Modeling and Life Cycle Assessment of a Membrane Bioreactor–Membrane Distillation Wastewater Treatment System for Potable Reuse
Abstract
:1. Introduction
2. Materials and Methods
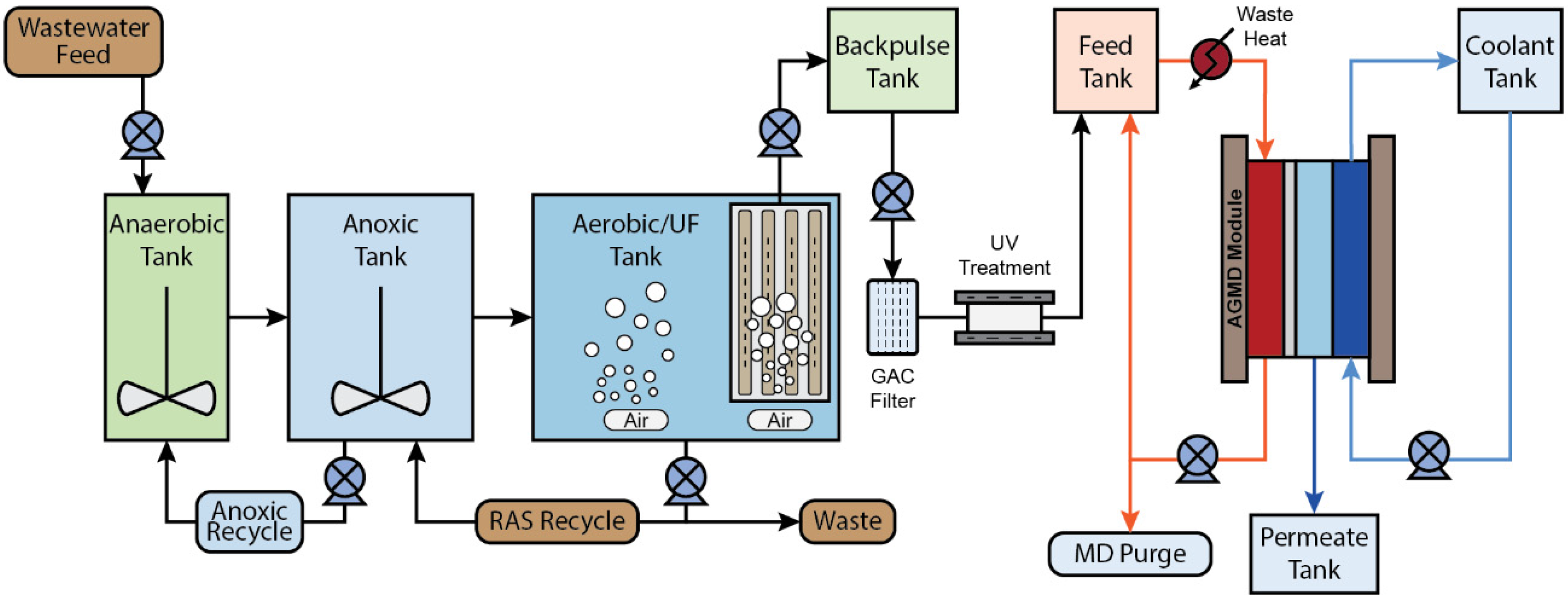
2.1. MBR-MD Process
2.2. MBR Modeling Process
2.3. MD Modeling Process
2.4. Baseline Modeling Process
2.5. Life Cycle Assessment
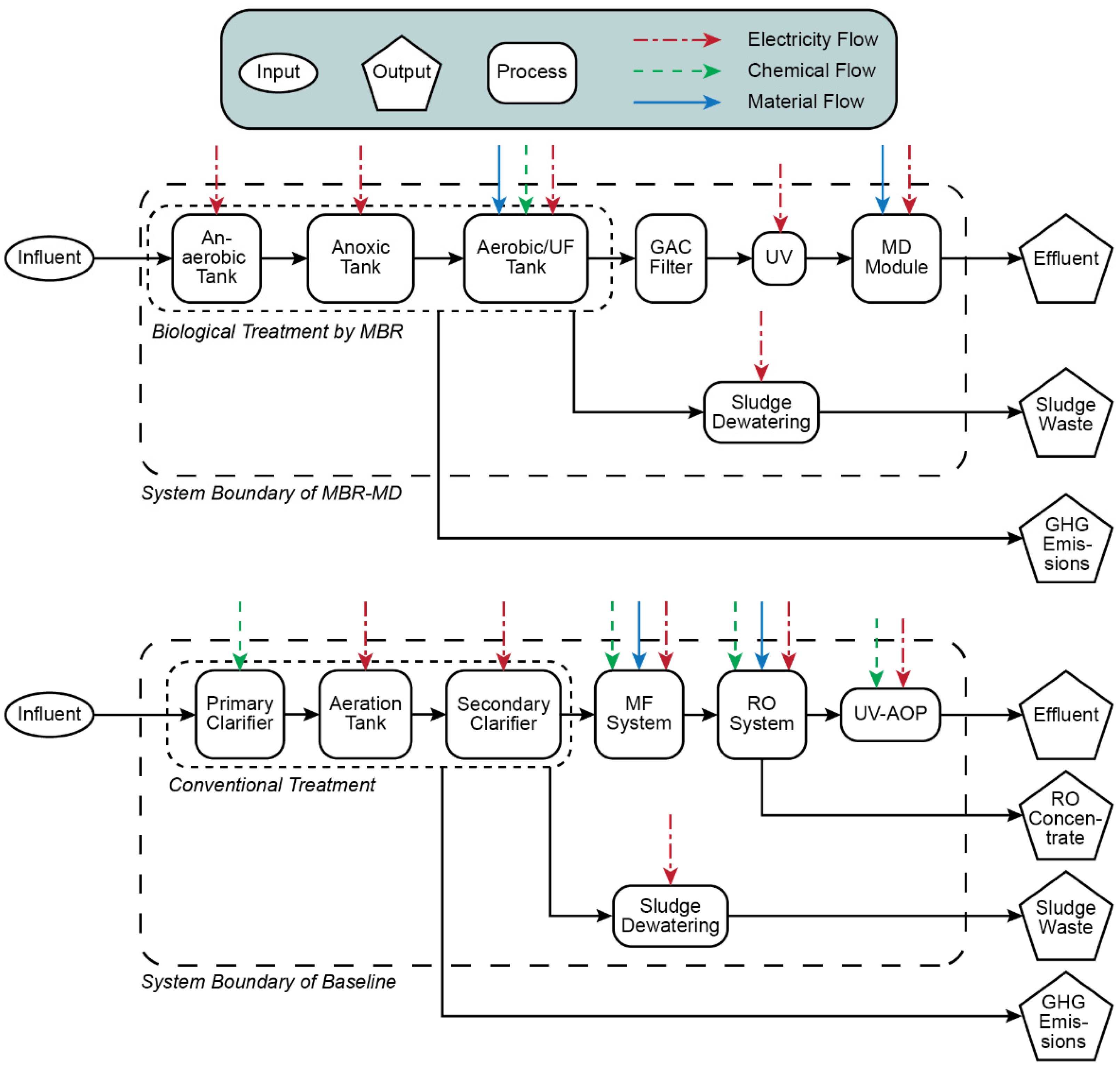
2.5.1. Goal and Scope
2.5.2. System Boundaries
2.5.3. Life Cycle Inventory
2.5.4. Impact Assessment Method
2.5.5. Uncertainty Analysis
3. Results and Discussion
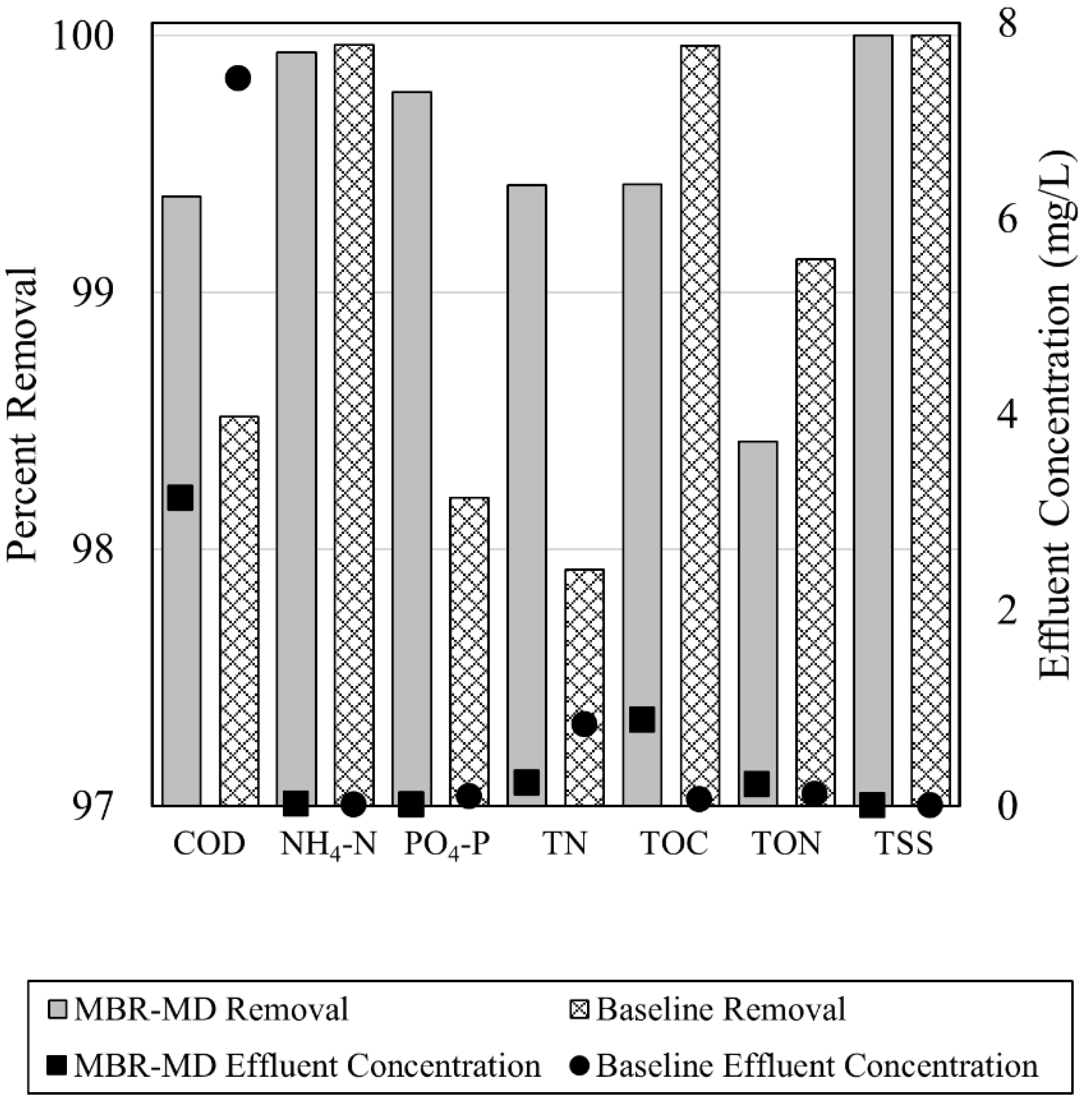
3.1. MBR-MD and Baseline Modeling
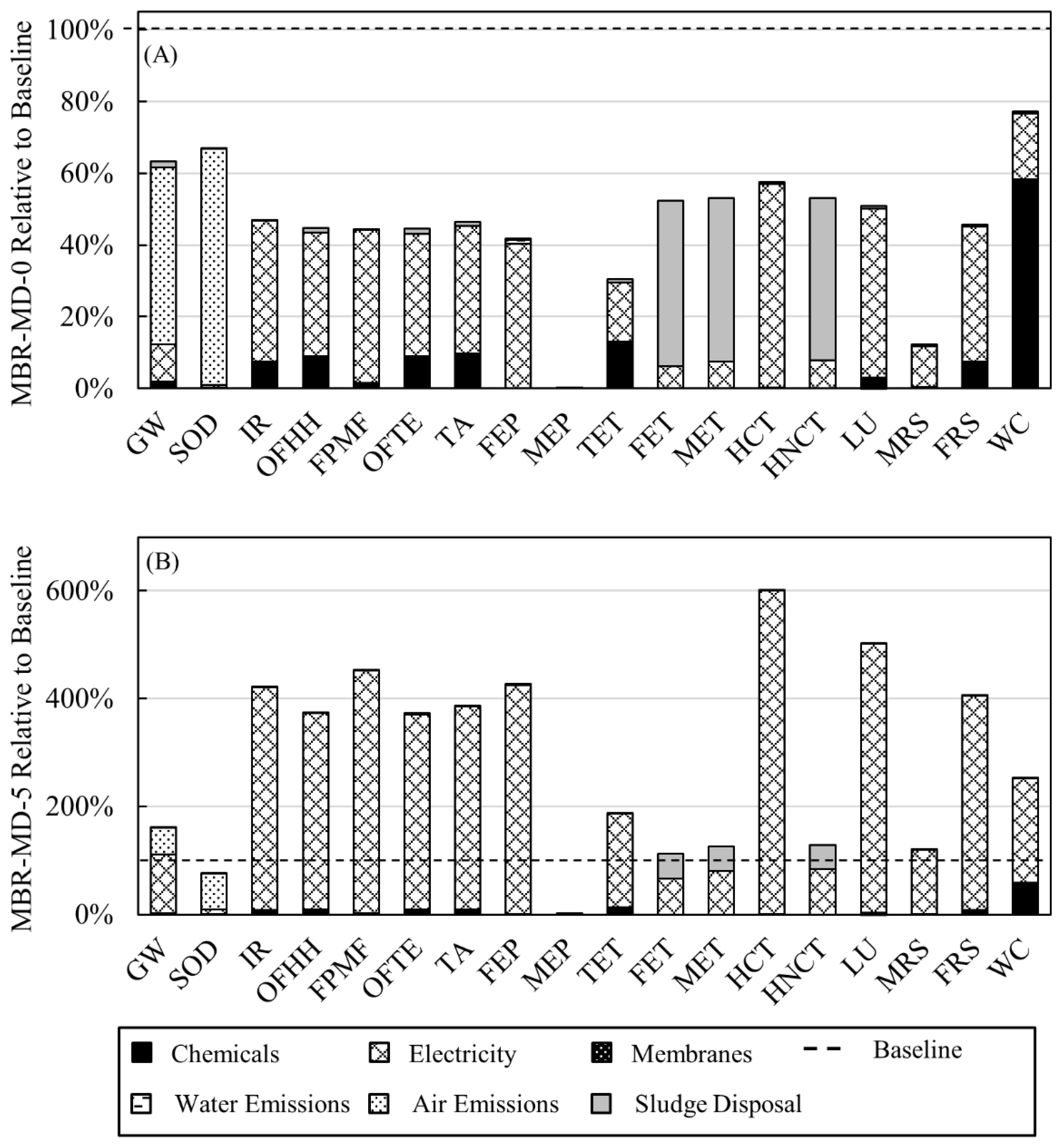
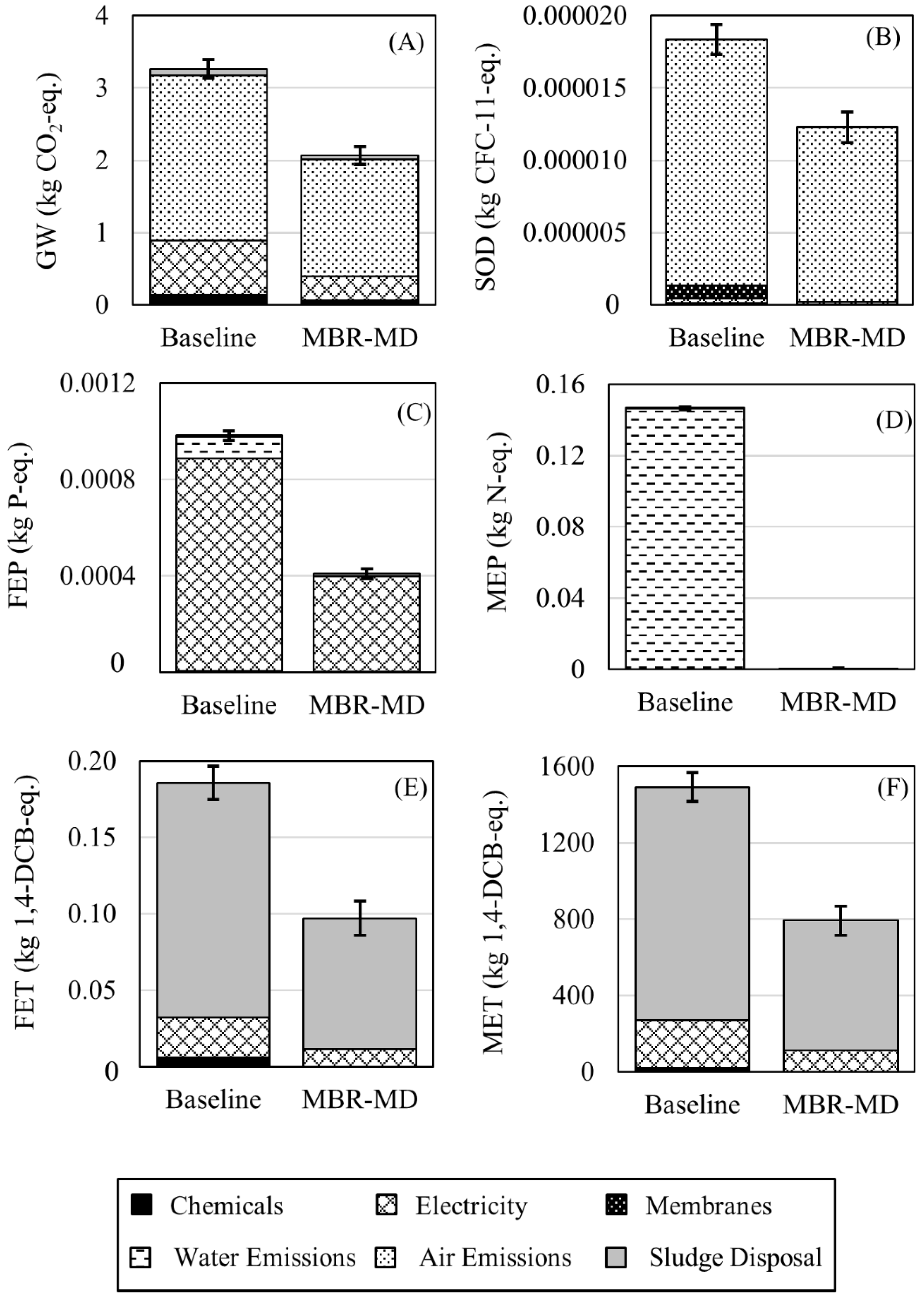
3.2. Environmental Impacts
4. Conclusions
- Effluent water quality of the MBR-MD and Baseline systems were comparable, with the MBR-MD system displaying generally lower concentrations of COD, TN, NH4–N, and PO4–P. Effluent water quality emissions were not a main contributor for any environmental impacts but did account for a small percentage of the FEP and MEP impact categories;
- Direct GHG emissions and sludge wasting for MBR-MD are both lower than the Baseline due to more efficient biotreatment by the MBR;
- Electricity consumption is the main driver behind the environmental impacts for both systems, with sludge wasting, chemical additions, and direct GHG emissions following behind;
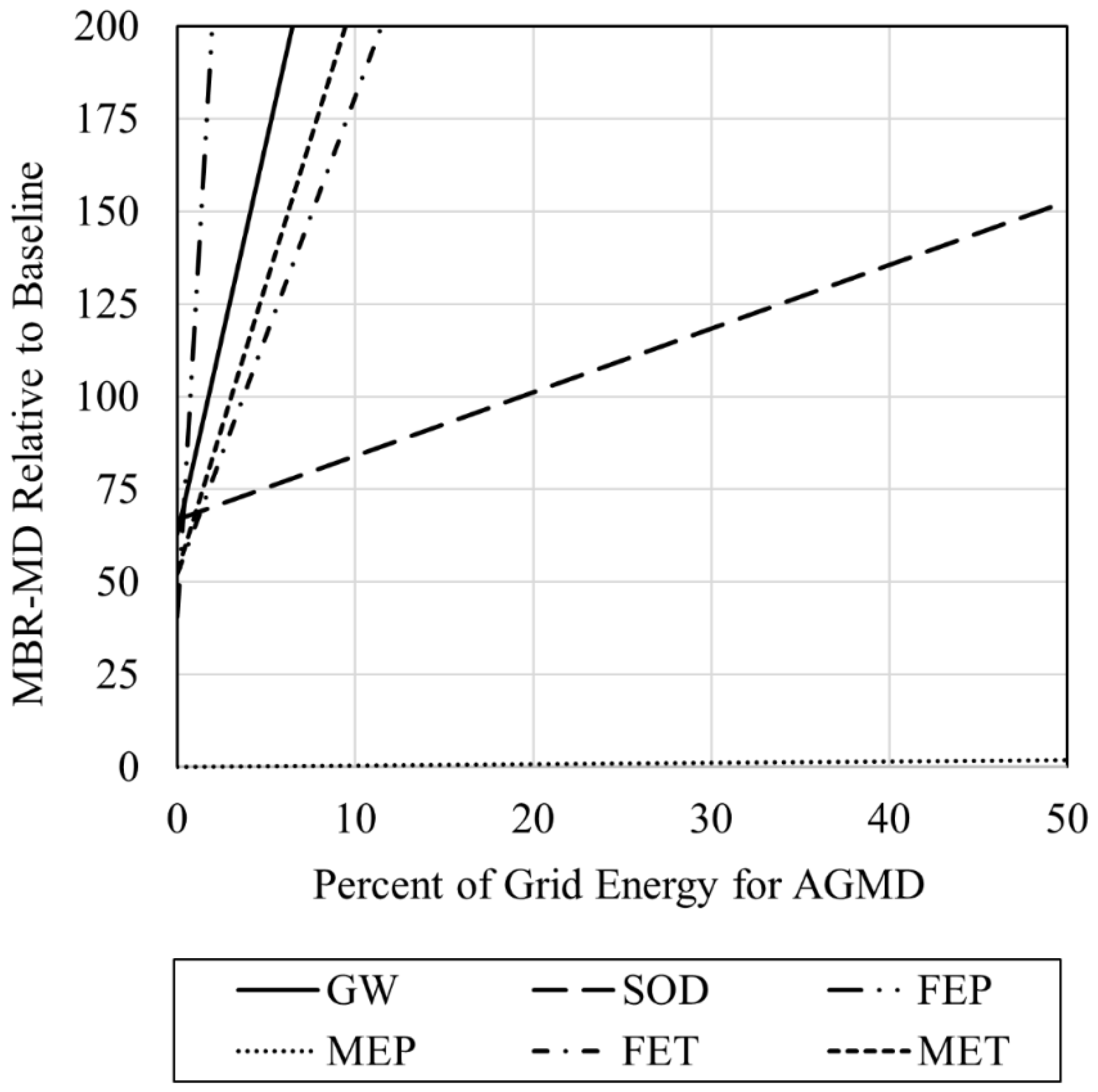
- RO is the highest consumer of electricity for the Baseline, while the specific thermal energy consumption of the air gap MD sub-system consumes the most electricity for the MBR-MD system;
- Using 0% grid energy (100% waste heat) for MD heating, environmental impacts for the MBR-MD system are lower for each impact category compared to the Baseline.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Richey, A.S.; Thomas, B.F.; Lo, M.-H.; Reager, J.T.; Famiglietti, J.S.; Voss, K.; Swenson, S.; Rodell, M. Quantifying Renewable Groundwater Stress with GRACE. Water Resour. Res. 2015, 51, 5217–5238. [Google Scholar] [CrossRef] [PubMed]
- Burek, P.; Satoh, Y.; Fischer, G.; Kahil, M.T.; Scherzer, A.; Tramberend, S.; Nava, L.F.; Wada, Y.; Eisner, S.; Flörke, M.; et al. Water Futures and Solution—Fast Track Initiative (Final Report); IIASA: Laxenburg, Austria, 2016. [Google Scholar]
- Kummu, M.; Guillaume, J.H.A.; de Moel, H.; Eisner, S.; Flörke, M.; Porkka, M.; Siebert, S.; Veldkamp, T.I.E.; Ward, P.J. The World’s Road to Water Scarcity: Shortage and Stress in the 20th Century and Pathways towards Sustainability. Sci. Rep. 2016, 6, 38495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mekonnen, M.M.; Hoekstra, A.Y. Sustainability: Four Billion People Facing Severe Water Scarcity. Sci. Adv. 2016, 2, e1500323. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, C.; Van Buynder, P.; Lugg, R.; Blair, P.; Devine, B.; Cook, A.; Weinstein, P. Indirect Potable Reuse: A Sustainable Water Supply Alternative. Int. J. Environ. Res. Public Health 2009, 6, 1174–1203. [Google Scholar] [CrossRef] [Green Version]
- Lautze, J.; Stander, E.; Drechsel, P.; da Silva, A.K.; Keraita, B. Global Experiences in Water Reuse; International Water Management Institute (IWMI): Colombo, Sri Lanka, 2014. [Google Scholar] [CrossRef]
- Holloway, R.W.; Miller-Robbie, L.; Patel, M.; Stokes, J.R.; Munakata-Marr, J.; Dadakis, J.; Cath, T.Y. Life-Cycle Assessment of Two Potable Water Reuse Technologies: MF/RO/UV-AOP Treatment and Hybrid Osmotic Membrane Bioreactors. J. Membr. Sci. 2016, 507, 165–178. [Google Scholar] [CrossRef] [Green Version]
- Bai, Y.; Shan, F.; Zhu, Y.; Xu, J.; Wu, Y.; Luo, X.; Wu, Y.; Hu, H.-Y.; Zhang, B. Long-Term Performance and Economic Evaluation of Full-Scale MF and RO Process—A Case Study of the Changi NEWater Project Phase 2 in Singapore. Water Cycle 2020, 1, 128–135. [Google Scholar] [CrossRef]
- Morrow, C.P.; McGaughey, A.L.; Hiibel, S.R.; Childress, A.E. Submerged or Sidestream? The Influence of Module Configuration on Fouling and Salinity in Osmotic Membrane Bioreactors. J. Membr. Sci. 2018, 548, 583–592. [Google Scholar] [CrossRef]
- Achilli, A.; Marchand, E.A.; Childress, A.E. A Performance Evaluation of Three Membrane Bioreactor Systems: Aerobic, Anaerobic, and Attached-Growth. Water Sci. Technol. 2011, 63, 2999–3005. [Google Scholar] [CrossRef]
- Carvajal, G.; Branch, A.; Sisson, S.A.; Roser, D.J.; van den Akker, B.; Monis, P.; Reeve, P.; Keegan, A.; Regel, R.; Khan, S.J. Virus Removal by Ultrafiltration: Understanding Long-Term Performance Change by Application of Bayesian Analysis. Water Res. 2017, 122, 269–279. [Google Scholar] [CrossRef]
- Isik, O.; Abdelrahman, A.M.; Ozgun, H.; Ersahin, M.E.; Demir, I.; Koyuncu, I. Comparative Evaluation of Ultrafiltration and Dynamic Membranes in an Aerobic Membrane Bioreactor for Municipal Wastewater Treatment. Environ. Sci. Pollut. Res. 2019, 26, 32723–32733. [Google Scholar] [CrossRef]
- Kitanou, S.; Hafida, A.; Soukaina, B.; Mahi, M.; Taky, M.; Elmidaoui, A. Performance of an Ultrafiltration Membrane Bioreactor (UF-MBR) in Wastewater Treatment. Desalin. Water Treat. 2019, 157, 393–398. [Google Scholar] [CrossRef] [Green Version]
- Phan, H.V.; Hai, F.I.; McDonald, J.A.; Khan, S.J.; Zhang, R.; Price, W.E.; Broeckmann, A.; Nghiem, L.D. Nutrient and Trace Organic Contaminant Removal from Wastewater of a Resort Town: Comparison between a Pilot and a Full Scale Membrane Bioreactor. Int. Biodeterior. Biodegrad. 2015, 102, 40–48. [Google Scholar] [CrossRef] [Green Version]
- Hoinkis, J.; Deowan, S.A.; Panten, V.; Figoli, A.; Huang, R.R.; Drioli, E. Membrane Bioreactor (MBR) Technology—A Promising Approach for Industrial Water Reuse. Procedia Eng. 2012, 33, 234–241. [Google Scholar] [CrossRef] [Green Version]
- Environmental Protection Agency. Wastewater Management Fact Sheet: Membrane Bioreactors; EPA: Washington, DC, USA, 2007. [Google Scholar]
- Davis, M.L. Water and Wastewater Engineering: Design Principles and Practice; McGraw-Hill Education: New York, NY, USA, 2010; ISBN 9780071713849. [Google Scholar]
- Viet, N.D.; Cho, J.; Yoon, Y.; Jang, A. Enhancing the Removal Efficiency of Osmotic Membrane Bioreactors: A Comprehensive Review of Influencing Parameters and Hybrid Configurations. Chemosphere 2019, 236, 124363. [Google Scholar] [CrossRef] [PubMed]
- Iorhemen, O.T.; Hamza, R.A.; Tay, J.H. Membrane Bioreactor (MBR) Technology for Wastewater Treatment and Reclamation: Membrane Fouling. Membranes 2016, 6, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shirazi, M.M.A.; Bazgir, S.; Meshkani, F. A Novel Dual-Layer, Gas-Assisted Electrospun, Nanofibrous SAN4-HIPS Membrane for Industrial Textile Wastewater Treatment by Direct Contact Membrane Distillation (DCMD). J. Water Process Eng. 2020, 36, 101315. [Google Scholar] [CrossRef]
- Song, X.; Luo, W.; McDonald, J.; Khan, S.J.; Hai, F.I.; Price, W.E.; Nghiem, L.D. An Anaerobic Membrane Bioreactor—Membrane Distillation Hybrid System for Energy Recovery and Water Reuse: Removal Performance of Organic Carbon, Nutrients, and Trace Organic Contaminants. Sci. Total Environ. 2018, 628–629, 358–365. [Google Scholar] [CrossRef] [Green Version]
- Luo, W.; Phan, H.V.; Li, G.; Hai, F.I.; Price, W.E.; Elimelech, M.; Nghiem, L.D. An Osmotic Membrane Bioreactor-Membrane Distillation System for Simultaneous Wastewater Reuse and Seawater Desalination: Performance and Implications. Environ. Sci. Technol. 2017, 51, 14311–14320. [Google Scholar] [CrossRef]
- Gustafson, R.D.; Hiibel, S.R.; Childress, A.E. Membrane Distillation Driven by Intermittent and Variable-Temperature Waste Heat: System Arrangements for Water Production and Heat Storage. Desalination 2018, 448, 49–59. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, D.; Sun, M.; Hao Ngo, H.; Guo, W.; Wu, G.; Jia, W.; Shi, L.; Wu, Q.; Guo, F.; et al. Sustainability Evaluation and Implication of a Large Scale Membrane Bioreactor Plant. Bioresour. Technol. 2018, 269, 246–254. [Google Scholar] [CrossRef]
- Ortiz, M.; Raluy, R.G.; Serra, L. Life Cycle Assessment of Water Treatment Technologies: Wastewater and Water-Reuse in a Small Town. Desalination 2007, 204, 121–131. [Google Scholar] [CrossRef]
- Banti, D.C.; Tsangas, M.; Samaras, P.; Zorpas, A. LCA of a Membrane Bioreactor Compared to Activated Sludge System for Municipal Wastewater Treatment. Membranes 2020, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Kamble, S.; Singh, A.; Kazmi, A.; Starkl, M. Environmental and Economic Performance Evaluation of Municipal Wastewater Treatment Plants in India: A Life Cycle Approach. Water Sci. Technol. 2019, 79, 1102–1112. [Google Scholar] [CrossRef] [Green Version]
- Akhoundi, A.; Nazif, S. Life-Cycle Assessment of Tertiary Treatment Technologies to Treat Secondary Municipal Wastewater for Reuse in Agricultural Irrigation, Artificial Recharge of Groundwater, and Industrial Usages. J. Environ. Eng. 2020, 146, 04020031. [Google Scholar] [CrossRef]
- Alsebaeai, M.K.; Ahmad, A.L. Membrane Distillation: Progress in the Improvement of Dedicated Membranes for Enhanced Hydrophobicity and Desalination Performance. J. Ind. Eng. Chem. 2020, 86, 13–34. [Google Scholar] [CrossRef]
- Tarnacki, K.; Meneses, M.; Melin, T.; van Medevoort, J.; Jansen, A. Environmental Assessment of Desalination Processes: Reverse Osmosis and Memstill®. Desalination 2012, 296, 69–80. [Google Scholar] [CrossRef]
- Siefan, A.; Rachid, E.; Elashwah, N.; AlMarzooqi, F.; Banat, F.; van der Merwe, R. Desalination via Solar Membrane Distillation and Conventional Membrane Distillation: Life Cycle Assessment Case Study in Jordan. Desalination 2022, 522, 115383. [Google Scholar] [CrossRef]
- Metcalf & Eddy. Wastewater Engineering Treatment & Reuse; McGraw Hill: New York, NY, USA, 2003. [Google Scholar]
- Bill & Melinda Gates Foundation. Sludge Thickening, Dewatering and Drying Technologies. Gates Open Res. 2021, 5, 14. [Google Scholar] [CrossRef]
- Krzeminski, P.; Van Der Graaf, J.H.J.M.; Van Lier, J.B. Specific Energy Consumption of Membrane Bioreactor (MBR) for Sewage Treatment. Water Sci. Technol. 2012, 65, 380–392. [Google Scholar] [CrossRef]
- Noamani, S.; Niroomand, S.; Rastgar, M.; Azhdarzadeh, M.; Sadrzadeh, M. Modeling of Air-Gap Membrane Distillation and Comparative Study with Direct Contact Membrane Distillation. Ind. Eng. Chem. Res. 2020, 59, 21930–21947. [Google Scholar] [CrossRef]
- Sharqawy, M.H.; Lienhard, J.H.; Zubair, S.M. Thermophysical Properties of Seawater: A Review of Existing Correlations and Data. Desalin. Water Treat. 2010, 16, 354–380. [Google Scholar] [CrossRef]
- Duong, H.C.; Cooper, P.; Nelemans, B.; Cath, T.Y.; Nghiem, L.D. Evaluating Energy Consumption of Air Gap Membrane Distillation for Seawater Desalination at Pilot Scale Level. Sep. Purif. Technol. 2016, 166, 55–62. [Google Scholar] [CrossRef] [Green Version]
- Hardikar, M.; Marquez, I.; Phakdon, T.; Sáez, A.E.; Achilli, A. Scale-up of Membrane Distillation Systems Using Bench-Scale Data. Desalination 2022, 530, 115654. [Google Scholar] [CrossRef]
- Orange County Sanitation District. District Overview and Compliance: Introduction Compliance with NPDES Permit Requirements Waste Discharge Requirements for Sewage Collection Agencies; Orange County Sanitation District: Fountain Valley, CA, USA, 2005. [Google Scholar]
- Yang, X.-L.; Song, H.-L.; Chen, M.; Cheng, B. Characterizing Membrane Foulants in MBR with Addition of Polyferric Chloride to Enhance Phosphorus Removal. Bioresour. Technol. 2011, 102, 9490–9496. [Google Scholar] [CrossRef]
- Orange County Water District. Groundwater Replenishment System 2019 Annual Report; Orange County Water District: Fountain Valley, CA, USA, 2020. [Google Scholar]
- Klüppel, H. The Revision of ISO Standards 14040-3. Int. J. Life Cycle Assess. 2005, 10, 165. [Google Scholar] [CrossRef]
- Fenu, A.; De Wilde, W.; Gaertner, M.; Weemaes, M.; de Gueldre, G.; Van De Steene, B. Elaborating the Membrane Life Concept in a Full Scale Hollow-Fibers MBR. J. Membr. Sci. 2012, 421–422, 349–354. [Google Scholar] [CrossRef]
- Coutinho de Paula, E.; Amaral, M.C.S. Extending the Life-Cycle of Reverse Osmosis Membranes: A Review. Waste Manag. Res. 2017, 35, 456–470. [Google Scholar] [CrossRef]
- Dow, N.; Gray, S.; Li, J.; Zhang, J.; Ostarcevic, E.; Liubinas, A.; Atherton, P.; Roeszler, G.; Gibbs, A.; Duke, M. Pilot Trial of Membrane Distillation Driven by Low Grade Waste Heat: Membrane Fouling and Energy Assessment. Desalination 2016, 391, 30–42. [Google Scholar] [CrossRef]
- Lokare, O.R.; Tavakkoli, S.; Rodriguez, G.; Khanna, V.; Vidic, R.D. Integrating Membrane Distillation with Waste Heat from Natural Gas Compressor Stations for Produced Water Treatment in Pennsylvania. Desalination 2017, 413, 144–153. [Google Scholar] [CrossRef] [Green Version]
- Robbins, C.A.; Carlson, K.H.; Garland, S.D.; Bandhauer, T.M.; Grauberger, B.M.; Tong, T. Spatial Analysis of Membrane Distillation Powered by Waste Heat from Natural Gas Compressor Stations for Unconventional Oil and Gas Wastewater Treatment in Weld County, Colorado. ACS ES&T Eng. 2020, 1, 192–203. [Google Scholar] [CrossRef]
- Agamaliyev, M.M.; Ahmadova, J.A.; Aliyeva, O.O. Waste Heat Utilization of Diesel Power Plant Cooling System for Seawater Desalination by Membrane Distillation. Membr. Membr. Technol. 2022, 4, 48–58. [Google Scholar] [CrossRef]
- National Renewable Energy Laboratory. U.S. Life Cycle Inventory Database; NREL: Golden, CO, USA, 2012. [Google Scholar]
- Wernet, G.; Bauer, C.; Steubing, B.; Reinhard, J.; Moreno-Ruiz, E.; Weidema, B. The Ecoinvent Database Version 3 (Part I): Overview and Methodology. Int. J. Life Cycle Assess. 2016, 21, 1218–1230. [Google Scholar] [CrossRef]
- The Intergovernmental Panel on Climate Change. 2006 IPCC Guidelines for National Greenhouse Gas Inventories; Institute for Global Environmental Strategies (IGES): Kanagawa, Japan, 2006. [Google Scholar]
- Huijbregts, M.A.J.; Steinmann, Z.J.N.; Elshout, P.M.F.; Stam, G.; Verones, F.; Vieira, M.; Zijp, M.; Hollander, A.; van Zelm, R. ReCiPe2016: A Harmonised Life Cycle Impact Assessment Method at Midpoint and Endpoint Level. Int. J. Life Cycle Assess. 2017, 22, 138–147. [Google Scholar] [CrossRef]
- Lee, S.; Kim, Y.; Kim, A.S.; Hong, S. Evaluation of Membrane-Based Desalting Processes for RO Brine Treatment. Desalin. Water Treat. 2015, 57, 7432–7439. [Google Scholar] [CrossRef]
- Couto, C.F.; Santos, A.V.; Amaral, M.C.S.; Lange, L.C.; de Andrade, L.H.; Foureaux, A.F.S.; Fernandes, B.S. Assessing Potential of Nanofiltration, Reverse Osmosis and Membrane Distillation Drinking Water Treatment for Pharmaceutically Active Compounds (PhACs) Removal. J. Water Process Eng. 2020, 33, 101029. [Google Scholar] [CrossRef]
- Miyoshi, T.; Nguyen, T.P.; Tsumuraya, T.; Tanaka, H.; Morita, T.; Itokawa, H.; Hashimoto, T. Energy Reduction of a Submerged Membrane Bioreactor Using a Polytetrafluoroethylene (PTFE) Hollow-Fiber Membrane. Front. Environ. Sci. Eng. 2018, 12, 1. [Google Scholar] [CrossRef]
- Electric Power Research Institute. Water & Sustainability (Volume 4): U.S. Electricity Consumption for Water Supply & Treatment—The Next Half Century; Technical Report; EPRI: Palo Alto, CA, USA, 2002. [Google Scholar]
- Livingston, D. Beyond Conventional MBRs: Oxygen Transfer Technology Revolutionizing MBR Applications. In Proceedings of the Membrane Technology Conference & Exposition, San Diego, CA, USA, 12–15 July 2010. [Google Scholar]
- Bailey, J.R.; Ahmad, S.; Batista, J.R. The Impact of Advanced Treatment Technologies on the Energy Use in Satellite Water Reuse Plants. Water 2020, 12, 366. [Google Scholar] [CrossRef] [Green Version]
- Koschikowski, J.; Wieghaus, M.; Rommel, M.; Ortin, V.S.; Suarez, B.P.; Betancort Rodríguez, J.R. Experimental Investigations on Solar Driven Stand-Alone Membrane Distillation Systems for Remote Areas. Desalination 2009, 248, 125–131. [Google Scholar] [CrossRef]
- Banat, F.; Jwaied, N.; Rommel, M.; Koschikowski, J.; Wieghaus, M. Desalination by a “Compact SMADES” Autonomous Solarpowered Membrane Distillation Unit. Desalination 2007, 217, 29–37. [Google Scholar] [CrossRef]
- Zaragoza, G.; Ruiz-Aguirre, A.; Guillén-Burrieza, E. Efficiency in the Use of Solar Thermal Energy of Small Membrane Desalination Systems for Decentralized Water Production. Appl. Energy 2014, 130, 491–499. [Google Scholar] [CrossRef]
- Tow, E.W.; Hartman, A.L.; Jaworowski, A.; Zucker, I.; Kum, S.; AzadiAghdam, M.; Blatchley, E.R.; Achilli, A.; Gu, H.; Urper, G.M.; et al. Modeling the Energy Consumption of Potable Water Reuse Schemes. Water Res. X 2021, 13, 100126. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, K.; Yang, D.R.; Hong, S. A Comprehensive Review of Energy Consumption of Seawater Reverse Osmosis Desalination Plants. Appl. Energy 2019, 254, 113652. [Google Scholar] [CrossRef]
- Electric Power Research Institute. Electricity Use and Management in the Municipal Water Supply and Wastewater Industries; EPRI: Palo Alto, CA, USA, 2013. [Google Scholar]
- Hao, X.; Liu, R.; Huang, X. Evaluation of the Potential for Operating Carbon Neutral WWTPs in China. Water Res. 2015, 87, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Awe, O.W.; Liu, R.; Zhao, Y. Analysis of Energy Consumption and Saving in Wastewater Treatment Plant: Case Study from Ireland. J. Water Sustain. 2016, 6, 63–76. [Google Scholar] [CrossRef]
- Jonasson, M. Energy Benchmark for Wastewater Treatment Processes—A Comparison between Sweden and Austria; Lund University: Lund, Sweden, 2007. [Google Scholar]
- Longo, S.; d’Antoni, B.M.; Bongards, M.; Chaparro, A.; Cronrath, A.; Fatone, F.; Lema, J.M.; Mauricio-Iglesias, M.; Soares, A.; Hospido, A. Monitoring and Diagnosis of Energy Consumption in Wastewater Treatment Plants. A State of the Art and Proposals for Improvement. Appl. Energy 2016, 179, 1251–1268. [Google Scholar] [CrossRef] [Green Version]
- Miyoshi, T.; Nguyen, T.P.; Tsumuraya, T.; Kimura, K.; Watanabe, Y. Energy Consumption in a Baffled Membrane Bioreactor (B-MBR): Estimation Based on Long-Term Continuous Operation. Water Sci. Technol. 2019, 80, 1011–1021. [Google Scholar] [CrossRef]
- The New York State Energy Research and Development Authority. Municipal Waste Water Treatment Plant Energy Evaluation for Town of Tonawanda WWTP—Agreement No. 7185; NYSERDA: Albany, NY, USA, 2005. [Google Scholar]
- Kato, H.; Fujimoto, H.; Yamashina, K. Operational Improvement of Main Pumps for Energy-Saving in Wastewater Treatment Plants. Water 2019, 11, 2438. [Google Scholar] [CrossRef] [Green Version]
- Ravishankara, A.R.; Daniel, J.S.; Portmann, R.W. Nitrous Oxide (N2O): The Dominant Ozone-Depleting Substance Emitted in the 21st Century. Science 2009, 326, 123–125. [Google Scholar] [CrossRef] [Green Version]
- Chu, T.; Abbassi, B.E.; Zytner, R.G. Life-Cycle Assessment of Full-Scale Membrane Bioreactor and Tertiary Treatment Technologies in the Fruit Processing Industry. Water Environ. Res. 2022, 94, e1661. [Google Scholar] [CrossRef]
- Ioannou-Ttofa, L.; Foteinis, S.; Chatzisymeon, E.; Fatta-Kassinos, D. The Environmental Footprint of a Membrane Bioreactor Treatment Process through Life Cycle Analysis. Sci. Total Environ. 2016, 568, 306–318. [Google Scholar] [CrossRef] [Green Version]
- Liao, X.; Tian, Y.; Gan, Y.; Ji, J. Quantifying Urban Wastewater Treatment Sector’s Greenhouse Gas Emissions Using a Hybrid Life Cycle Analysis Method—An Application on Shenzhen City in China. Sci. Total Environ. 2020, 745, 141176. [Google Scholar] [CrossRef] [PubMed]
- Environmental Protection Agency. Waste Heat to Power Systems; Fact Sheet; US Environmental Protection Agency: Washington, DC, USA, 2012. [Google Scholar]
- Volpin, F.; Jiang, J.; El Saliby, I.; Preire, M.; Lim, S.; Hasan Johir, M.A.; Cho, J.; Han, D.S.; Phuntsho, S.; Shon, H.K. Sanitation and Dewatering of Human Urine via Membrane Bioreactor and Membrane Distillation and Its Reuse for Fertigation. J. Clean. Prod. 2020, 270, 122390. [Google Scholar] [CrossRef]
- Nguyen, N.C.; Nguyen, H.T.; Chen, S.S.; Ngo, H.H.; Guo, W.; Chan, W.H.; Ray, S.S.; Li, C.W.; Hsu, H. Te A Novel Osmosis Membrane Bioreactor-Membrane Distillation Hybrid System for Wastewater Treatment and Reuse. Bioresour. Technol. 2016, 209, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Huang, M.; Deng, Q.; Cai, T. Combination and Performance of Forward Osmosis and Membrane Distillation (FO-MD) for Treatment of High Salinity Landfill Leachate. Desalination 2017, 420, 99–105. [Google Scholar] [CrossRef]
- Han, L.; Tan, Y.Z.; Netke, T.; Fane, A.G.; Chew, J.W. Understanding Oily Wastewater Treatment via Membrane Distillation. J. Membr. Sci. 2017, 539, 284–294. [Google Scholar] [CrossRef]
- Lu, D.; Liu, Q.; Zhao, Y.; Liu, H.; Ma, J. Treatment and Energy Utilization of Oily Water via Integrated Ultrafiltration-Forward Osmosis–Membrane Distillation (UF-FO-MD) System. J. Membr. Sci. 2018, 548, 275–287. [Google Scholar] [CrossRef]
- Mikielewicz, D.; Wajs, J.; Ziółkowski, P.; Mikielewicz, J. Utilisation of Waste Heat from the Power Plant by Use of the ORC Aided with Bleed Steam and Extra Source of Heat. Energy 2016, 97, 11–19. [Google Scholar] [CrossRef]
- Tai, C.; Tian, G.; Lei, W. A Water-Heat Combined Supply System Based on Waste Heat from a Coastal Nuclear Power Plant in Northern China. Appl. Therm. Eng. 2022, 200, 117684. [Google Scholar] [CrossRef]
- Su, Z.; Yang, L. A Novel and Efficient Cogeneration System of Waste Heat Recovery Integrated Carbon Capture and Dehumidification for Coal-Fired Power Plants. Energy Convers. Manag. 2022, 255, 115358. [Google Scholar] [CrossRef]
- Fathi, N.; McDaniel, P.; Aleyasin, S.S.; Robinson, M.; Vorobieff, P.; Rodriguez, S.; de Oliveira, C. Efficiency Enhancement of Solar Chimney Power Plant by Use of Waste Heat from Nuclear Power Plant. J. Clean. Prod. 2018, 180, 407–416. [Google Scholar] [CrossRef]
- Obara, S.; Tanaka, R. Waste Heat Recovery System for Nuclear Power Plants Using the Gas Hydrate Heat Cycle. Appl. Energy 2021, 292, 116667. [Google Scholar] [CrossRef]
- Wealer, B.; Bauer, S.; Landry, N.; Seiß, H.; von Hirschhausen, C. Navigating the Roadmap for Clean, Secure and Efficient Energy Innovation: Nuclear Power Reactors Worldwide-Technology Developments, Diffusion Patterns, and Country-by-Country Analysis of Implementation (1951–2017); DIW Berlin: Berlin, Germany, 2019. [Google Scholar]
- Yu, M.G.; Nam, Y. Feasibility Assessment of Using Power Plant Waste Heat in Large Scale Horticulture Facility Energy Supply Systems. Energies 2016, 9, 112. [Google Scholar] [CrossRef] [Green Version]
- Jacob, P.; Phungsai, P.; Fukushi, K.; Visvanathan, C. Direct Contact Membrane Distillation for Anaerobic Effluent Treatment. J. Membr. Sci. 2015, 475, 330–339. [Google Scholar] [CrossRef]
- Czepiel, P.M.; Crill, P.M.; Harriss, R.C. Methane Emissions from Municipal Wastewater Treatment Processes. Environ. Sci. Technol. 1993, 27, 2472–2477. [Google Scholar] [CrossRef]
- Czepiel, P.; Crill, P.; Harriss, R. Nitrous Oxide Emissions from Municipal Wastewater Treatment. Environ. Sci. Technol. 1995, 29, 2352–2356. [Google Scholar] [CrossRef]
- Sümer, E.; Weiske, A.; Benckiser, G.; Ottow, J.C.G. Influence of Environmental Conditions on the Amount of N2O Released from Activated Sludge in a Domestic Waste Water Treatment Plant. Experientia 1995, 51, 419–422. [Google Scholar] [CrossRef]
- Benckiser, G.; Eilts, R.; Linn, A.; Lorch, H.J.; Sümer, E.; Weiske, A.; Wenzhöfer, F. N2O Emissions from Different Cropping Systems and from Aerated, Nitrifying and Denitrifying Tanks of a Municipal Waste Water Treatment Plant. Biol. Fertil. Soils 1996, 23, 257–265. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, J.; Xie, H.; Qi, P.; Ren, Y.; Hu, Z. Methane Emissions from a Full-Scale A/A/O Wastewater Treatment Plant. Bioresour. Technol. 2011, 102, 5479–5485. [Google Scholar] [CrossRef]
- STOWA. Emissies van Broeikasgassen van Rwzi’s; STOWA: Engelsbrand, Germany, 2010. [Google Scholar]
- VROM Protocol. 8136 Afvalwater, t.b.v NIR 2008 Uitgave Maart 2008 6B: CH4 En N2O Uit Afvalwater; Directie Klimaatverandering en Industrie: The Hague, The Netherlands, 2008. [Google Scholar]
- Daelman, M.R.J.; van Voorthuizen, E.M.; van Dongen, U.G.J.M.; Volcke, E.I.P.; van Loosdrecht, M.C.M. Methane Emission during Municipal Wastewater Treatment. Water Res. 2012, 46, 3657–3670. [Google Scholar] [CrossRef]
- Tumendelger, A.; Alshboul, Z.; Lorke, A. Methane and Nitrous Oxide Emission from Different Treatment Units of Municipal Wastewater Treatment Plants in Southwest Germany. PLoS ONE 2019, 14, e0209763. [Google Scholar] [CrossRef]
- Tibi, F.; Guo, J.; Ahmad, R.; Lim, M.; Kim, M.; Kim, J. Membrane Distillation as Post-Treatment for Anaerobic Fluidized Bed Membrane Bioreactor for Organic and Nitrogen Removal. Chemosphere 2019, 234, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.; Bae, W.; Kim, J. Hybrid Forward Osmosis/Membrane Distillation Integrated with Anaerobic Fluidized Bed Bioreactor for Advanced Wastewater Treatment. J. Hazard. Mater. 2021, 404, 124160. [Google Scholar] [CrossRef] [PubMed]
- Mokhtar, N.M.; Lau, W.J.; Ismail, A.F.; Kartohardjono, S.; Lai, S.O.; Teoh, H.C. The Potential of Direct Contact Membrane Distillation for Industrial Textile Wastewater Treatment Using PVDF-Cloisite 15A Nanocomposite Membrane. Chem. Eng. Res. Des. 2016, 111, 284–293. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Kang, Y.; Zhang, L.; Qu, D.; Cheng, X.; Feng, L. Performance and Fouling Mechanism of Direct Contact Membrane Distillation (DCMD) Treating Fermentation Wastewater with High Organic Concentrations. J. Environ. Sci. 2018, 65, 253–261. [Google Scholar] [CrossRef]
- Davey, C.J.; Liu, P.; Kamranvand, F.; Williams, L.; Jiang, Y.; Parker, A.; Tyrrel, S.; McAdam, E.J. Membrane Distillation for Concentrated Blackwater: Influence of Configuration (Air Gap, Direct Contact, Vacuum) on Selectivity and Water Productivity. Sep. Purif. Technol. 2021, 263, 118390. [Google Scholar] [CrossRef]
- Zoungrana, A.; Zengin, İ.H.; Elcik, H.; Özkaya, B.; Çakmakci, M. The Treatability of Landfill Leachate by Direct Contact Membrane Distillation and Factors Influencing the Efficiency of the Process. Desalin. Water Treat. 2017, 71, 233–243. [Google Scholar] [CrossRef]
- Zoungrana, A.; Zengin, I.; Karadag, D.; Cakmakci, M. Treatability of Municipal Wastewater with Direct Contact Membrane Distillation. Sigma J. Eng. Nat. Sci. 2017, 8, 245–254. [Google Scholar]
- Li, F.; Huang, J.; Zia, Q.; Lou, M.; Yang, B.; Tian, Q.; Liu, Y. Direct Contact Membrane Distillation for the Treatment of Industrial Dyeing Wastewater and Characteristic Pollutants. Sep. Purif. Technol. 2018, 195, 83–91. [Google Scholar] [CrossRef]
| Parameter | Value | Units |
|---|---|---|
| Influent Flow | 2500 | m3/day |
| Total COD | 500 | mg/L |
| TKN | 40 | mg/L |
| Total P | 10 | mg/L |
| Nitrate N | 0 | mg/L |
| pH | 7.04 | - |
| VSS | 198 | mg/L |
| TSS | 218 | mg/L |
| Parameter | Average | Minimum | Maximum | Number of Sources |
|---|---|---|---|---|
| Ammonia N | 72.19 | 34.00 | 99.89 | 9 |
| COD | 89.51 | 46.20 | 99.99 | 11 |
| Nitrate N | 99.81 | 99.81 | 99.81 | 1 |
| Nitrite N | 99.99 | 99.99 | 99.99 | 1 |
| Phosphate P | 95.50 | 91.00 | 99.99 | 2 |
| TN | 88.90 | 63.70 | 99.82 | 4 |
| TOC | 88.49 | 49.10 | 99.50 | 6 |
| Inputs | Units | MBR-MD | Baseline |
|---|---|---|---|
| Energy Inputs | |||
| MBR Pumping | kWh/m3 | 0.049 | - |
| Aerobic/UF tank | kWh/m3 | 0.520 | - |
| Anoxic tank | kWh/m3 | 0.055 | - |
| Anaerobic tank | kWh/m3 | 0.055 | - |
| MD Pumping | kWh/m3 | 0.076 | - |
| UV | kWh/m3 | 0.055 | - |
| MD Heating | kWh/m3 | 174 | - |
| Conventional treatment pumping | kWh/m3 | - | 0.009 |
| Aeration tank | kWh/m3 | - | 0.403 |
| Sludge dewatering | kWh/m3 | 0.027 | 0.030 |
| MF | kWh/m3 | - | 0.274 |
| RO | kWh/m3 | - | 1.08 |
| UV-AOP | kWh/m3 | - | 0.070 |
| Chemical Inputs | |||
| NaClO | mg/L | 11 | 11 |
| FeCl3 | mg/L | - | 12 |
| H2SO4 | mg/L | - | 24 |
| Citric acid | mg/L | - | 0.2 |
| NaOH | mg/L | - | 3.6 |
| Ca(OH)2 | mg/L | - | 15 |
| H2O2 | mg/L | - | 3 |
| Gas Emissions | |||
| CH4 | g/day | 7.91 × 105 | 7.91 × 105 |
| N2O | g/day | 1710 | 1710 |
| Influent & Effluent Flows | |||
| Influent flow | m3/day | 2500 | 2500 |
| Effluent flow | m3/day | 2416 | 1840 |
| Water Emissions | |||
| Effluent | |||
| Ammonia-N | mg/L | 0.017 | 0.010 |
| COD | mg/L | 3.14 | 7.43 |
| Organic Nitrogen | mg/L | 0.215 | 0.118 |
| Phosphate-P | mg/L | 0.011 | 0.09 |
| Total Nitrogen | mg/L | 0.233 | 0.83 |
| Total Organic Carbon | mg/L | 0.872 | 0.06 |
| RO Concentrate | |||
| Ammonia-N | mg/L | - | 1.44 |
| COD | mg/L | - | 273 |
| Organic Nitrogen | mg/L | - | 9.87 |
| Phosphate-P | mg/L | - | 17.6 |
| Total Nitrogen | mg/L | - | 146 |
| Total Organic Carbon | mg/L | - | 88.9 |
| Membranes | |||
| MF/UF | m2 | 213 | 365 |
| MD | m2 | 7.2 | - |
| RO | m2 | - | 341 |
| Waste | |||
| Sludge waste | kg/day | 479 | 608 |
| RO Concentrate | m3/day | - | 324 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glover, C.J.; Phillips, J.A.; Marchand, E.A.; Hiibel, S.R. Modeling and Life Cycle Assessment of a Membrane Bioreactor–Membrane Distillation Wastewater Treatment System for Potable Reuse. Separations 2022, 9, 151. https://doi.org/10.3390/separations9060151
Glover CJ, Phillips JA, Marchand EA, Hiibel SR. Modeling and Life Cycle Assessment of a Membrane Bioreactor–Membrane Distillation Wastewater Treatment System for Potable Reuse. Separations. 2022; 9(6):151. https://doi.org/10.3390/separations9060151
Chicago/Turabian StyleGlover, Callan J., James A. Phillips, Eric A. Marchand, and Sage R. Hiibel. 2022. "Modeling and Life Cycle Assessment of a Membrane Bioreactor–Membrane Distillation Wastewater Treatment System for Potable Reuse" Separations 9, no. 6: 151. https://doi.org/10.3390/separations9060151
APA StyleGlover, C. J., Phillips, J. A., Marchand, E. A., & Hiibel, S. R. (2022). Modeling and Life Cycle Assessment of a Membrane Bioreactor–Membrane Distillation Wastewater Treatment System for Potable Reuse. Separations, 9(6), 151. https://doi.org/10.3390/separations9060151






