Multivariate Optimization of Chromatographic Conditions for Rapid Simultaneous Quantification of Antidiarrheal Drugs in Formulation Using Surface Response Methodology
Abstract
1. Introduction
2. Materials and Methods
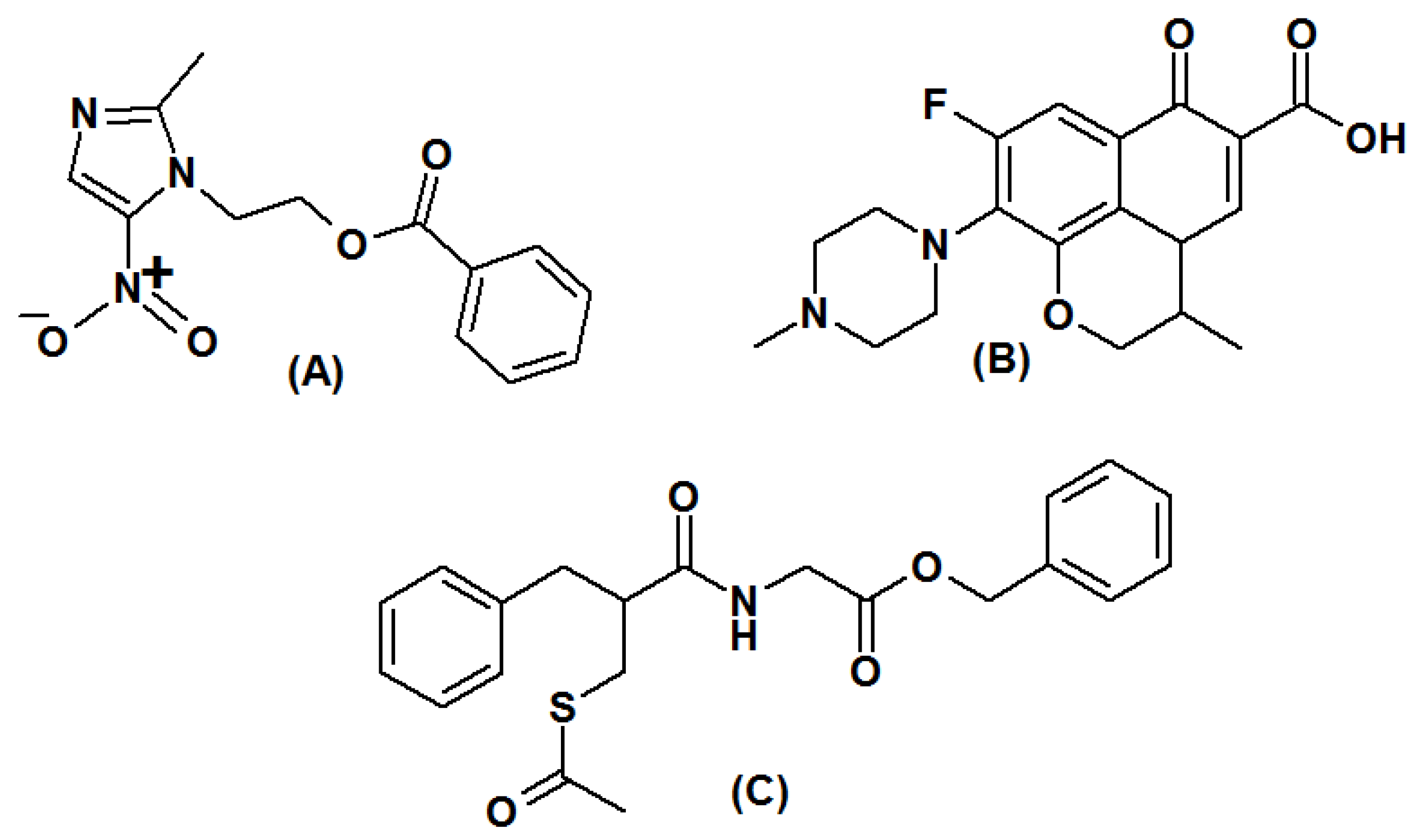
2.1. Materials
2.2. Instrumentation
2.3. Chromatographic Procedure
2.4. Preparation of Primary Standard Solutions
2.5. Preparation of Sample Solution
3. Results and Discussion
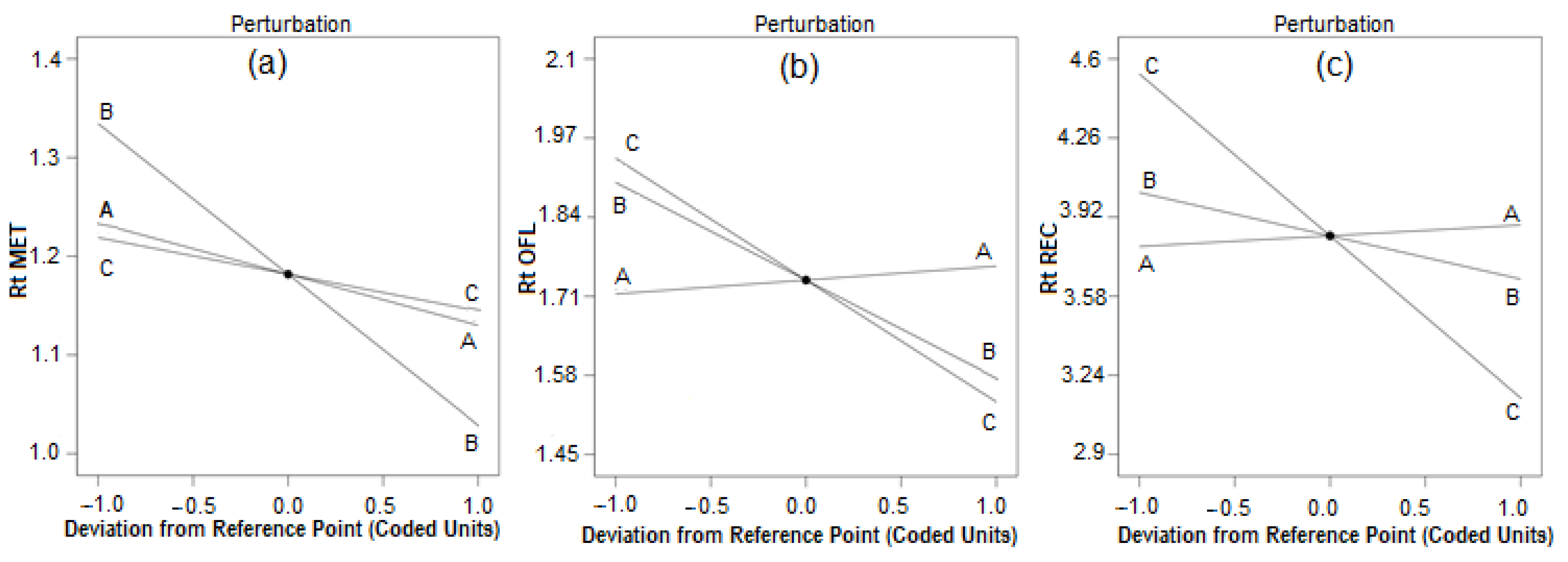
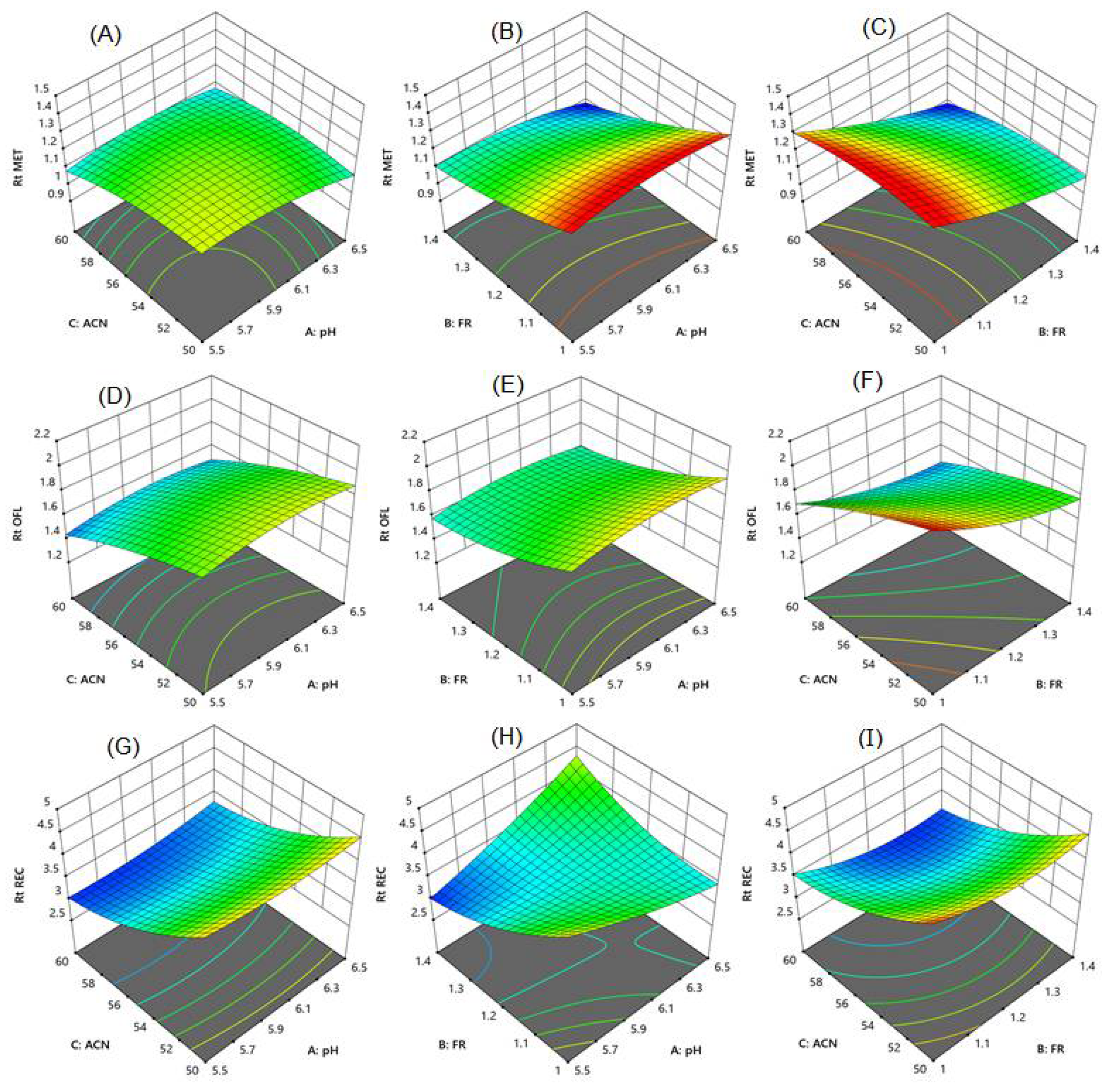
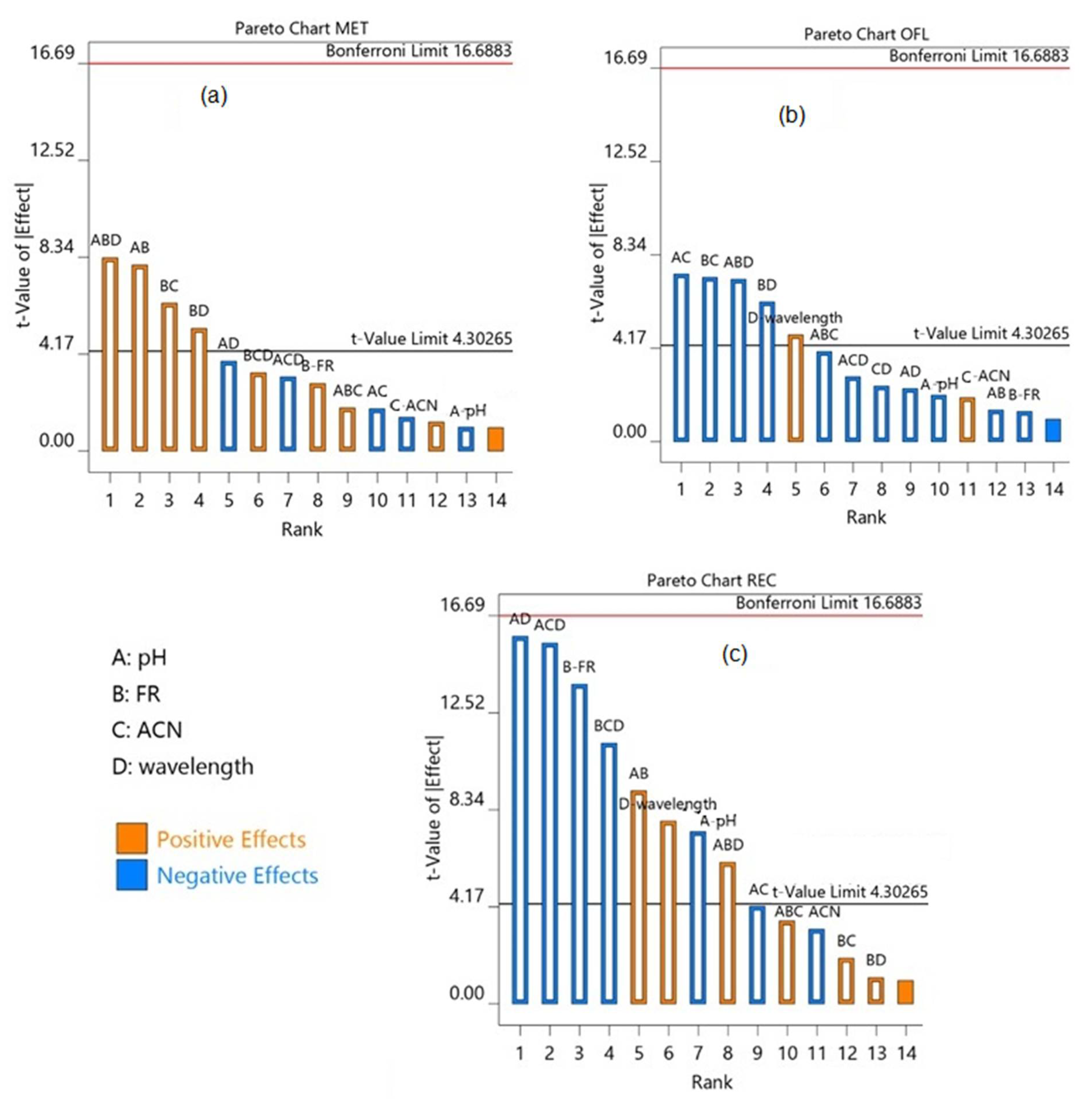
3.1. Optimization of Chromatographic Condition
3.2. Selection of Wavelength
3.3. Method Validation
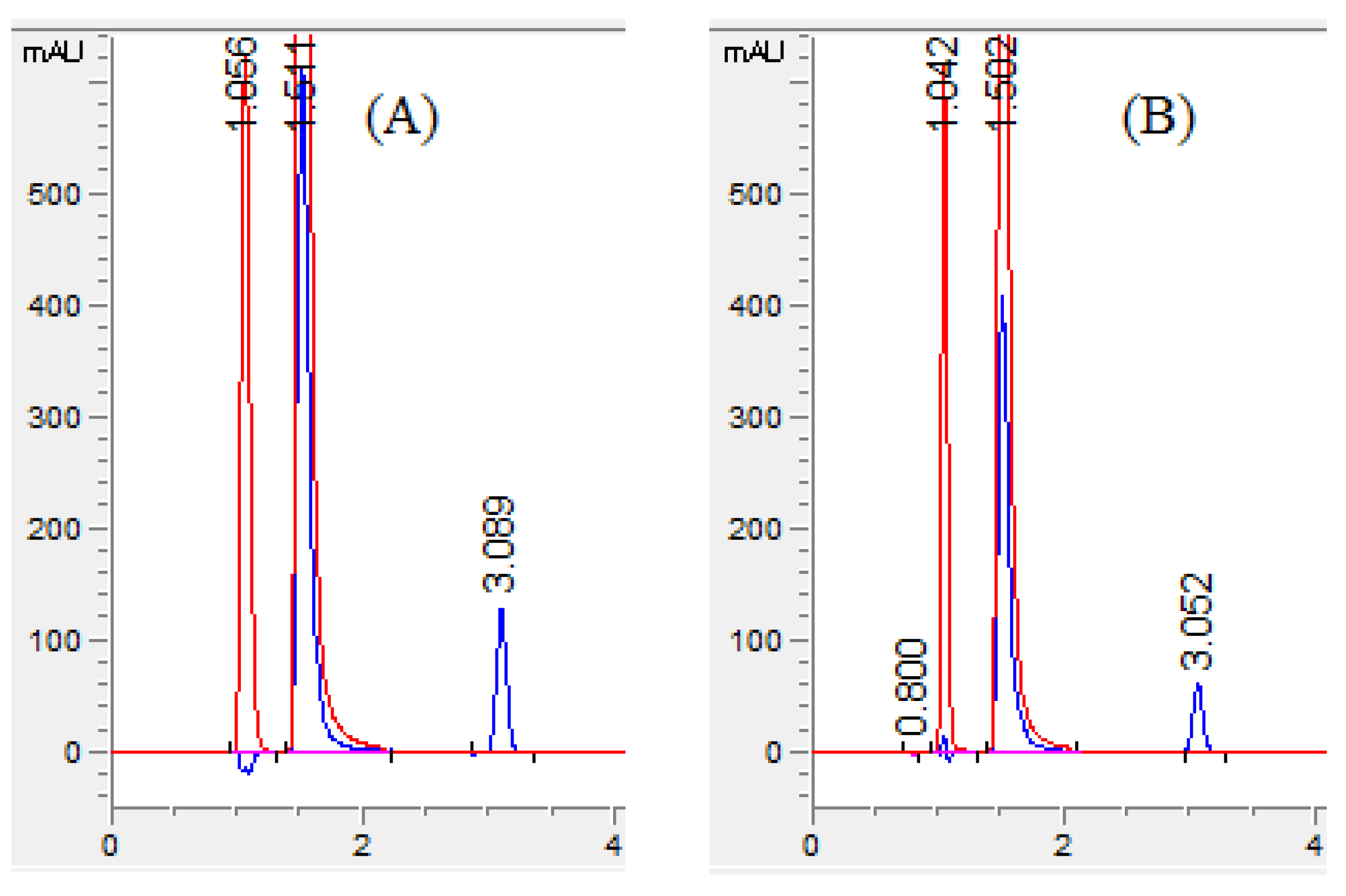
3.3.1. System Suitability Test
3.3.2. Linearity of a Calibration Curve
3.3.3. Sensitivity
3.3.4. Precision and Accuracy
3.3.5. Selectivity
3.3.6. Stability of Solutions
3.3.7. Robustness
3.4. Analysis of Formulation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Gastroenterology Global Guidelines: Acute Diarrhea in Adults and Children: A Global Perspective. Available online: https://www.worldgastroenterology.org/guidelines/acute-diarrhea/acute-diarrhea-english (accessed on 20 April 2022).
- Bhaveshaikh, N.; Sukumaran, S.; Vyas, U. Drug prescribing pattern in acute gastroenteritis in an in–patient setting in a private hospital. Int. J. Res. Med. Sci. 2017, 5, 1256–1259. [Google Scholar] [CrossRef][Green Version]
- Joshi, A.S.; Shah, R.K.; Suthar, M.H.; Keharia, U.H. Evaluation of rationality of antimicrobial and antidiarrheal fixed dose combination available in Indian market. Natl. J. Physiol. Pharm. Pharmacol. 2021, 11, 222–226. [Google Scholar] [CrossRef]
- Fischbach, W.; Andresen, V.; Eberlin, M.; Mueck, T.; Layer, P. A Comprehensive Comparison of the Efficacy and Tolerability of Racecadotril with Other Treatments of Acute Diarrhea in Adults. Front. Med. 2016, 4, 44. [Google Scholar] [CrossRef]
- Blondeau, J.M. Fluoroquinolones: Mechanism of action, classification, and development of resistance. Surv. Ophthalmol. 2004, 49, S73–S78. [Google Scholar] [CrossRef] [PubMed]
- Hooper, D.C. Mechanisms of action of antimicrobials: Focus on fluoroquinolones. Clin. Infect. Dis. 2001, 32, S9–S15. [Google Scholar] [CrossRef]
- Dingsdag, S.A.; Hunter, N. Metronidazole: An update on metabolism, structure–cytotoxicity and resistance mechanisms. J. Antimicrob. Chemother. 2018, 73, 265–279. [Google Scholar] [CrossRef]
- Eberlin, M.; Chen, M.; Mueck, T.; Dabritz, J. Racecadotril in the treatment of acute diarrhea in children: A systematic, comprehensive review and meta–analysis of randomized controlled trials. BMC Pediatr. 2018, 18, 124. [Google Scholar] [CrossRef]
- Eberlin, M.; Mück, T.; Michel, M.C. A comprehensive review of the pharmacodynamics, pharmacokinetics, and clinical effects of the neutral endopeptidase inhibitor racecadotril. Front. Pharmacol. 2012, 3, 93. [Google Scholar] [CrossRef]
- Gandhi, S.V.; Patil, D.; Baravkar, A.A. Comparison of Chemometric assisted UV Spectrophotometric and RP–HPLC Method for the simultaneous determination of Ofloxacin and Tinidazole in their Combined dosage form. Res. J. Pharm. Tech. 2021, 14, 5713–5718. [Google Scholar] [CrossRef]
- Bodiwala, K.; Shah, S.; Patel, Y.; Prajapati, P.; Marolia, B.; Kalyankar, G. Simultaneous Estimation of Ofloxacin, Clotrimazole, and Lignocaine Hydrochloride in Their Combined Ear–Drop Formulation by Two Spectrophotometric Methods. J. AOAC Int. 2017, 100, 38–44. [Google Scholar] [CrossRef]
- Pulgarín, J.A.; Molina, A.A.; Boras, N. Development of a spectrofluorimetric method for the determination of ofloxacin in urine. Appl. Spectrosc. 2013, 67, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Attimarad, M.V.; Sreeharsha, N.; Setty, R.S. Simultaneous Determination Of Ofloxacin Additionally, Flavoxate Hydrochloride In Human Plasma By RP HPLC. J. Liq. Chromatogr. Relat. Technol. 2012, 35, 768–777. [Google Scholar] [CrossRef]
- D’Souza, K.; Syeda, A.; Khatal, P.; Muddukrishna, B.S.; Vasantharaju, S.G. Stability Indicating Assay Method Development and Validation for Simultaneous Estimation of Ofloxacin and Ornidazole by RP–HPLC in Bulk: An Application to Tablet Formulation and Dissolution Studies. Indian J. Pharm. Edu. Res. 2021, 55, 607–613. [Google Scholar] [CrossRef]
- Patel, D.M.; Soneji, J.A.; Patel, P.B.; Patel, C.N. Development and validation of a method for simultaneous estimation of ofloxacin and ornidazole in different dissolution media. Pharm. Methods. 2012, 3, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Hussein, L.A.; Hussien, E.M.; Magdy, N.; Mohamed, H.S. Simultaneous estimation of Ofloxacin and Cefixime in tablet form in presence of the inactive Ofloxacin USP Related compound A. Bull. Fac. Pharm. Cairo Univ. 2017, 55, 171–176. [Google Scholar] [CrossRef]
- Ko´ska, I.; Purgat, K.; Głowacki, R.; Kubalczyk, P. Simultaneous Determination of Ciprofloxacin and Ofloxacin in Animal Tissues with the Use of Capillary Electrophoresis with Transient Pseudo–Isotachophoresis. Molecules 2021, 26, 6931. [Google Scholar] [CrossRef]
- Alnajjar, A.O. Simultaneous Determination Of Ofloxacin Additionally, Cefixime In Tablet Formulation Using Capillary Electrophoresis. J. Liq. Chromatogr. Rel. Technol. 2013, 36, 2687–2697. [Google Scholar] [CrossRef]
- Attimarad, M.V.; Alnajjar, A.O. A conventional HPLCMS method for the simultaneous determination of ofloxacin and cefixime in plasma: Development and validation. J. Basic Clin. Pharm. 2013, 4, 36–41. [Google Scholar] [CrossRef]
- Patel, N.B.; Aravdiya, A.C.; Desai, H.T. Development Additionally, Validation Of Two Spectrophotometric Method For Simultaneous Estimation Of Metronidazole Additionally, Ofloxacin In Suspension. Int. J. Pharm. Sci. Res. 2011, 2, 3202–3206. [Google Scholar] [CrossRef]
- Maslarska, V.; Tsvetkova, B.; Peikova, L.; Bozhanov, S. RP-HPLC Method For Simultaneous Determination Of Metronidazole Additionally, Ofloxacin In Synthetic Mixture. CBU Int. Conf. Proc. ISE Res. Inst. 2016, 4, 900–905. [Google Scholar] [CrossRef]
- Challa, R.; Ramachandra, B.; Naidu, N.V.S. Development and validation of stability indicating assay method for simultaneous determination of Metronidazole and Ofloxacin in pharmaceutical dosage form by using RP-HPLC. Ana. Chem. An. Indian J. 2016, 16, 519–531. [Google Scholar]
- Sakira, A.K.; Mees, C.; Braekeleer, K.D.; Delporte, C.; Yameogo, J.; Yabre, M.; Some, T.I.; Antwerpen, P.V.; Mertens, D.; Kauffmann, J.M. Determination of the quality of metronidazole formulations by near–infrared spectrophotometric analysis. Talanta Open 2021, 3, 100027. [Google Scholar] [CrossRef]
- Zemanová, N.; Anzenbacher, P.; Hudcovic, T.; Anzenbacherová, E. Rapid Determination of Metronidazole and 2–Hydroxymetronidazole in Murine Blood Plasma. J. Chromatogr. Sci. 2022, 60, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Kamel, M. Application of High–Performance Liquid Chromatographic Method For Simultaneous Determination of Racecadotril and Ofloxacin in Their Pharmaceutical Dosage Form. Al–Azhar J. Pharm. Sci. 2019, 60, 95–110. [Google Scholar] [CrossRef][Green Version]
- Makadiya, A.S.; Prajapati, L.M.; Joshi, A.K.; Jethara, S.H.I.; Kharodiya, M.A.L. Development and Validation of Simultaneous Estimation of Racecadotril and Ofloxacin in Bulk and Combined Tablet Dosage Form. Inventi. Rapid Pharm. Ana. Qua. Assu. 2015, 2, 1–5. [Google Scholar]
- Gupta, K.; Sharma, D.; Chawla, P. Simultaneous Estimation of Racecadotril and Ofloxacin by Reverse Phase High Performance Liquid Chromatography Method in Pharmaceutical Dosage Forms. J. Drug Deliv. Ther. 2019, 9, 165–170. [Google Scholar] [CrossRef]
- Singh, N.P.; Damahe, D.P.; Narkhede, S.B. Analytical method development & validation for simultaneous estimation of ofloxacin, ornidazole & racecadotril in pharmaceutical dosage form by HPTLC. Pharm. Innov. J. 2019, 8, 228–234. [Google Scholar]
- Salama, F.M.; El–Abasawi, N.M.; El–Olemy, A.; Hasan, M.A.; Kamel, M. Application of High–Performance Thin–layer Chromatographic Method for Simultaneous Determination of Co–formulated Ofloxacin and Racecadotril in their Oral Dosage Form. J. Adv. Pharm. Res. 2020, 4, 25–32. [Google Scholar] [CrossRef]
- Ukirde, R.D.; Sawant, R.L.; Barkade, G.D. Development and validation of simple uv spectrophotometric method for the determination of racecodotril both in bulk and marketed dosage formulations. Int. J. Pharm. Qual. Assur. 2020, 11, 334–337. [Google Scholar] [CrossRef]
- Ali, N.W.; Elghobashy, M.R.; Mahmoud, M.G.; Mohammed, M.A. Spectrophotometric and spectrofluorimetric methods for determination of Racecadotril. Pak. J. Pharm. Sci. 2011, 24, 19–23. [Google Scholar]
- Mohamed, A.O.; Fouad, M.M.; Hasan, M.M.; Abdel Razeq, S.A.; Elsherif, Z.A. Stability–indicating methods for the determination of racecadotril in the presence of its degradation products. Biosci. Trends 2009, 3, 247–252. [Google Scholar] [PubMed]
- Yu, X.; Jinchang, H.; Fei, L.; Shu, G.; Guo, Q. Quantitative analysis of racecadotril metabolite in human plasma using a liquid chromatography/tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007, 852, 101–107. [Google Scholar] [CrossRef]
- Lopes, W.A.; da Rocha, G.O.; Pereira, P.A.; Oliveira, F.S.; Carvalho, L.S.; Bahia, N.; Conceição, L.S.; de Andrade, J.B. Multivariate optimization of a GC–MS method for determination of sixteen priority polycyclic aromatic hydrocarbons in environmental samples. J. Sep. Sci. 2008, 31, 1787–1796. [Google Scholar] [CrossRef] [PubMed]
- Gurrala, S.; Raj, S.; Subrahmanyam, C.V.S.; Anumolu, P.D. Multivariate optimization of liquid chromatographic conditions for determination of dapagliflozin and saxagliptin, application to an in vitro dissolution and stability studies. Future J. Pharm. Sci. 2021, 7, 85. [Google Scholar] [CrossRef]
- Hemdan, A.; Al–Tannak, N.F.; Mohamed, E.H. Development of a multivariate model with desirability–based optimization for determination of atenolol and hydrochlorothiazide by eco–friendly HPLC method with fluorescence detection. J. Sep. Sci. 2022, 45, 824–831. [Google Scholar] [CrossRef]
- Orlandini, S.; Gotti, R.; Furlanetto, S. Multivariate optimization of capillary electrophoresis methods: A critical review. J. Pharm. Biomed. Anal. 2014, 87, 290–307. [Google Scholar] [CrossRef]
- Attimarad, M. Multivariate optimization of a capillary zone electrophoresis assay method for simultaneous quantification of metformin and vildagliptin from a formulation. J. Liq. Chromatogr. Relat. Technol. 2016, 39, 401–407. [Google Scholar] [CrossRef]
- ICH. Q2B, Validation of Analytical Procedures: Methodology. In Proceedings of the International Conference on Harmonization, Geneva, Switzerland, 15–16 November 1996; pp. 1–17. [Google Scholar]
| Parameters | MET | OFL | RAC |
|---|---|---|---|
| System suitability results | |||
| Retention time ± SD | 1.05 ± 0.011 | 1.51 ± 0.013 | 3.05 ± 0.035 |
| Peak area ± SD | 1365.4 ± 9.64 a | 4117.8 ± 21.56 b | 178.37 ± 2.18 c |
| Resolution ± SD | -- | 2.12± 0.05 d | 5.33 ± 0.08 e |
| Tailing factor ± SD | 1.08 ± 0.012 | 0.96 ± 0.016 | 1.04 ± 0.011 |
| Theoretical plate ± SD | 2714.41 ± 12.45 | 2532.77 ± 10.78 | 11,995.42 ± 124.38 |
| Linearity | |||
| Linearity range (µg/mL) | 20–250 | 10–150 | 5–80 |
| Slope | 12.111 | 72.272 | 8.991 |
| Intercept | 15.442 | 454.494 | 2.691 |
| Regression coefficient (r2) | 0.9991 | 0.9997 | 0.9995 |
| Sensitivity | |||
| LOD (µg/mL) | 3.06 | 2.88 | 1.25 |
| LOQ (µg/mL) | 10.21 | 9.63 | 4.18 |
| Drug | Within-Day | Between-Day | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Amount [µg/mL] | Amount Found Mean [n = 3] ± SD | % RSD | % Recovery | % RE | Amount Found Mean [n = 9] ± SD | % RSD | % Recovery | % RE | |
| MET | 20 | 19.69 ± 0.20 | 1.02 | 98.45 | −1.55 | 20.02 ± 0.31 | 1.55 | 100.10 | 0.10 |
| 100 | 98.96 ± 1.13 | 1.14 | 98.96 | 1.04 | 99.47 ± 1.45 | 1.46 | 99.47 | −0.53 | |
| 250 | 247.38 ± 1.89 | 0.76 | 98.95 | 1.05 | 246.94 ± 2.03 | 0.82 | 98.78 | −1.22 | |
| OFL | 10 | 10.06 ± 0.16 | 1.59 | 100.60 | −0.60 | 10.03 ± 0.14 | 1.40 | 100.30 | 0.30 |
| 75 | 74.51 ± 1.46 | 1.96 | 99.35 | 0.65 | 74.38 ± 0.97 | 1.30 | 99.17 | −0.83 | |
| 150 | 148.99 ± 0.83 | 0.56 | 99.33 | 0.67 | 148.83 ± 1.09 | 0.73 | 99.22 | −0.78 | |
| RAC | 5 | 5.01 ± 0.08 | 1.60 | 100.20 | −0.20 | 4.98 ± 0.06 | 1.20 | 99.60 | −0.40 |
| 40 | 39.72 ± 0.64 | 1.61 | 99.30 | 0.70 | 39.38 ± 0.23 | 0.59 | 98.05 | −1.92 | |
| 80 | 79.08 ± 0.93 | 1.18 | 98.85 | 1.15 | 79.83 ± 0.96 | 1.20 | 99.79 | −0.21 | |
| Amount of Sample Taken a [µg mL−1] | % Found by Proposed Method | % Found by Reference Method | ||||||
|---|---|---|---|---|---|---|---|---|
| MET | OFL | RAC | MET | OFL | RAC | MET [24] | OFL [25] | RAC [25] |
| 80 | 40 | 12 | 99.64 | 99.47 | 99.46 | 99.47 | 98.37 | 100.33 |
| 100 | 50 | 15 | 100.27 | 100.94 | 98.09 | 101.62 | 99.33 | 101.67 |
| 160 | 80 | 24 | 99.37 | 101.07 | 100.77 | 98.37 | 100.43 | 98.27 |
| 200 | 100 | 30 | 100.47 | 99.48 | 99.52 | 99.39 | 100.76 | 99.61 |
| Across Mean | 99.93 | 100.24 | 99.46 | 99.71 | 99.72 | 99.97 | ||
| %RSD | 0.52 | 0.88 | 1.09 | 1.37 | 1.09 | 1.42 | ||
| t (2.446) b | 0.307 | 0.737 | 0.569 | |||||
| F (9.276) c | 6.963 | 1.515 | 1.67 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Attimarad, M.; Venugopala, K.N.; Chohan, M.S.; Shinu, P.; David, M.; Molina, E.I.P.; Nair, A.B.; Sreeharsha, N.; Altaysan, A.I.; Balgoname, A.A. Multivariate Optimization of Chromatographic Conditions for Rapid Simultaneous Quantification of Antidiarrheal Drugs in Formulation Using Surface Response Methodology. Separations 2022, 9, 103. https://doi.org/10.3390/separations9050103
Attimarad M, Venugopala KN, Chohan MS, Shinu P, David M, Molina EIP, Nair AB, Sreeharsha N, Altaysan AI, Balgoname AA. Multivariate Optimization of Chromatographic Conditions for Rapid Simultaneous Quantification of Antidiarrheal Drugs in Formulation Using Surface Response Methodology. Separations. 2022; 9(5):103. https://doi.org/10.3390/separations9050103
Chicago/Turabian StyleAttimarad, Mahesh, Katharigatta Narayanaswamy Venugopala, Muhammad S. Chohan, Pottathil Shinu, Marysheela David, Effren II Plaza Molina, Anroop Balachandran Nair, Nagaraja Sreeharsha, Abdulrahman Ibrahim Altaysan, and Abdulmalek Ahmed Balgoname. 2022. "Multivariate Optimization of Chromatographic Conditions for Rapid Simultaneous Quantification of Antidiarrheal Drugs in Formulation Using Surface Response Methodology" Separations 9, no. 5: 103. https://doi.org/10.3390/separations9050103
APA StyleAttimarad, M., Venugopala, K. N., Chohan, M. S., Shinu, P., David, M., Molina, E. I. P., Nair, A. B., Sreeharsha, N., Altaysan, A. I., & Balgoname, A. A. (2022). Multivariate Optimization of Chromatographic Conditions for Rapid Simultaneous Quantification of Antidiarrheal Drugs in Formulation Using Surface Response Methodology. Separations, 9(5), 103. https://doi.org/10.3390/separations9050103








