z.; Chen, J.; Chen, J. Bioaccumulation and trophic transfer of short chain chlorinated paraffins in a marine food web from Liaodong Bay, North China. Environ. Sci. Technol. 2014, 48, 5964–5971. [Google Scholar] [CrossRef] [PubMed]
- Halse, A.K.; Schlabach, M.; Schuster, J.K.; Jones, K.C.; Steinnes, E.; Breivik, K. Endosulfan, pentachlorobenzene and short-chain chlorinated paraffins in background soils from Western Europe. Environ. Pollut. 2015, 196, 21–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogdal, C.; Niggeler, N.; Gluge, J.; Diefenbacher, P.S.; Wachter, D.; Hungerbuhler, K. Temporal trends of chlorinated paraffins and polychlorinated biphenyls in Swiss soils. Environ. Pollut. 2017, 220, 891–899. [Google Scholar] [CrossRef]
- Li, H.; Fu, J.; Zhang, A.; Zhang, Q.; Wang, Y. Occurrence, bioaccumulation and long-range transport of short-chain chlorinated paraffins on the Fildes Peninsula at King George Island, Antarctica. Environ. Int. 2016, 94, 408–414. [Google Scholar] [CrossRef]
- Wang, K.; Gao, L.; Zhu, S.; Cui, L.; Qiao, L.; Xu, C.; Huang, D.; Zheng, M. Spatial distributions and homolog profiles of chlorinated nonane paraffins, and short and medium chain chlorinated paraffins in soils from Yunnan, China. Chemosphere 2020, 247, 125855. [Google Scholar] [CrossRef]
- Li, H.; Bu, D.; Fu, J.; Gao, Y.; Cong, Z.; Zhang, G.; Wang, Y.; Chen, X.; Zhang, A.; Jiang, G. Trophic dilution of short-chain chlorinated paraffins in a plant-plateau pika-eagle food chain from the Tibetan Plateau. Environ. Sci. Technol. 2019, 53, 9472–9480. [Google Scholar] [CrossRef]
- Wu, J.; Gao, W.; Liang, Y.; Fu, J.; Shi, J.; Lu, Y.; Wang, Y.; Jiang, G. Short- and medium-chain chlorinated paraffins in multi-environmental matrices in the Tibetan Plateau environment of China: A regional scale study. Environ. Int. 2020, 140, 105767. [Google Scholar] [CrossRef]
- Wania, F.; Westgate, J.N. On the mechanism of mountain cold-trapping of organic chemicals. Environ. Sci. Technol. 2008, 42, 9092–9098. [Google Scholar] [CrossRef]
- Marvin, C.H.; Painter, S.; Tomy, G.T.; Stern, G.A.; Braekevelt, E.; Muir, D.C.G. Spatial and temporal trends in short-chain chlorinated paraffins in Lake Ontario sediments. Environ. Sci. Technol. 2003, 37, 4561–4568. [Google Scholar] [CrossRef]
- Tomy, G.T.; Stern, G.A.; Lockhart, W.L.; Muir, D.C.G. Occurrence of C10–C13 polychlorinated n-alkanes in Canadian midlatitude and arctic lake sediments. Environ. Sci. Technol. 1999, 33, 2858–2863. [Google Scholar] [CrossRef]
- Hussy, I.; Webster, L.; Russell, M.; Moffat, C. Determination of chlorinated paraffins in sediments from the Firth of Clyde by gas chromatography with electron capture negative ionisation mass spectrometry and carbon skeleton analysis by gas chromatography with flame ionisation detection. Chemosphere 2012, 88, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Pribylova, P.; Klanova, J.; Holoubek, I. Screening of short- and medium-chain chlorinated paraffins in selected riverine sediments and sludge from the Czech Republic. Environ. Pollut. 2006, 144, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Iozza, S.; Mueller, C.E.; Schmid, P.; Bogdal, C.; Oehme, M. Historical profiles of chlorinated paraffins and polychlorinated biphenyls in a dated sediment core from Lake Thun (Switzerland). Environ. Sci. Technol. 2008, 42, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Lam, J.C.W.; Horii, Y.; Li, X.; Chen, W.; Qiu, J.-W.; Leung, K.M.Y.; Yamazaki, E.; Yamashita, N.; Lam, P.K.S. Spatial and temporal trends of short- and medium-chain chlorinated paraffins in sediments off the urbanized coastal zones in China and Japan: A comparison study. Environ. Pollut. 2017, 224, 357–367. [Google Scholar] [CrossRef]
- Huttig, J.; Oehme, M. Presence of chlorinated paraffins in sediments from the North and Baltic Seas. Arch. Environ. Contam. Toxicol. 2005, 49, 449–456. [Google Scholar] [CrossRef] [Green Version]
- Castells, P.; Parera, J.; Santos, F.J.; Galceran, M.T. Occurrence of polychlorinated naphthalenes, polychlorinated biphenyls and short-chain chlorinated paraffins in marine sediments from Barcelona (Spain). Chemosphere 2008, 70, 1552–1562. [Google Scholar] [CrossRef]
- Castells, P.; Santos, F.J.; Galceran, M.T. Evaluation of three ionisation modes for the analysis of chlorinated paraffins by gas chromatography/ion-trap mass spectrometry. Rapid Commun. Mass Spectrom. 2004, 18, 529–536. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, C.; Zhang, H.-J.; Zou, L.-L.; Tian, Y.-Z.; Chen, J.-P. Analysis of short-chain chlorinated paraffins in sediment samples from the mouth of the Daliao River by HRGC/ECNI-LRMS. Environ. Sci. 2010, 31, 1904–1908. (In Chinese) [Google Scholar]
- Zeng, L.; Zhao, Z.; Li, H.; Thanh, W.; Liu, Q.; Xiao, K.; Du, Y.; Wang, Y.; Jiang, G. Distribution of short chain chlorinated paraffins in marine sediments of the East China Sea: Influencing factors, transport and implications. Environ. Sci. Technol. 2012, 46, 9898–9906. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, H.; Wang, Y.; Li, G.; Cao, Y.; Zeng, L.; Lan, J.; Wang, T.; Jiang, G. Source and migration of short-chain chlorinated paraffins in the coastal East China Sea using multiproxies of marine organic geochemistry. Environ. Sci. Technol. 2013, 47, 5013–5022. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Gao, L.; Xia, D.; Qiao, L. Bioaccumulation and biomagnification of short and medium chain polychlorinated paraffins in different species of fish from Liaodong Bay, North China. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, L.; Lam, J.C.W.; Chen, H.; Du, B.; Leung, K.M.Y.; Lam, P.K.S. Tracking dietary sources of short- and medium-chain chlorinated paraffins in marine mammals through a subtropical marine food web. Environ. Sci. Technol. 2017, 51, 9543–9552. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yu, J.; Han, S.; Wang, Y.; Jiang, G. Levels of short chain chlorinated paraffins in pine needles and bark and their vegetation-air partitioning in urban areas. Environ. Pollut. 2015, 196, 309–312. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-T.; Zhou, J.; Lei, B.-L.; Zhou, J.-M.; Xu, S.-Y.; Hu, B.-P.; Wang, D.-Q.; Zhang, D.-P.; Wu, M.-H. Atmospheric occurrence, homologue patterns and source apportionment of short- and medium-chain chlorinated paraffins in Shanghai, China: Biomonitoring with Masson pine (Pinus massoniana L.) needles. Sci. Total Environ. 2016, 560, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Thanh, W.; Zhu, N.; Zhang, K.; Zeng, L.; Fu, J.; Wang, Y.; Jiang, G. Short chain chlorinated paraffins in mollusks from coastal waters in the Chinese Bohai Sea. Environ. Sci. Technol. 2012, 46, 6489–6496. [Google Scholar] [CrossRef]
- Guan, K.-L.; Liu, Y.; Luo, X.-J.; Zeng, Y.-H.; Mai, B.-X. Short- and medium-chain chlorinated paraffins in aquatic organisms from an e-waste site: Biomagnification and maternal transfer. Sci. Total Environ. 2020, 708, 134840. [Google Scholar] [CrossRef]
- Luo, X.-J.; Sun, Y.-X.; Wu, J.-P.; Chen, S.-J.; Mai, B.-X. Short-chain chlorinated paraffins in terrestrial bird species inhabiting an e-waste recycling site in South China. Environ. Pollut. 2015, 198, 41–46. [Google Scholar] [CrossRef]
- Ren, Z.; Zeng, Y.; Tang, B.; Luo, X.; Huang, C.; Mai, B. Bioaccumulative characteristics of halogenated flame retardants in aquatic and terrestrial biotas: A case study of catfish and pigeons. Asian J. Ecotoxicol. 2018, 13, 163–168. (In Chinese) [Google Scholar]
- Yuan, B.; Fu, J.; Wang, Y.; Jiang, G. Short-chain chlorinated paraffins in soil, paddy seeds (Oryza sativa) and snails (Ampullariidae) in an e-waste dismantling area in China: Homologue group pattern, spatial distribution and risk assessment. Environ. Pollut. 2017, 220, 608–615. [Google Scholar] [CrossRef]
- Zhou, Y.; Yin, G.; Du, X.; Xu, M.; Qiu, Y.; Ahlqvist, P.; Chen, Q.; Zhao, J. Short-chain chlorinated paraffins (SCCPs) in a freshwater food web from Dianshan Lake: Occurrence level, congener pattern and trophic transfer. Sci. Total Environ. 2018, 615, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Chen, H.; Huang, T.; Lian, L.; Li, J.; Jia, C.; Gao, H.; Mao, X.; Ma, J. Tagged sources of short-chain chlorinated paraffins in China’s marine environment and fish. Chemosphere 2019, 229, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ma, X.; Guo, W.; Zhao, Y.; Jingcai, L.U.; Wang, Z.; Yao, Z. Congener specific distribution and bioaccumulation of short-chain chlorinated paraffins in Liao estuary. Chin. Sci. Bull. 2014, 59, 578–585. (In Chinese) [Google Scholar]
- Sun, R.; Luo, X.; Tang, B.; Li, Z.; Huang, L.; Wang, T.; Mai, B. Short-chain chlorinated paraffins in marine organisms from the Pearl River Estuary in South China: Residue levels and interspecies differences. Sci. Total Environ. 2016, 553, 196–203. [Google Scholar] [CrossRef]
- Li, H.; Fu, J.; Pan, W.; Wang, P.; Li, Y.; Zhang, Q.; Wang, Y.; Zhang, A.; Liang, Y.; Jiang, G. Environmental behaviour of short-chain chlorinated paraffins in aquatic and terrestrial ecosystems of Ny-Alesund and London Island, Svalbard, in the Arctic. Sci. Total Environ. 2017, 590, 163–170. [Google Scholar] [CrossRef]
- Sun, R.; Luo, X.; Tang, B.; Chen, L.; Liu, Y.; Mai, B. Bioaccumulation of short chain chlorinated paraffins in a typical freshwater food web contaminated by e-waste in south china: Bioaccumulation factors, tissue distribution, and trophic transfer. Environ. Pollut. 2017, 222, 165–174. [Google Scholar] [CrossRef]
- Du, B.; Ge, J.; Yang, R.; Han, X.; Chen, H.; Li, J.; Zeng, L. Altitude-dependent accumulation of short chain chlorinated paraffins in fish from alpine lakes and Lhasa river on the Tibetan Plateau. Environ. Pollut. 2019, 250, 594–600. [Google Scholar] [CrossRef]
- Chen, C.; Li, L.; Liu, J.; Liu, J. Global environmental fate of short-chain chlorinated paraffins: Modeling with a single vs. multiple sets of physicochemical properties. Sci. Total Environ. 2019, 666, 423–430. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, Q.; Gao, L.; Zheng, M.; Qiao, L.; Cui, L.; Wang, R.; Cheng, J. Spatial distributions and transport implications of short- and medium-chain chlorinated paraffins in soils and sediments from an e-waste dismantling area in China. Sci. Total Environ. 2019, 649, 821–828. [Google Scholar] [CrossRef]
- Li, T.; Gao, S.; Ben, Y.; Zhang, H.; Kang, Q.; Wan, Y. Screening of chlorinated paraffins and unsaturated analogues in commercial mixtures: Confirmation of their occurrences in the atmosphere. Environ. Sci. Technol. 2018, 52, 1862–1870. [Google Scholar] [CrossRef]
- Sun, R.; Chen, J.; Shao, H.; Tang, L.; Zheng, X.; Li, Q.X.; Wang, Y.; Luo, X.; Mai, B. Bioaccumulation of short-chain chlorinated paraffins in chicken (Gallus domesticus): Comparison to fish. J. Hazard. Mater. 2020, 396, 122590. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Huang, C.; Luo, X.; Liu, Y.; Ren, Z.; Mai, B. Polychlorinated biphenyls and chlorinated paraffins in home-produced eggs from an e-waste polluted area in South China: Occurrence and human dietary exposure. Environ. Int. 2018, 116, 52–59. [Google Scholar] [CrossRef]
- Zeng, Y.-H.; Luo, X.-J.; Tang, B.; Mai, B.-X. Habitat- and species-dependent accumulation of organohalogen pollutants in home-produced eggs from an electronic waste recycling site in South China: Levels, profiles, and human dietary exposure. Environ. Pollut. 2016, 216, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Gao, L.; Zheng, M.; Li, J.; Zhang, L.; Wu, Y.; Qiao, L.; Xu, C.; Wang, K.; Huang, D. Bioaccessibility of short chain chlorinated paraffins in meat and seafood. Sci. Total Environ. 2019, 668, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Gao, S.; Yang, M.; Zhang, F.; Cao, L.; Xie, H.; Chen, X.; Cai, Z. Dietary exposure and risk assessment of short-chain chlorinated paraffins in supermarket fresh products in Jinan, China. Chemosphere 2020, 244, 125393. [Google Scholar] [CrossRef]
- Yu, J.; Wang, T.; Wang, Y.; Meng, M.; Chen, R.; Jiang, G. Levels and distribution of short chain chlorinated paraffins in seafood from Dalian, China. Environ. Sci. 2014, 35, 1955–1961. (In Chinese) [Google Scholar]
- Huang, Y.; Qing, X.; Jiang, G.; Chen, L.; He, Q.; Meng, X.-Z.; Gao, B. Short-chain chlorinated paraffins in fish from two developed regions of China: Occurrence, influencing factors and implication for human exposure via consumption. Chemosphere 2019, 236, 124317. [Google Scholar] [CrossRef]
- Jiang, G.; Chen, L.-G.; He, Q.-S.; Meng, X.-Z.; Feng, Y.-B.; Huang, Y.-M.; Tang, C.-M. Contamination characteristics of short-chain chlorinated paraffins in edible fish of Shanghai. Environ. Sci. 2013, 34, 3374–3380. (In Chinese) [Google Scholar]
- Gao, W.; Cao, D.; Lv, K.; Wu, J.; Wang, Y.; Wang, C.; Wang, Y.; Jiang, G. Elimination of short-chain chlorinated paraffins in diet after Chinese traditional cooking-a cooking case study. Environ. Int. 2019, 122, 340–345. [Google Scholar] [CrossRef]
- Aamir, M.; Yin, S.; Guo, F.; Liu, K.; Xu, C.; Liu, W. Congener-specific mother-fetus distribution, placental retention, and transport of C10–13 and C14–17 chlorinated paraffins in pregnant women. Environ. Sci. Technol. 2019, 53, 11458–11466. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Aamir, M.; Li, M.; Liu, K.; Hu, Y.; Liu, N.; Xu, Y.; Du, J.; Xu, J.; Liu, W. Prenatal and postnatal exposure risk assessment of chlorinated paraffins in mothers and neonates: Occurrence, congener profile, and transfer behavior. J. Hazard. Mater. 2020, 395, 122660. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Harada, K.H.; Hitomi, T.; Niisoe, T.; Wang, P.; Shi, Y.; Yang, H.-R.; Takasuga, T.; Koizumi, A. Lactational exposure to short-chain chlorinated paraffins in China, Korea, and Japan. Chemosphere 2017, 173, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yuan, B.; Nyberg, E.; Yin, G.; Bignert, A.; Glynn, A.; Odland, J.O.; Qu, Y.; Sun, Y.; Wu, Y.; et al. Chlorinated paraffins in human milk from urban sites in China, Sweden, and Norway. Environ. Sci. Technol. 2020, 54, 4356–4366. [Google Scholar] [CrossRef]
- Xia, D.; Gao, L.; Zheng, M.; Li, J.; Zhang, L.; Wu, Y.; Tian, Q.; Huang, H.; Qiao, L. Human exposure to short- and medium-chain chlorinated paraffins via mothers’ milk in Chinese urban population. Environ. Sci. Technol. 2017, 51, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Cao, D.; Wang, Y.; Wu, J.; Wang, Y.; Wang, Y.; Jiang, G. External exposure to short- and medium-chain chlorinated paraffins for the general population in Beijing, China. Environ. Sci. Technol. 2018, 52, 32–39. [Google Scholar] [CrossRef]
- Zhou, W.; Shen, M.; Lam, J.C.W.; Zhu, M.; Liu, L.; Chen, H.; Du, B.; Zeng, L.; Zeng, E.Y. Size-dependent distribution and inhalation exposure characteristics of particle-bound chlorinated paraffins in indoor air in Guangzhou, China. Environ. Int. 2018, 121, 675–682. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Z.; Li, J.; Ma, Y.; Kong, L.; Yang, H.; Wang, L.; Liu, Y.; Lu, Y.; Zhang, J. Evaluation of short chain chlorinated paraffins in human milk and their intake by infants in Hebei Province, China. Food Addit. Contam. Part A-Chem. Anal. Control. Expo. Risk Assess. 2018, 35, 2011–2021. [Google Scholar] [CrossRef]
- Huang, H.; Gao, L.; Zheng, M.; Li, J.; Zhang, L.; Wu, Y.; Wang, R.; Xia, D.; Qiao, L.; Cui, L.; et al. Dietary exposure to short- and medium-chain chlorinated paraffins in meat and meat products from 20 provinces of China. Environ. Pollut. 2018, 233, 439–445. [Google Scholar] [CrossRef]
- Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.R.; Leblanc, J.-C.; Nebbia, C.S.; et al. Risk assessment of chlorinated paraffins in feed and food. EFSA J. 2020, 18, 5991. [Google Scholar]
- Wang, R.; Gao, L.; Zheng, M.; Li, J.; Zhang, L.; Wu, Y.; Wang, G.; Xiong, L.; Ding, D.; Lu, D.; et al. Characterization of short- and medium-chain chlorinated paraffins in cereals and legumes from 19 Chinese provinces. Chemosphere 2019, 226, 282–289. [Google Scholar] [CrossRef]
- Wang, R.; Gao, L.; Zheng, M.; Tian, Y.; Li, J.; Zhang, L.; Wu, Y.; Huang, H.; Qiao, L.; Liu, W.; et al. Short- and medium-chain chlorinated paraffins in aquatic foods from 18 Chinese provinces: Occurrence, spatial distributions, and risk assessment. Sci. Total Environ. 2018, 615, 1199–1206. [Google Scholar] [CrossRef] [PubMed]
- Kodell, R.L. Replace the NOAEL and LOAEL with the BMDL01 and BMDL10. Environ. Ecol. Stat. 2009, 16, 3–12. [Google Scholar] [CrossRef]
- WCC. International Chlorinated Alkanes Industry Association (ICAIA) Newsletter. World Chlorine Council. 2013. Available online: https://www.eurochlor.org/media/88258/20130712_icaia_newsletter_02_final.pdf (accessed on 22 July 2020).
- Gao, Y.; Zhang, H.; Su, F.; Tian, Y.; Chen, J. Environmental occurrence and distribution of short chain chlorinated paraffins in sediments and soils from the Liaohe River Basin, P.R. China. Environ. Sci. Technol. 2012, 46, 3771–3778. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Huang, T.; Mao, X.; Wang, L.; Zhao, Y.; Jia, C.; Wang, Y.; Gao, H.; Ma, J. Gridded emission inventory of short-chain chlorinated paraffins and its validation in China. Environ. Pollut. 2017, 220, 132–141. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, W.; Jiang, G. Strengthening the Study on the Behavior and Transformation of Medium-Chain Chlorinated Paraffins in the Environment. Environ. Sci. Technol. 2017, 51, 10282–10283. [Google Scholar] [CrossRef]
- Gluege, J.; Schinkel, L.; Hungerbuehler, K.; Cariou, R.; Bogdal, C. Environmental Risks of Medium-Chain Chlorinated Paraffins (MCCPs): A Review. Environ. Sci. Technol. 2018, 52, 6743–6760. [Google Scholar] [CrossRef]
- Liu, D.; Li, Q.; Cheng, Z.; Li, K.; Li, J.; Zhang, G. Spatiotemporal variations of chlorinated paraffins in PM2.5 from Chinese cities: Implication of the shifting and upgrading of its industries. Environ. Pollut. 2020, 259, 113853. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, X.; Gao, Y.; Bai, H.; Wang, P.; Chen, J.; Yuan, H.; Wang, L.; Li, X.; Wang, W. Monitoring gas- and particulate-phase short-chain polychlorinated paraffins in the urban air of Dalian by a self-developed passive sampler. J. Environ. Sci. 2019, 80, 287–295. [Google Scholar] [CrossRef]
- Wu, J.; Gao, W.; Liang, Y.; Fu, J.; Gao, Y.; Wang, Y.; Jiang, G. Spatiotemporal Distribution and Alpine Behavior of Short Chain Chlorinated Paraffins in Air at Shergyla Mountain and Lhasa on the Tibetan Plateau of China. Environ. Sci. Technol. 2017, 51, 11136–11144. [Google Scholar] [CrossRef]
- Yu, G. Study on Method and Application for Analysis of Short Chain Chlorinated Paraffins in Marine Environment; Dlian Maritime University: Dalian, China, 2012; p. 74. (In Chinese) [Google Scholar]
- Wan, W. Distribution Characteristics of Short-Chain Chlorinated Paraffin in Baiyangdian Lake and the Middle Reaches of the Yangtze River; Shijiazhuang; Hebei Normal University: Shijiazhuang, China, 2017; pp. 24–50. (In Chinese) [Google Scholar]
- Xu, J.; Gao, Y.; Zhang, H.; Zhan, F.; Chen, J. Dispersion of Short- and Medium-Chain Chlorinated Paraffins (CPs) from a CP Production Plant to the Surrounding Surface Soils and Coniferous Leaves. Environ. Sci. Technol. 2016, 50, 12759–12766. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Wang, T.; Han, W.; Yuan, B.; Liu, Q.; Wang, Y.; Jiang, G. Spatial and Vertical Distribution of Short Chain Chlorinated Paraffins in Soils from Wastewater Irrigated Farmlands. Environ. Sci. Technol. 2011, 45, 2100–2106. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Gao, L.; Qiao, L.; Cui, L.; Xu, C.; Wang, K.; Zheng, M. Concentrations of and risks posed by short-chain and medium-chain chlorinated paraffins in soil at a chemical industrial park on the southeast coast of China. Environ. Pollut. 2020, 258, 113704. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-T.; Wang, X.-K.; Zhang, Y.; Chen, L.; Sun, Y.-F.; Li, M.; Wu, M.-H. Short- and medium-chain chlorinated paraffins in urban soils of Shanghai: Spatial distribution, homologue group patterns and ecological risk assessment. Sci. Total Environ. 2014, 490, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q. The Research about SCCPs Level in the Zhejiang Province Soil and Sediments; Zhejiang University of Technology: Hangzhou, China, 2012; p. 71. (In Chinese) [Google Scholar]
- Chen, R.; Wang, Y.; Wang, P.; Jiang, G. Spatial distribution of short chain chlorinated paraffins in soils from Taizhou, an e-waste dismantling area. Environ. Chem. 2014, 33, 873–879. (In Chinese) [Google Scholar]
- Han, S.; Huang, Y.; Chen, L.; Ye, Z.; Feng, Y.; Zhang, S. The short-chain chlorinated paraffins content and distribution in soil of Guangzhou. Sichuan Environ. 2012, 31, 56–60. (In Chinese) [Google Scholar]
- Huang, Y.; Chen, L.; Feng, Y.; Ye, Z.; He, Q.; Feng, Q.; Qing, X.; Liu, M.; Gao, B. Short-chain chlorinated paraffins in the soils of two different Chinese cities: Occurrence, homologue patterns and vertical migration. Sci. Total Environ. 2016, 557, 644–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Wu, J.; Tan, H.; Song, Q.; Zhang, J.; Zhong, X.; Zhou, J.; Wu, W.; Cai, X.; Zhang, W.; et al. Distributions of chlorinated paraffins and the effects on soil microbial community structure in a production plant brownfield site. Environ. Pollut. 2020, 262, 114328. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Chen, L.; Huang, Y.; Ye, Z.; Jiang, G.; Wang, X. Preliminary study on short-chain chlorinated paraffins in different types of soils of Chengdu. Environ. Sci. Technol. 2014, 37, 33–37. (In Chinese) [Google Scholar]
- Pan, X.; Tang, J.; Tian, C.; Li, J.; Zhang, G. Short- and medium-chain chlorinated paraffins in sediments from the Laizhou Bay area, North China: Implications for transportation from rivers to marine environment. Environ. Pollut. 2018, 243, 1460–1468. [Google Scholar] [CrossRef]
- Qiao, L.; Xia, D.; Gao, L.; Huang, H.; Zheng, M. Occurrences, sources and risk assessment of short- and medium-chain chlorinated paraffins in sediments from the middle reaches of the Yellow River, China. Environ. Pollut. 2016, 219, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Cheng, X.; Cui, Y.; Sun, J.; Li, J.; Zhang, G. Short- and medium-chain chlorinated paraffins in the Henan section of the Yellow River: Occurrences, fates, and fluxes. Sci. Total Environ. 2018, 640, 1312–1319. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Gao, L.; Xia, D.; Huang, H.; Zheng, M. Short- and medium-chain chlorinated paraffins in sediments from the middle reaches of the Yangtze River: Spatial distributions, source apportionment and risk assessment. Sci. Total Environ. 2017, 575, 1177–1182. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-Y.; Luo, X.-J.; Zhang, X.-L.; He, M.-J.; Chen, S.-J.; Mi, B.-X. Chlorinated Paraffins in Sediments from the Pearl River Delta, South China: Spatial and Temporal Distributions and Implication for Processes. Environ. Sci. Technol. 2011, 45, 9936–9943. [Google Scholar] [CrossRef]
- Lu, F.; Chen, M.; Chen, Y.; Liu, F.; Luo, X.; Mai, B. Distribution of chlorinated paraffins and polychlorinated biphenyls in e-waste, residues and sediment from e-waste areas of Qingyuan. Environ. Chem. 2015, 34, 1297–1303. (In Chinese) [Google Scholar]
- Zeng, L.; Chen, R.; Zhao, Z.; Wang, T.; Gao, Y.; Li, A.; Wang, Y.; Jiang, G.; Sun, L. Spatial Distributions and Deposition Chronology of Short Chain Chlorinated Paraffins in Marine Sediments across the Chinese Bohai and Yellow Seas. Environ. Sci. Technol. 2013, 47, 11449–11456. [Google Scholar] [CrossRef]
- Li, H.; Lan, J.; Zeng, L.; Cao, Y.; Zhao, Z. Sedimentary records of short chain chlorinated paraffins in the Zhejiang-Fujian mud area of the East China Sea. Fresenius Environ. Bull. 2014, 23, 105–112. [Google Scholar]
- Du, X.; Yuan, B.; Zhou, Y.; de Wit, C.A.; Zheng, Z.; Yin, G. Chlorinated Paraffins in Two Snake Species from the Yangtze River Delta: Tissue Distribution and Biomagnification. Environ. Sci. Technol. 2020, 54, 2753–2762. [Google Scholar] [CrossRef]
- Jiang, G. The Contamination Characteristic of Short-Chain Chlorinated Paraffins in Edible Fish and Preliminary Study on Their Rish Exposure; Taiyuan Uniersity of Science and Technology: Taiyuan, China, 2013; p. 59. (In Chinese) [Google Scholar]
- Zeng, L.; Lam, J.C.W.; Wang, Y.; Jiang, G.; Lam, P.K.S. Temporal Trends and Pattern Changes of Short- and Medium-Chain Chlorinated Paraffins in Marine Mammals from the South China Sea over the Past Decade. Environ. Sci. Technol. 2015, 49, 11348–11355. [Google Scholar] [CrossRef]
- Chen, L.; Huang, Y.; Han, S.; Feng, Y.; Jiang, G.; Tang, C.; Ye, Z.; Zhan, W.; Liu, M.; Zhang, S. Sample pretreatment optimization for the analysis of short chain chlorinated paraffins in soil with gas chromatography-electron capture negative ion-mass spectrometry. J. Chromatogr. A 2013, 1274, 36–43. [Google Scholar] [CrossRef]
- Harada, K.H.; Takasuga, T.; Hitomi, T.; Wang, P.; Matsukami, H.; Koizumi, A. Dietary Exposure to Short-Chain Chlorinated Paraffins Has Increased in Beijing, China. Environ. Sci. Technol. 2011, 45, 7019–7027. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Harada, K.H.; Liu, W.; Yan, J.; Zhao, C.; Niisoe, T.; Adachi, A.; Fujii, Y.; Nouda, C.; Takasuga, T.; et al. Short-chain chlorinated paraffins in cooking oil and related products from China. Chemosphere 2015, 138, 104–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Shen, J.; Wang, X.; Yang, L. Dietary Pollution Status and Exposure Risk Assessment of Short-Chain Chlorinated Paraffins. Food Sci. 2019, 40, 143–149. (In Chinese) [Google Scholar]
- Dong, S.; Zhang, S.; Li, X.; Wei, S.; Li, T.; Zou, Y.; Zhang, W.; Cheng, J.; Wang, R.; Wang, P.; et al. Occurrence of short- and medium-chain chlorinated paraffins in raw dairy cow milk from five Chinese provinces. Environ. Int. 2020, 136, 105466. [Google Scholar] [CrossRef]
- Dong, S.; Li, X.; Su, X.; Wang, P. Concentrations and congener group profiles of short-and medium-chain chlorinated paraffins in animal feed materials. Sci. Total Environ. 2019, 647, 676–681. [Google Scholar] [CrossRef]
- Chen, R. Environmental Behavior of Short-Chain Chlorinated Paraffins in Multi-Medium; Shandong University: Jinan, China, 2014; p. 72. (In Chinese) [Google Scholar]
- Li, T.; Wan, Y.; Gao, S.; Wang, B.; Hu, J. High-Throughput Determination and Characterization of Short-, Medium-, and Long-Chain Chlorinated Paraffins in Human Blood. Environ. Sci. Technol. 2017, 51, 3346–3354. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wu, H.; Huang, X.; Hang, F.; Luo, H. Development a simple and rapid HPLC-ESI-Q-TOF/MS method for determination of short- and medium-chain chlorinated paraffins in human serum. J. Chromatogr. B-Anal. Technol. Biomed. Life Sci. 2019, 1126, 121722. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Guo, W.; Wei, L.; Gao, Y.; Zhang, H.; Zhang, Y.; Sun, M.; Chen, J. Validation of a HRGC-ECNI/LRMS method to monitor short-chain chlorinated paraffins in human plasma. J. Environ. Sci. 2019, 75, 289–295. [Google Scholar] [CrossRef]
- Ding, L.; Luo, N.; Liu, Y.; Fang, X.; Zhang, S.; Li, S.; Jiang, W.; Zhao, N. Short and medium -chain chlorinated paraffins in serum from residents aged from 50 to 84 in Jinan, China: Occurrence, composition and association with hematologic parameters. Sci. Total Environ. 2020, 728, 137998. [Google Scholar] [CrossRef]
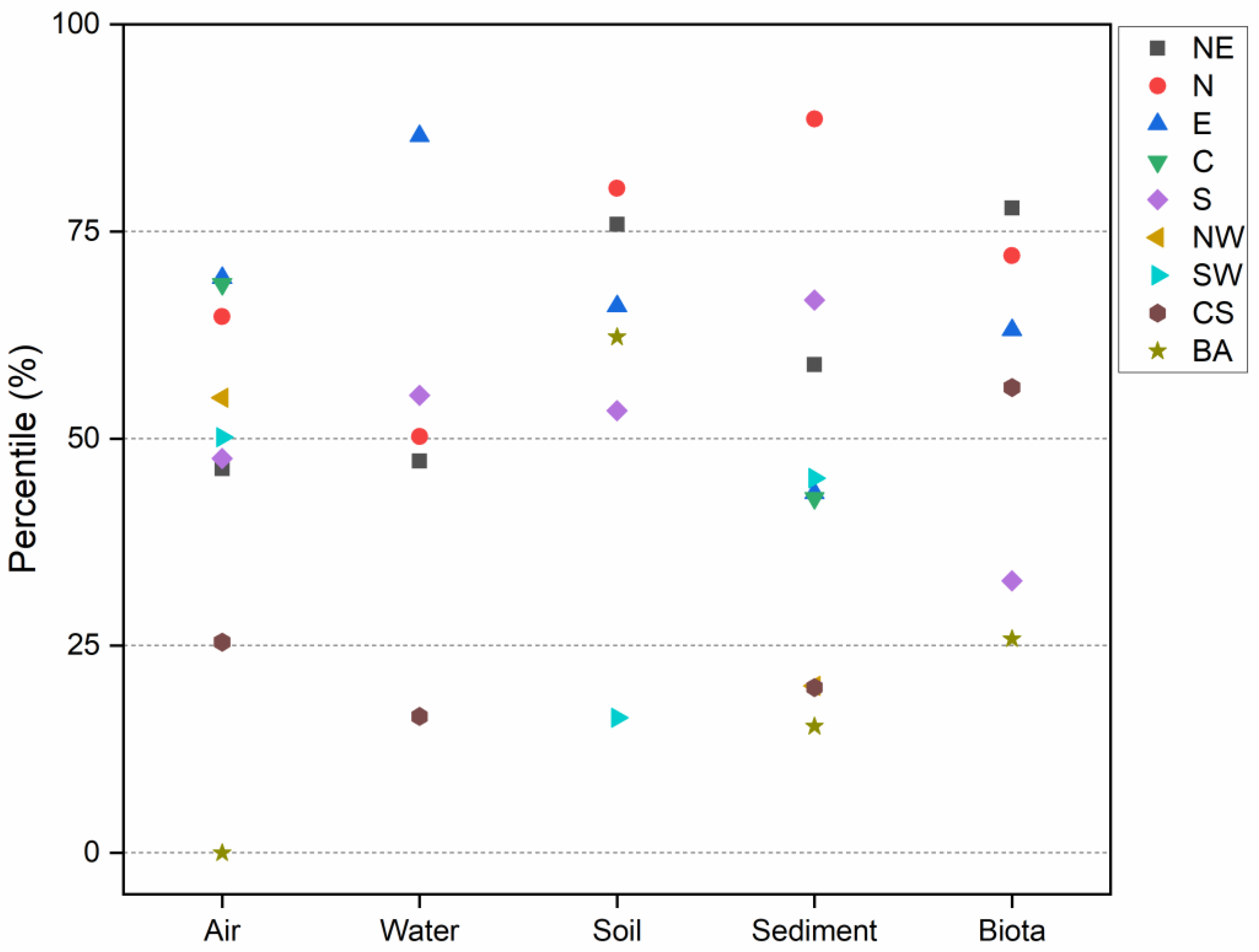

| Region | Air (ng/m3) | Water (ng/L) | Soil (ng/g dw a) | Sediment (ng/g dw) | Biota (ng/g dw) | |
|---|---|---|---|---|---|---|
| Gas Phase | Particle Phase | |||||
| Northeastern China | 4.04–165 | 0.52–10.5 | 4.10–1490 | 56.9–189 | ND–13,800 | 374–20,320 |
| Northern China | 0.40–1350 | 1.40–87.7 | 162–176 | 160–1450 | 690–9120 | 320–4700 |
| Eastern China | 6.08–63.2 | 2.36–105 | 15.0–1978 | ND–697 | ND–2020 | ND–30,000 |
| Central China | - | 2.98–89.4 | - | - | 4.19–9760 | - |
| Southern China | 0.95–106 | 1.60–51.8 | 61.0–460 | 1.45–541 | ND–6600 | 11.1–2000 |
| Northwestern China | - | 4.36–27.5 | - | - | ND–100 | - |
| Southwestern China | 1.10–14.4 | 3.82–39.9 | - | 0.22–948 | ND–680 | - |
| China sea areas | 2.80–29.0 | 0.31–3.60 | 11.0–110 | - | 4.40–1757 | 9.30–9100 |
| Background area | 0.13–1.27 | - | - | 81.6 ± 31.1 | ND–59.0 | 3.90–300 |
| Emission region | 81.7–988 | 7.40–454 | 27.0–4700 | 30.4–554,161 | 32.5–350,000 | 4.90–2197 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Fan, R.; Xu, Y.; Gao, Y.-Z.; Bizimana, A.; Naidoo, A.R.; Han, B.-C.; Meng, X.-Z. Occurrence, Distribution and Health Risk of Short-Chain Chlorinated Paraffins (SCCPs) in China: A Critical Review. Separations 2022, 9, 208. https://doi.org/10.3390/separations9080208
Zhang X, Fan R, Xu Y, Gao Y-Z, Bizimana A, Naidoo AR, Han B-C, Meng X-Z. Occurrence, Distribution and Health Risk of Short-Chain Chlorinated Paraffins (SCCPs) in China: A Critical Review. Separations. 2022; 9(8):208. https://doi.org/10.3390/separations9080208
Chicago/Turabian StyleZhang, Xufeng, Ru Fan, Yang Xu, Yun-Ze Gao, Aaron Bizimana, Anastacia Rochelle Naidoo, Bao-Cang Han, and Xiang-Zhou Meng. 2022. "Occurrence, Distribution and Health Risk of Short-Chain Chlorinated Paraffins (SCCPs) in China: A Critical Review" Separations 9, no. 8: 208. https://doi.org/10.3390/separations9080208
APA StyleZhang, X., Fan, R., Xu, Y., Gao, Y.-Z., Bizimana, A., Naidoo, A. R., Han, B.-C., & Meng, X.-Z. (2022). Occurrence, Distribution and Health Risk of Short-Chain Chlorinated Paraffins (SCCPs) in China: A Critical Review. Separations, 9(8), 208. https://doi.org/10.3390/separations9080208