Influence and Mechanism of Polar Solvents on the Retention Time of Short-Chain Fatty Acids in Gas Chromatography
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
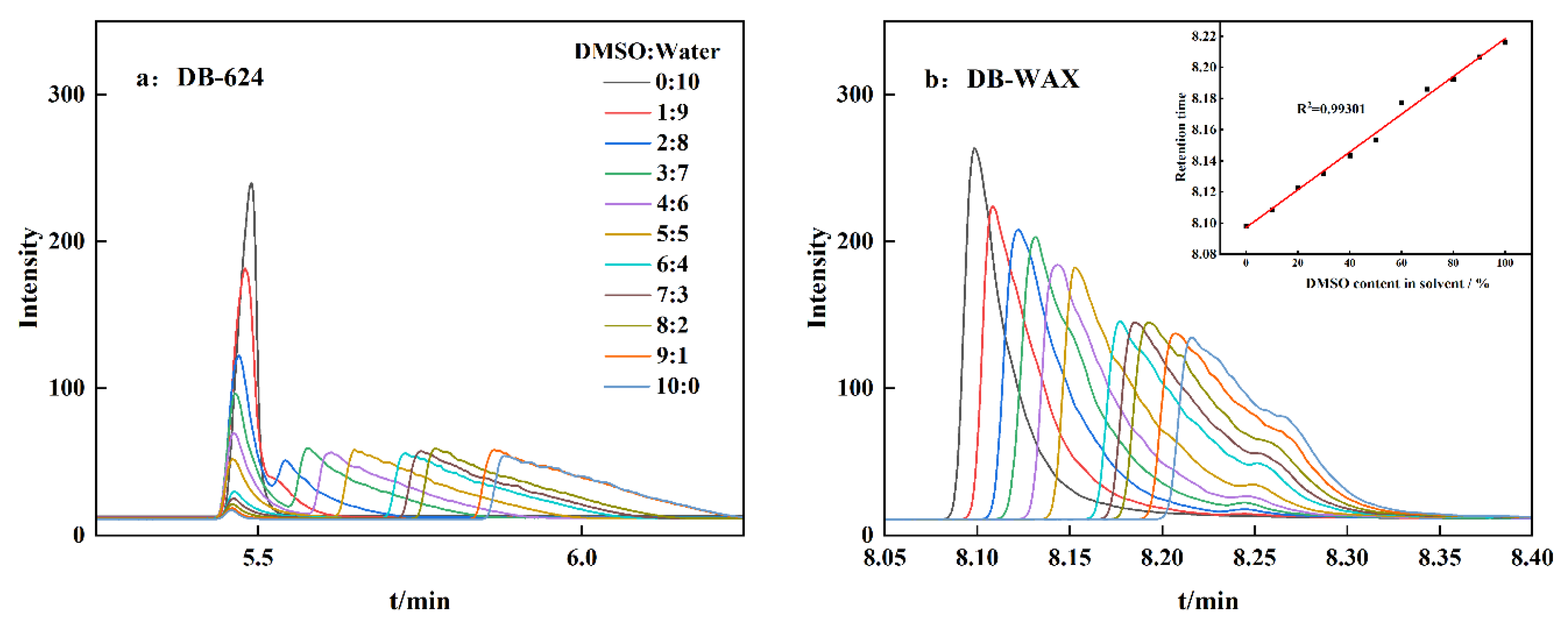
3.1. Acetic Acid Separation Results in Capillary Columns with Different Polarities
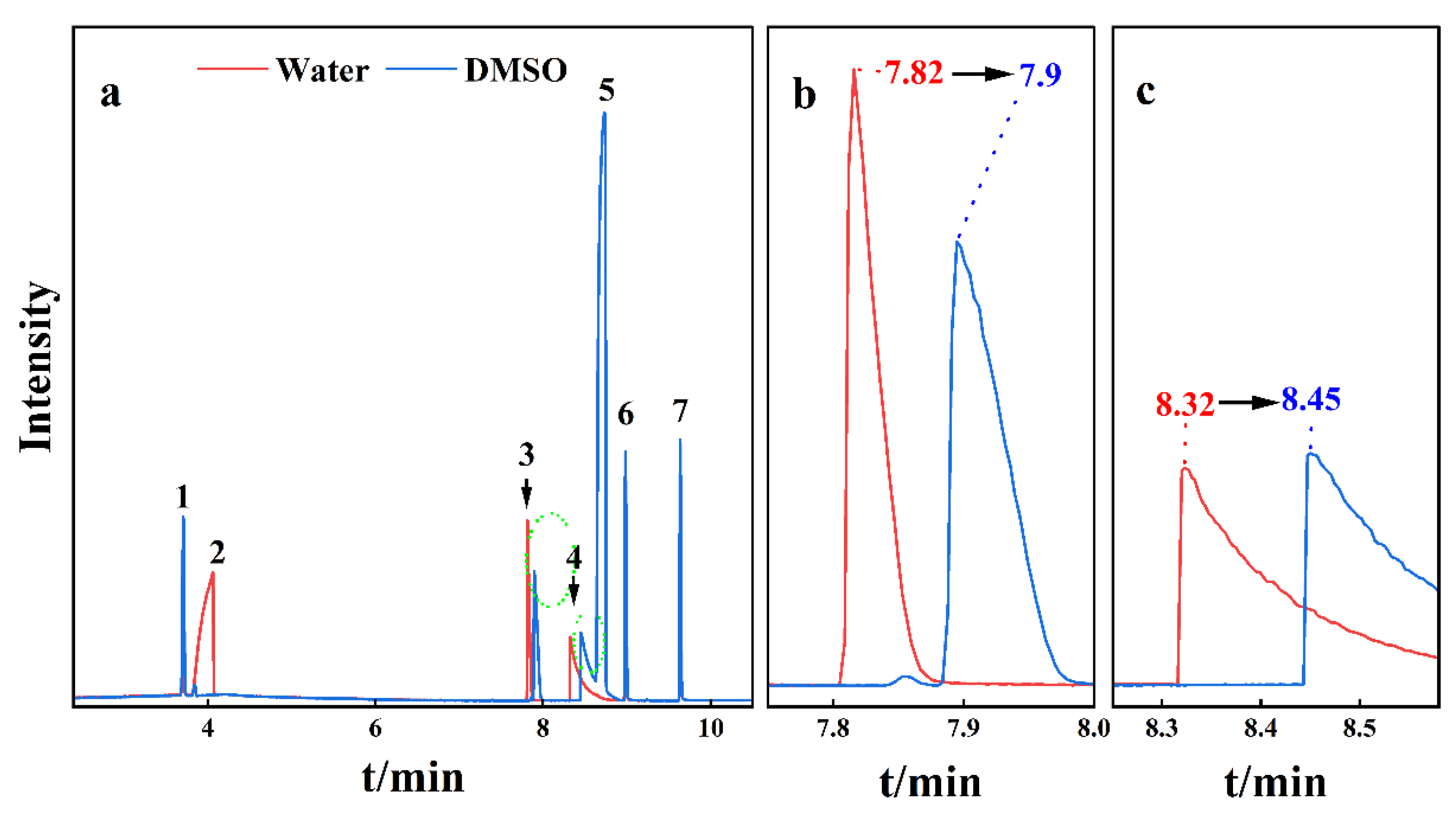
3.2. Gas Chromatographic Separation of SCFAs in Water and DMSO
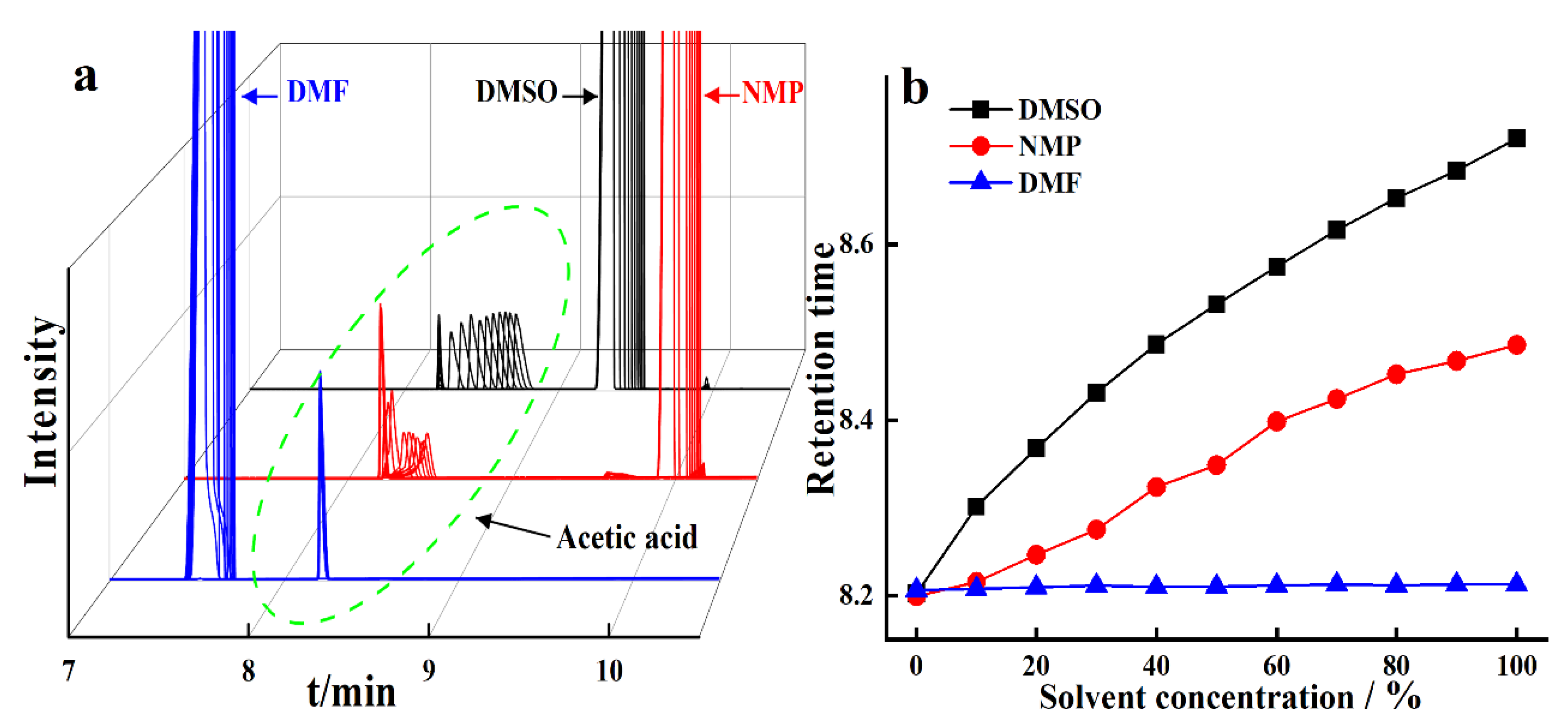
3.3. Chromatographic Separation of Acetic Acid in Three Polar Aprotic Solvents
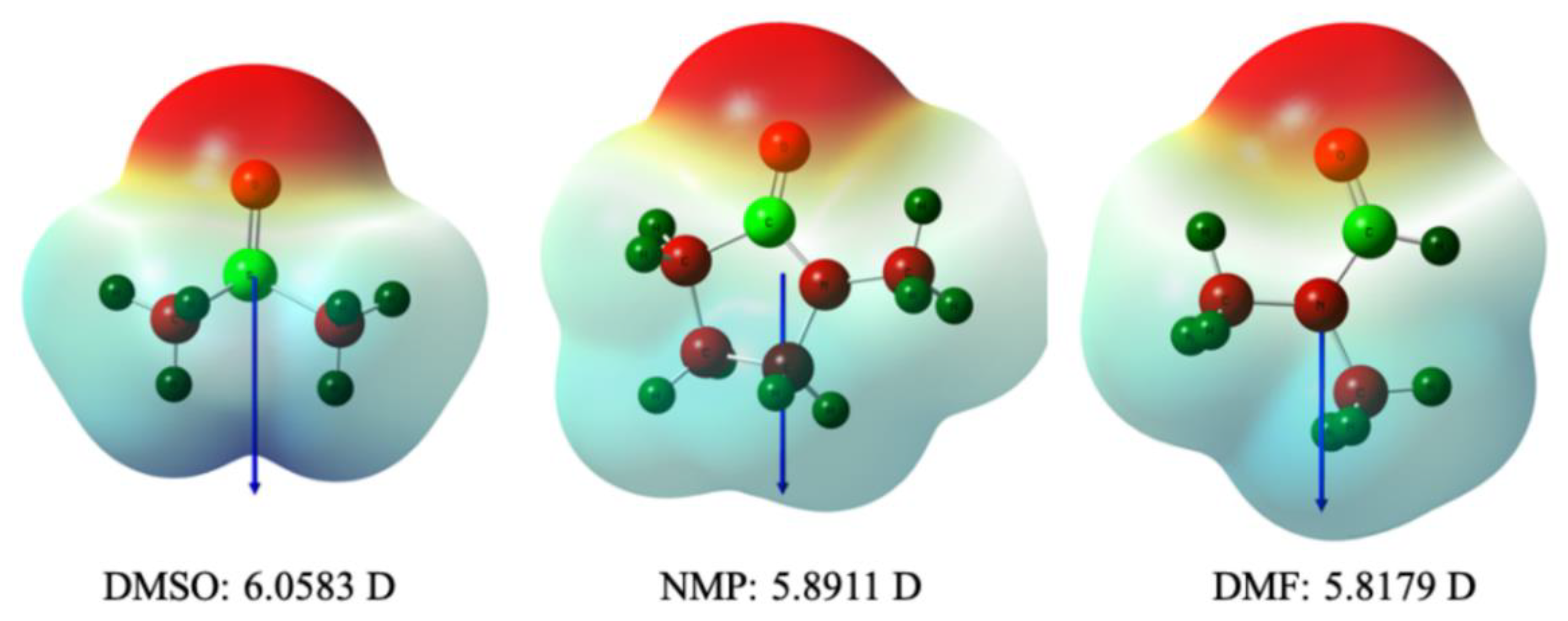
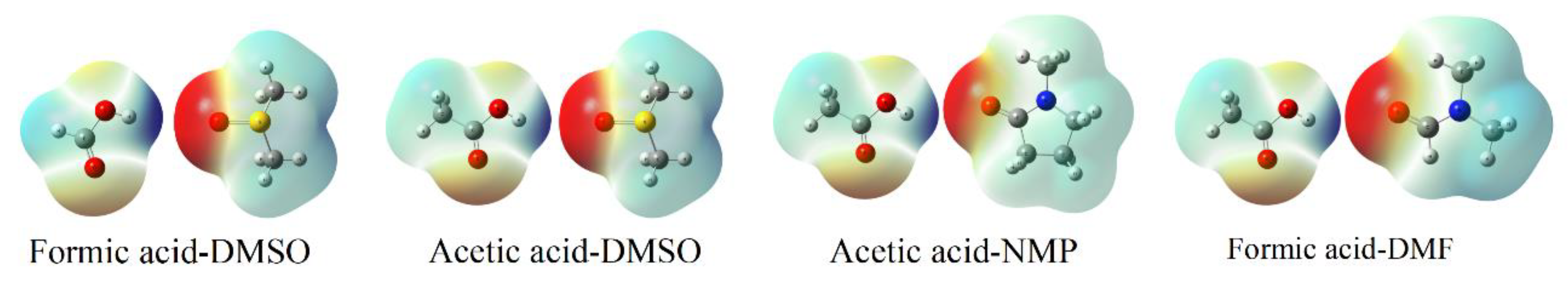
3.4. Hydrogen Bonding between Acetic Acid and Polar Aprotic Solvents
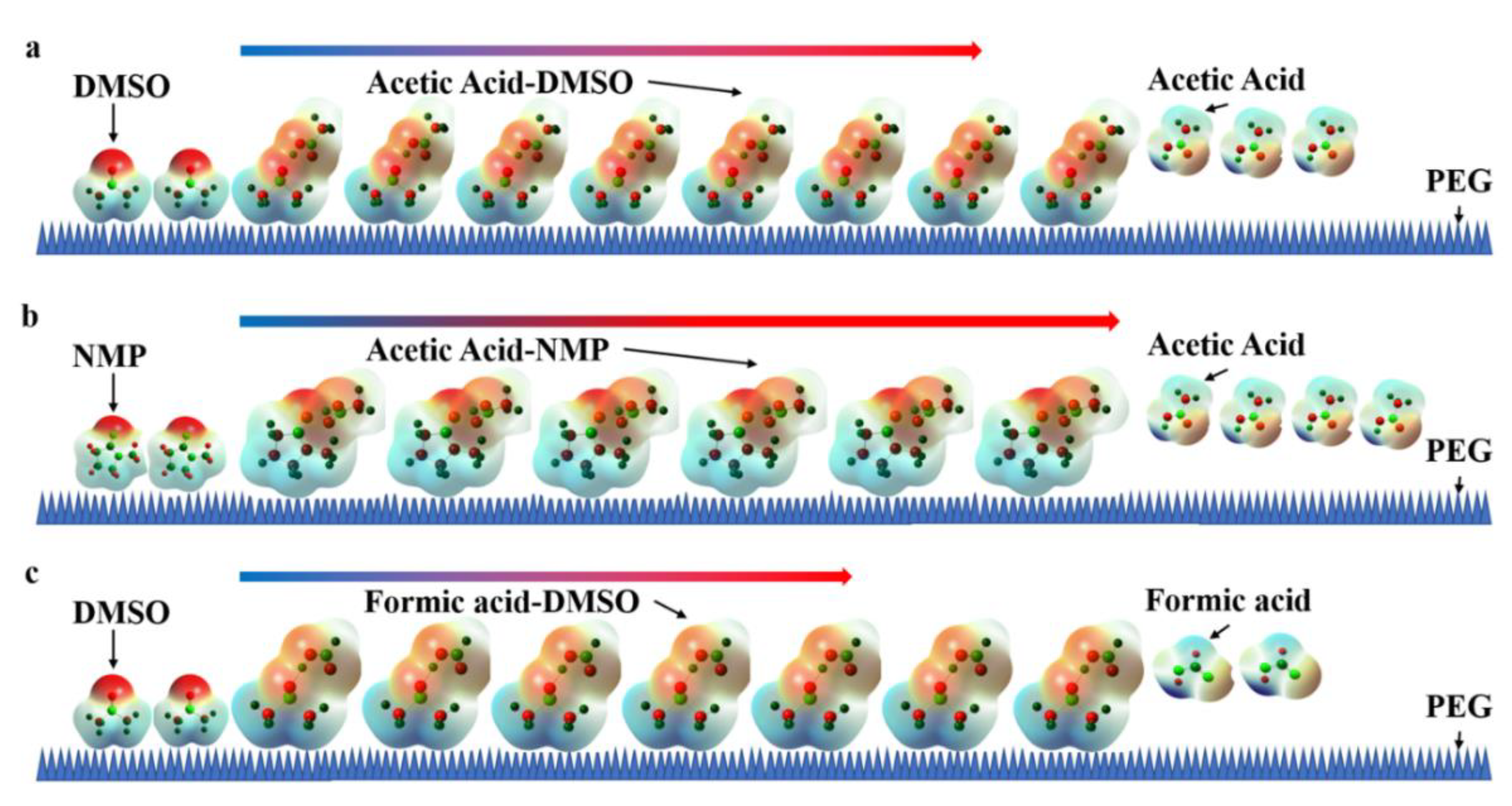
3.5. Mechanism of Change in Retention Time for SCFAs in Polar Solvents
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mihaylova, M.M.; Stratton, M.S. Short chain fatty acids as epigenetic and metabolic regulators of neurocognitive health and disease. In Nutritional Epigenomics; Elsevier: London, UK, 2019; pp. 381–397. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids from Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aho, V.T.E.; Houser, M.C.; Pereira, P.A.B.; Chang, J.; Rudi, K.; Paulin, L.; Hertzberg, V.; Auvinen, P.; Tansey, M.G.; Scheperjans, F. Relationships of gut microbiota, short-chain fatty acids, inflammation, and the gut barrier in Parkinson’s disease. Mol. Neurodegener. 2021, 16, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The microbiota-gut-brain axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef] [PubMed]
- García-Gutiérrez, M.S.; Navarrete, F.; Sala, F.; Gasparyan, A.; Austrich-Olivares, A.; Manzanares, J. Biomarkers in Psychiatry: Concept, Definition, Types and Relevance to the Clinical Reality. Front. Psychiatry 2020, 11, 432. [Google Scholar] [CrossRef] [PubMed]
- Liebisch, G.; Ecker, J.; Roth, S.; Schweizer, S.; Öttl, V.; Schött, H.-F.; Yoon, H.; Haller, D.; Holler, E.; Burkhardt, R.; et al. Quantification of Fecal Short Chain Fatty Acids by Liquid Chromatography Tandem Mass Spectrometry—Investigation of Pre-Analytic Stability. Biomolecules 2019, 9, 121. [Google Scholar] [CrossRef] [Green Version]
- Van Eijk, H.M.; Bloemen, J.G.; Dejong, C.H. Application of liquid chromatography–mass spectrometry to measure short chain fatty acids in blood. J. Chromatogr. B 2009, 877, 719–724. [Google Scholar] [CrossRef]
- Wang, L.-L.; Guo, H.-H.; Huang, S.; Feng, C.-L.; Han, Y.-X.; Jiang, J.-D. Comprehensive evaluation of SCFA production in the intestinal bacteria regulated by berberine using gas-chromatography combined with polymerase chain reaction. J. Chromatogr. B 2017, 1057, 70–80. [Google Scholar] [CrossRef]
- Yongru, L.; Xu, L.; Shengnan, T.; Yunxiao, Z.; Ruijie, L.; Qingzhe, J. Determination of short-chain fatty acids in Rancimat measuring cell water by reversed-phase high-performance liquid chromatography. China Oils Fats 2017, 42, 126–128. [Google Scholar]
- Meimei, G.; Liwei, X.; Hongzhao, Y.; Jiurong, W.; Xianfeng, K.; Min, W. A Determination Method Based on Gas Chromatography for Analysis of Short-chain Fatty Acids in Colonic Contents of Piglet. Prog. Mod. Biomed. 2015, 15, 1010–1014. [Google Scholar]
- Zhao, X.-Y.; Wang, Y.-F.; Yang, F.; Jiang, Z.-Z.; Wang, Y.; Yang, L.; Pan, Z.-H. Rapid Analysis of Seven Short-Chain Fatty Acids in Human Saliva by Gas Chromatography. Chin. J. Anal. Chem. 2016, 44, 1009–1014. [Google Scholar]
- Pharmacopoeia, C. Pharmacopoeia of the People’s Republic of China 2020; China Medical Science and Technology Press: Beijing, China, 2020. [Google Scholar]
- Tan, L.; Ju, H.; Li, J. Extraction and determination of short-chain fatty acids in biological samples. Chin. J. Chromatogr. 2006, 24, 81–87. [Google Scholar]
- Poole, C.F.; Berezkin, V.G.; Borisova, L.V.; Demin, Y.V.; Gatinskaya, N.G.; Ermakov, V.V.; Ryabukhin, V.A.; Bozhkov, O.D. The essence of chromatography. J. Anal. Chem. 2005, 60, 193. [Google Scholar] [CrossRef]
- Dai, Z.Y.; Chen, W.H.; Zhou, H.; Yang, H.Y. Studies on quantitative chromatographic retention-structure relationships. Chin. J. Chromatogr. 2000, 18, 125–127. [Google Scholar]
- Nie, J.; Zhi, D.; Zhou, Y. Magnetic biochar-based composites for removal of recalcitrant pollutants in water. In Sorbents Materials for Controlling Environmental Pollution; Elsevier: San Diego, CA, USA, 2021; pp. 163–187. [Google Scholar] [CrossRef]
- Dereka, B.; Yu, Q.; Lewis, N.H.; Carpenter, W.B.; Bowman, J.M.; Tokmakoff, A. Crossover from hydrogen to chemical bonding. Science 2021, 371, 160–164. [Google Scholar]
- Majid, H.A.; Cole, J.; Emery, P.W.; Whelan, K. Additional oligofructose/inulin does not increase faecal bifidobacteria in critically ill patients receiving enteral nutrition: A randomised controlled trial. Clin. Nutr. 2014, 33, 966–972. [Google Scholar] [CrossRef]
- Ou, J.; Carbonero, F.; Zoetendal, E.G.; DeLany, J.; Wang, M.; Newton, K.; Gaskins, H.R.; O’Keefe, S.J.D. Diet, microbiota, and microbial metabolites in colon cancer risk in rural Africans and African Americans. Am. J. Clin. Nutr. 2013, 98, 111–120. [Google Scholar] [CrossRef] [Green Version]
- Yu-jian, S.; Hong, S.; Jin-di, X.; Dong-zhou, K.; Song-lin, L. Advance in determination of short-chain fatty acids of gut bacterial metabolites in feces. Chin. J. Pharm. Anal. 2019, 39, 967–974. [Google Scholar]
- Zhou, D.; Hou, Q.; Liu, W.; Ren, X. Rapid determination of formic and acetic acids in biomass hydrolysate by headspace gas chromatography. J. Ind. Eng. Chem. 2017, 47, 281–287. [Google Scholar] [CrossRef]
- Fu, Z.; Jia, Q.; Zhang, H.; Kang, L.; Sun, X.; Zhang, M.; Wang, Y.; Hu, P. Simultaneous quantification of eleven short-chain fatty acids by derivatization and solid phase microextraction—Gas chromatography tandem mass spectrometry. J. Chromatogr. A 2021, 1661, 462680. [Google Scholar] [CrossRef]
- Klecker, C.; Nair, L.S. Matrix Chemistry Controlling Stem Cell Behavior. In Biology and Engineering of Stem Cell Niches; Elsevier: London, UK, 2017; pp. 195–213. [Google Scholar] [CrossRef]
- Kurt, M.; Babu, P.C.; Sundaraganesan, N.; Cinar, M.; Karabacak, M. Molecular structure, vibrational, UV and NBO analysis of 4-chloro-7-nitrobenzofurazan by DFT calculations. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 79, 1162–1170. [Google Scholar]
- Singh, D.K.; Jagannathan, R.; Khandelwal, P.; Abraham, P.M.; Poddar, P. In situ synthesis and surface functionalization of gold nanoparticles with curcumin and their antioxidant properties: An experimental and density functional theory investigation. Nanoscale 2012, 5, 1882–1893. [Google Scholar] [CrossRef]
- Orozco, M.; Alhambra, C.; Barril, X.; Luque, F.J. Theoretical Methods for the Representation of Solvent. J. Mol. Model. 1996, 2, 1–15. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hertwig, R.H.; Koch, W. On the parameterization of the local correlation functional. What is Becke-3-LYP? Chem. Phys. Lett. 1997, 268, 345–351. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef] [Green Version]
- Kohn, W.; Sham, L.J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef] [Green Version]

| Stationary Phases | Column Type | Polarity Level |
|---|---|---|
| 100% dimethylpolysiloxane | DB-1 | non-polar |
| 6% cyanopropylphenyl—94% dimethylpolysiloxane | DB-624 | mid-polar |
| 100% PEG (polyethylene glycol) | DB-WAX | high-polar |
| Solvent | MW | Boiling Point (°C) | Density (g/mL) | Solubility in H2O (g/100 g) | Dielectric Constant (20 °C) | Flash Point (°C) |
|---|---|---|---|---|---|---|
| DMSO | 78 | 189 | 1.092 | Miscible | 47 | 95 |
| NMP | 99 | 202 | 1.033 | Miscible | 32 | 91 |
| DMF | 73 | 153 | 0.9445 | Miscible | 38.25 | 58 |
| Project | Formic Acid-DMSO | Acetic Acid-DMSO | Acetic Acid-NMP | Acetic Acid-DMF |
|---|---|---|---|---|
| NPA charge (Hydrogen atom) * | 0.534 | 0.540 | 0.546 | 0.539 |
| NPA charge (Oxygen atom) * | −1.028 | −0.997 | −0.69 | −0.732 |
| Hydrogen bond length (Å) | 1.530 | 1.560 | 1.605 | 1.634 |
| Hydrogen bond energy (kJ/mol) | 45.668 | 39.987 | 31.734 | 28.794 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, M.; Natsagdorj, N.; Shun, N. Influence and Mechanism of Polar Solvents on the Retention Time of Short-Chain Fatty Acids in Gas Chromatography. Separations 2022, 9, 124. https://doi.org/10.3390/separations9050124
Ren M, Natsagdorj N, Shun N. Influence and Mechanism of Polar Solvents on the Retention Time of Short-Chain Fatty Acids in Gas Chromatography. Separations. 2022; 9(5):124. https://doi.org/10.3390/separations9050124
Chicago/Turabian StyleRen, Mu, Narantsogt Natsagdorj, and Na Shun. 2022. "Influence and Mechanism of Polar Solvents on the Retention Time of Short-Chain Fatty Acids in Gas Chromatography" Separations 9, no. 5: 124. https://doi.org/10.3390/separations9050124
APA StyleRen, M., Natsagdorj, N., & Shun, N. (2022). Influence and Mechanism of Polar Solvents on the Retention Time of Short-Chain Fatty Acids in Gas Chromatography. Separations, 9(5), 124. https://doi.org/10.3390/separations9050124





