Abstract
Lichens are symbiotic organisms between algae and fungi, which are makers of secondary compounds named as lichen substances. Hyphenated techniques have significantly helped natural product chemistry, especially UHPLC/ESI/MS/MS in the identification, separation, and tentative characterization of secondary metabolites from natural sources. Twenty-five compounds were detected from the Antarctic lichen Cladonia metacorallifera for the first time using UHPLC-PDA/ESI/Orbitrap/MS/MS. Compounds 5 and 7 are reported as new compounds, based on their MS/MS fragmentation routes, and considered as fumarprotocetraric acid derivatives. Besides, ten known phenolic identified as orsellinic acid, ethyl 4-carboxyorsellinate, psoromic acid isomer, succinprotocetraric acid, siphulellic acid, connorstictic acid, cryptostictic acid, lecanoric acid, lobaric acid and gyrophoric acid are noticed for the first time in the Cladonia genus.
Keywords:
Antarctica; Cladonia; depsides; depsidones; Fumarprotocetraric acid; lichens; UHPLC; ESI/MS/MS 1. Introduction
Lichens are symbiotic organisms between algae or cyanobacteria and fungi. Lichens are organisms that can grow everywhere on soil, on and within rocks, on tree barks, as well as on any inanimate object. Lichens grow in tropical rainforests, deserts, and polar zones, including the Arctic and Antarctic regions [1,2,3]. From lichens, depsides, depsidones, dibenzofurans, depsones, anthraquinones, lactones and pulvinic acid derivatives have been isolated [4,5]. They have displayed multiple biological activities; for instance: antiulcer, gastroprotective, antibiotic, antiviral, antitumor, allergenic, plant growth inhibitory, antiherbivore, antileishmanial, anti-inflammatory, antioxidants, anti-trypanosoma and enzyme inhibitory activities [5,6,7].
Analyses of crude extracts were performed using chromatographic methods such as high-performance liquid chromatography (HPLC) in combination with various detection spectroscopic methods [8,9,10,11,12]. However, HPLC coupled to UV spectroscopy provide limited structural information when compounds are unknown [13]. Mass spectrometry (MS) provides rapid identification of unknown compounds while electro-spray ionization (ESI) is the most successful interface used in LC-MS coupled to time of flight (ToF), quadrupole or orbitrap [14]. Currently, tandem mass spectrometers operate within 1–5 ppm mass accuracy with high resolution and the quality of MS/MS spectra depend on the parameters such as precursor ion isolation width, intensity threshold, collision energy, total acquisition speed, accumulation time on MS/MS spectrum, and others [14]. Therefore, LC-MS is a dominant analytical technique to identify secondary metabolites, and unknown constituents, in plant extracts giving information on elemental composition and structural fragmentation patterns, but are unable to distinguish positional isomers [14]. The hyphenated Q-exactive instrument with a high-resolution collision cell has significantly contributed in the area of lichens chemistry for the identification of unknown compounds based on structural characterization by MS/MS [8,12,15].
Herein, we describe the chromatographic fingerprinting of the Antarctic lichen Cladonia metacorallifera by UHPLC/PDA/ESI/MS/MS, which revealed the presence of two new metabolites.
2. Materials and Methods
2.1. Collection and Identification of Lichen
A 5 g aliquot of C. metacorallifera was collected in “Peninsula Fildes”, King George Island, Antarctica during March, 2020. Vouchers specimens (reference numbers: CM-010420) were deposited at the Extreme natural product laboratory, Universidad de Chile.
2.2. UHPLC-Q/Orbitrap/ESI/MS/MS
2.2.1. Sample Preparation
A 1.0 g aliquot of C. metacorallifera was extracted with methanol (3 times, 10 mL each time, using a sonicator 30 min). The organic solutions were evaporated to obtain 18.0 mg of dark green gummy extract.
2.2.2. Instrument
The Thermo Scientific Dionex Ultimate 3000 UHPLC system, hyphenated with a Thermo Q exactive focus, was already reported [8,12]. For the analysis, 2 mg of each lichen extracts were first dissolved in 2 mL of methanol, then filtered (PTFE filter) and, finally, 10 µL were injected in the instrument, with all specifications set as previously reported [8].
2.2.3. LC Parameters
A UHPLC C18 column (150 mm × 4.6 mm ID, Thermo Fisher Scientific, Bremen, Germany) at 25 °C in an oven was used. Four detection UV systems were performed at 254, 280, 320 and 440 nm, and PDA from 180 to 800 nm. Mobile phases were 1% formic aqueous solution (A) and acetonitrile (B). The gradient (time (min), % B) was: (0.00, 5); (5.00, 5); (10.00, 30); (15.00, 30); (20.00, 70); (25.00, 70); (35.00, 5) and 12 min for column equilibration before each injection. The flow rate was setup at 1.00 mL min−1, and the injection volume by 10 μL. Usnic acid and gyrophoric acid were use as standards of spiking experiments to perform the qualitative analysis. Standards and lichen extracts were kept at 10 °C during storage in the auto sampler.
2.2.4. MS Parameters
The HESI parameters were setup as follows: gas flow rate 75 units; capillary temperature at 400 °C; auxiliary gas unit flow rate at 20 unit (N2); auxiliary gas heater temperature 500 °C; spray voltage 2500 V (for ESI−); and S lens RF level 30. For the compounds of interest, a scan range of m/z 100–1000 was chosen; the automatic gain control (AGC) was set at 3 × 106 and the injection time was set to 200 ms. Collision energy was setup at 30 kV. Detection was performed based on calculated exact mass and on retention time. The mass tolerance window was set to 5 ppm for the two analysis modes.
3. Results and Discussion
The identification of unknown secondary metabolites in metabolomics is the main bottleneck on the structural interpretation based on MS/MS spectra, making the identification an arduous time-consuming task. As a strategy, many researches are limited to library matching, software, databases, algoritms and matching learning. Among the most known, both publicly and commercially, MS/MS software tools and databases including Mass Frontier, SmileMS, Mass++, XCMS2, NIST, METLIN, MassBank, MoNA, Wiley MSforID, CFM-ID, and MS-DIAL. For the generation of molecular structures of unknown compounds, it is important to follow the MS fragmentation ruler analysing the main small fragments. The biosynthetic pathway of secondary metabolite should also be considered if it applies [13,14].
During our study, twenty-five compounds were characterized for the first time in the methanolic extract of C. metacorallifera (Table 1) using UHPLC/PDA/ESI/MS/MS in negative ion mode.

Table 1.
UHPLC/Orbitrap/ESI/MS/MS data for Antarctic lichen C. metacorallifera.
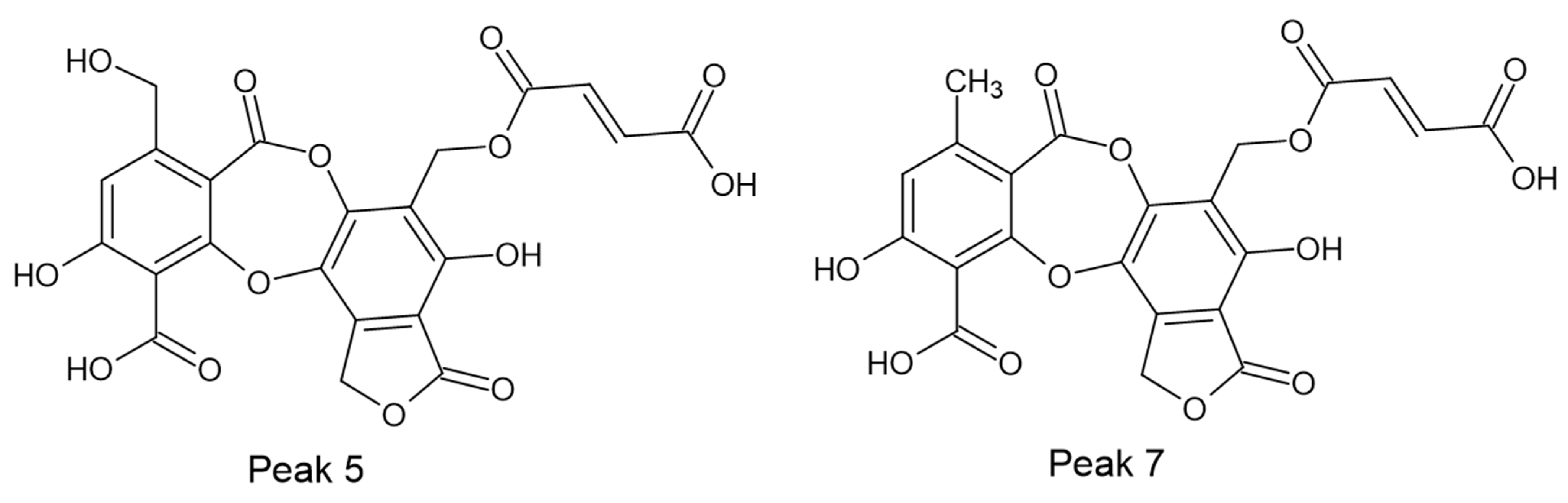
Among the identified compounds, two fumarprotocetraric acid lactones (peaks 5 and 7) are reported here for the first time. Their structures (Figure 1) are proposed based on biosynthetic pathways, UV data and MS/MS fragmentations.
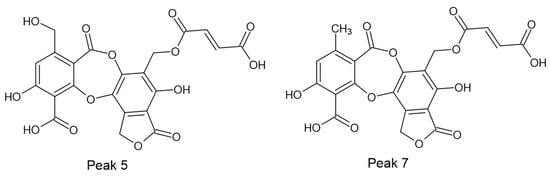
Figure 1.
Chemical structures of new compounds (5 and 7) identified by UHPLC/ESI/MS/MS from C. metacorallifera. It proposes interconversion mediated by natural oxidation from peak 13 (fumarprotocetraric acid) to peak 7 and after to peak 5, as supported by mass spectrometry and UV spectroscopy.
Peak 1 was identified as orsellinic acid (C8H8O4). The electrospray TOF mass fragmentation of this metabolite has been previously reported [10,15]. In the present study, orsellinic acid is identified, for the first time, in Cladonia genus. Orsellinic acid showed antioxidant activity in the β-carotene-linoleate model, and in the nitric oxide radical scavenging assay [6]. Peak 2 with an ion at m/z 239.0546 showed UV absorbance at λmax 249, 308 nm, which is a similar to the one showed by ethyl orsellinate (λmax 219, 265, 302 nm). Their fragmentation yielded a diagnostic MS ion at m/z 195.0649 and 149.0229 (see Supplementary Material). Considering that peak 2 displayed diagnostic losses of CO2 and C2H6O, we identified peak 2 as ethyl 4-carboxyorsellinate. From Picea schrenkiana has isolated this compound, and it is reported for the first time from lichens [16].
Peak 3 was characterized as squamatic acid (molecular anion at m/z 389.0860), whose fragmentation indicated diagnostic MS ions at m/z 209.0442 and 181.0490 [9,15]. This depside has been identified in Cladonia uncialis, C. crispata, C. bellidiflora, C. squamosa, and C. cenotea from Poland samples [17,18]. So far, squamatic acid has shown to be inactive against Staphylococcus aureus, Escherichia coli and Candida albican [19]. Peak 4 with a [M–H]− ion at m/z 357.0598 was characterized as a psoromic acid isomer. The fragmentation of this peak produced ions at m/z 313.0701 and 179.0335. This compound showed UV absorbance at λmax 249, 382 and 319 nm, which is similar to the one of psoromic acid (λmax 240, 271, 317 nm).
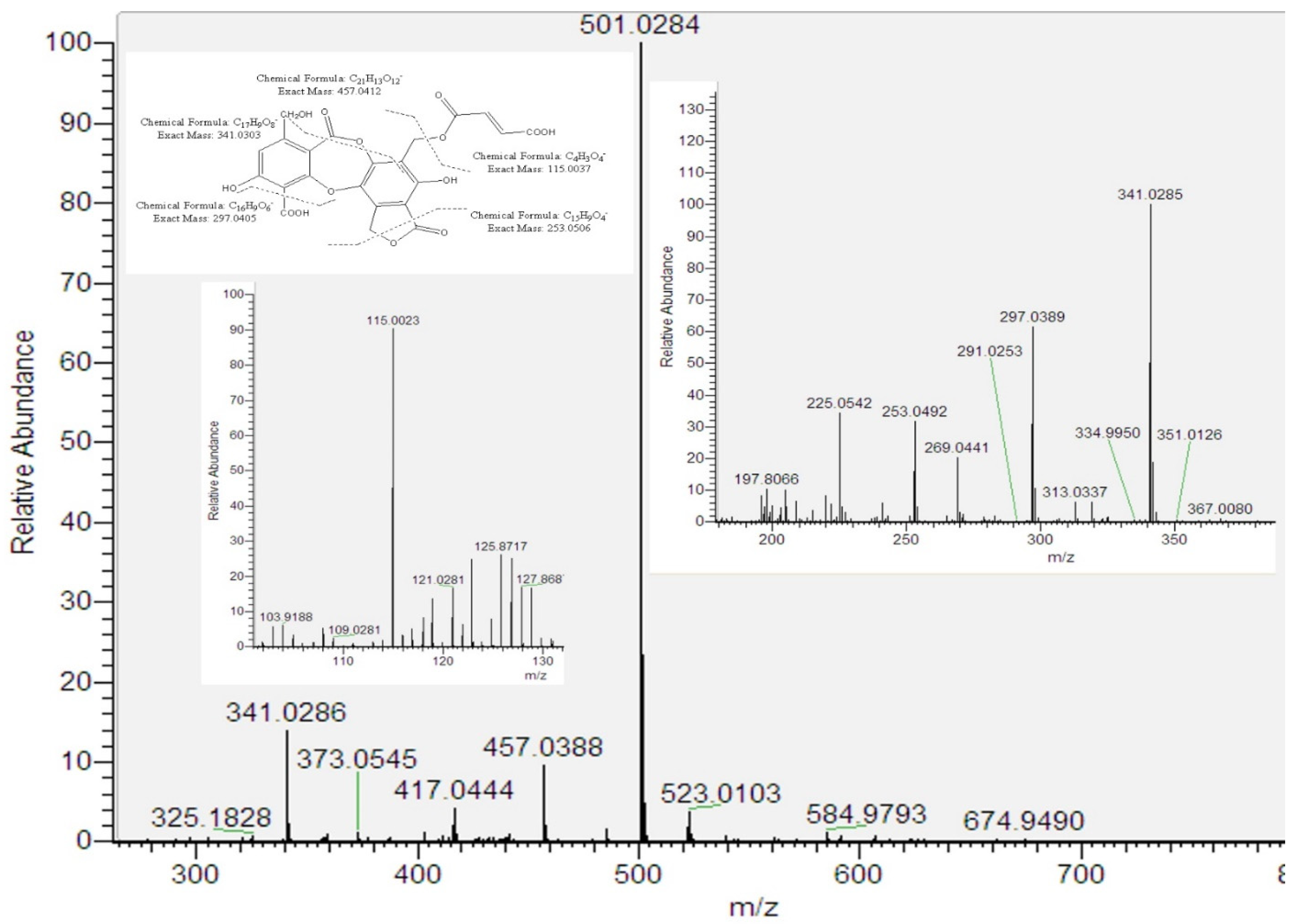
Peak 5 provided an [M–H]− ion at m/z 501.0284 (C22H13O14). This fragmentation indicated that peak 5 is a new compound related to fumarprotocetraric acid (peak 13). The key for this proposal implied the presence of the residue butendioic acid (C4H3O4—; 115.0023) along with the residue C17H9O8— (341.0303). Considering that peak 5 showed UV absorbance at λmax 215, 248 and 315 nm, which is similar to the one of peak 13 (λmax 212, 238, 314 nm), and according to biosynthetic considerations, we tentatively identified peak 5 as hydroxy fumarprotocetraric acid lactone (Figure 2). Finally, the presence of fumarprotocetraric acid in Cladonia species supported this idea to be a derivative [17,18].
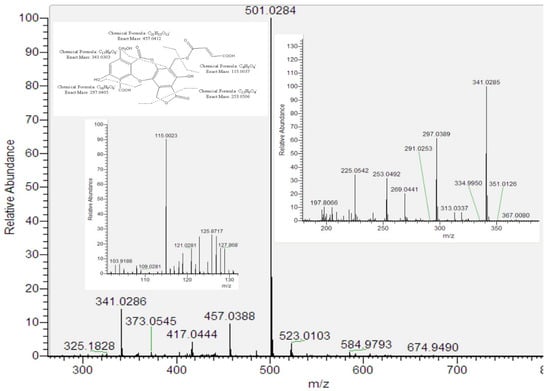
Figure 2.
ESI/MS/MS spectra of peak 5 and its proposed fragmentation pathway.
Peak 6 was identified as the depsidone connorstictic acid (m/z 373.0547) and its fragmentation produced ions at 329.0666 and 181.0555 [9,10,15]. To the best of our knowledge, there is no paper of connorstictic acid being present Cladonia genus. To support this fact, the presence of a related compound norstictic acid has been reported in the Cladonia genus [17,18]. No biological activity has been reported so far.
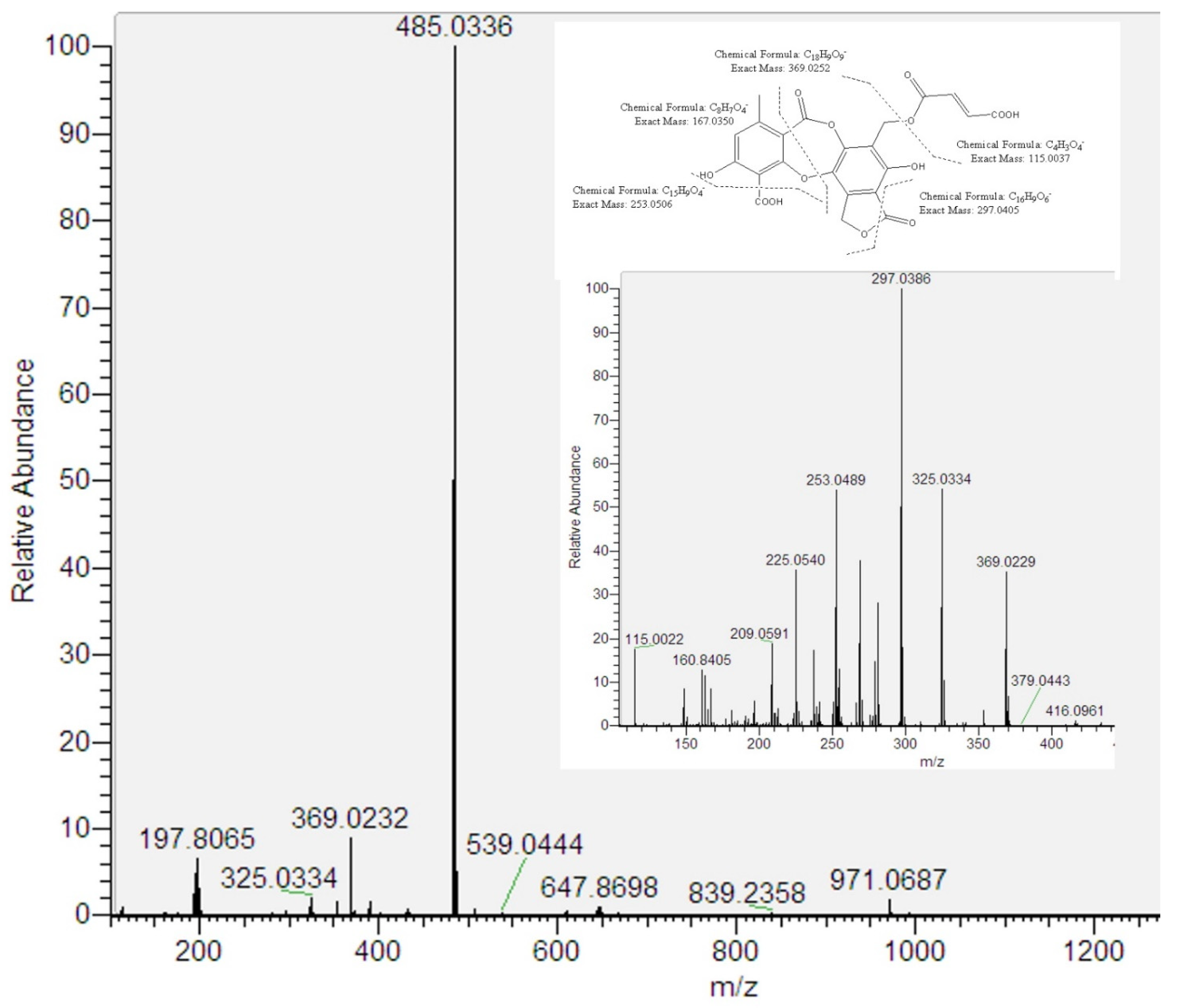
Peak 7 showed an [M–H]− ion at m/z 485.0336 (C22H13O13). According to their fragmentation pathway indicated that peak 7 is a new depsidone related to fumarprotocetraric acid (peak 13). The proposal implied the presence of three daughter fragments at m/z 369.0252 (C18H9O9−), m/z 115.0023 (butendioic acid; C4H3O4−) and the residue C17H9O7− (325.0354). Besides, peak 7 showed UV absorbance at λmax 248 and 311 nm, which is similar to peak 13. Considering this evidence and based on the biosynthesis of lichen metabolites, we tentatively identified peak 7 as a fumarprotocetraric acid lactone (Figure 3). It is well known that depsides as fumarprotocetraric acid are synthesized by the acetate-polymalonate pathway, which are formed by the bonding of two β-orcinol-type phenolic unit with ether, ester and C-C link [20]. In this context, both peak 5 and peak 7 derived from fumarprotocetraric acid are probably formed by lactonisation for peak 7 and oxidation and lactonisation for peak 5.
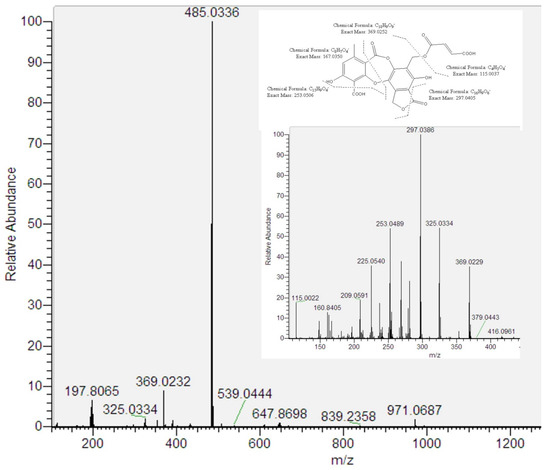
Figure 3.
ESI/MS/MS spectra of [M–H]− of peak 7 and its proposed fragmentation pathway.
Peak 8 with an ion at m/z 401.0514 was identified as siphulellic acid, which showed diagnostic daughter ions at m/z 123.0444, 149.0238, and 253.0505. To the best of our knowledge, this is the first information of its presence in the Cladonia genus [17,18]. No biological activity has been reported according to Scopus so far. Peak 9 was detected as lecanoric acid, which showed an [M–H]− ion at m/z 317.0668 according to published data [9,10,11]. Therefore, this is the first report of the presence of lecanoric acid in the Cladonia genus [17,18]. Lecanoric acid has displayed antioxidant, antibacterial and anticancer activities [5,6,7]. Peak 10 showed an [M–H]− ion at m/z 473.0701 (λmax 250 and 311 nm). Their fragmentation produced daughter ions at m/z 355.0441, 311.0545 and 117.0179 indicating that peak 10 is not confumarprotocetraric acid [17]. Considering these fragments, we identified as succinprotocetraric acid [21] (see Supplementary Material).
Peaks 11, 12, 14, 20 and 25 showed an [M–H]− ion at m/z 447.3305, 403.3040, 417.3201, 385.2940 and 295.1916, respectively. They were tentatively characterized as the fatty acids pentahydroxytetracosanoic acid (C24H48O7), tetrahydroxydocosanoic acid (C22H44O6), tetrahydroxytricosanoic acid (C23H46O6), dihydroxyoxodocosanoic acid (C22H42O5), and dihydroxyheptadecatrienoic acid (C17H28O4), respectively. Compounds related to these peaks have also been reported [17].
Peak 13 was detected as fumarprotocetraric acid (C22H15O12), which displayed an [M–H]− ion at m/z 471.0544. The fragmentation of this peak produced ions at m/z 355.0441, 311.0545 and 115.0023 according to reported [21]. This depside has been identified in Cladonia verticillata, C. cariosa, C. phyllophora, C. merochlorophaea, C. trassii, C. symphycarpia, C. subulata, C. pyxidata, C. fimbriata, C. chlorophaea and C. stricta from Poland samples [18]. Fumarprotocetraric acid has considerably shown antimicrobial, antioxidant, anticarcinogenic and immunoestimulatory activities [4,5,6,7].
Peak 15 was identified as the depsidone cryptostictic acid ([M–H]− ion at m/z 387.0726) and its fragmentation showed ions at m/z 343.0826, 311.0566 and 267.0661. These fragments have been reported [22]. This is the first report of the presence of cryptostictic acid in the Cladonia genus. No significant antioxidant activity has been found for this compound [6]. Peak 16 was tentatively identified as 6-ethyl-6-n-pentylpentadecan-4,5,7,8,15-pentol-15-acetate (C24H48O6) [17]. Peak 17 with a [M–H]− ion at m/z 419.0963 was identified as thamnolic acid, which showed daughter ions at m/z 375.0722, 211.0238 and 167.0345 [11]. The presence of thamnolic acid in Cladonia species has been previously informed. No important biological activity has been reported so far. Peak 18 was identified as gyrophoric acid showing a molecular anion at m/z 467.0959. Its fragmentation informed ions at m/z 317.0647 [M–H−, C8H6O3]−, 167.0336 [M–H–, C16H12O6]−, 151.0387 [M–H–, C16H12O7]−, and 123.0438 [M–H–, C17H12O8]− confirming this tridepside [9,10,11]. This is the first report of the presence of gyrophoric acid in the Cladonia genus. Gyrophoric acid demostrated to be cytotoxic, anticarcinogenic, antibacterial, and antiproliferative [4,5,6]. Peak 19 was identified as psoromic acid according to data reported previously [15]. This depside has been identified in Cladonia symphycarpia, and C. macrophyllodes from Poland samples [18]. Psoromic acid has shown antioxidant, cardioprotective, cytotoxic and apoptotic activities [4,5,6]. Peak 21 was assigned as lobaric acid (m/z 455.1712). The fragmentation produced ions at m/z 411.1808 [M–H– CO2]−, 367.1909 [M–H–, 2CO2]−, 352.1675 [M–H–, 2CO2–CH3]−, and 296.1049 [M–H–, 2CO2–C5H11]−. Such fragmentations have been reported previously [8,9,10]. This is the first report of the presence of lobaric acid in Cladonia genus. Lobaric acid has shown antibacterial, antioxidant, and anticarcinogenic activities. Also, the inhibition of arachidonate-5-lipooxygenase, cyclooxygenase, DNA synthesis, and tubulin polymerization has also been reported [4,5,6,7]. An aromatic compound was identified as ethyl-4-O-methylolivetolcarboxylate at the peak 22, which was reported from C. macaronesica previously [17]. No important enzymatic activity has been reported as 5-lipooxygenase inhibitor [23]. Peak 23 was identified as usnic acid according to the studies [4,8,10]. This dibenzofuran has been identified and isolated in many Cladonia samples [17,18]. Usnic acid has been the most evaluated metabolite in lichens regarding biological activity. Among them, we can mention anti-inflammatory, antioxidant, antimicrobial, immunoestimulatory, gastroprotective, cytoprotective, cardioprotective and anticarcinogenic activities [4,5,6,7,24]. Peak 24 was assigned to atranorin, and their major diagnostic daughter ions were at m/z 177.0187 and 163.0394 a.m.u. These findings are in good agreement with previous reported studies [10,11]. This depside has been identified and isolated in many Cladonia samples [17,18]. Atranorin showed interesting biological activities such as antioxidant, antimicrobial, cytoprotective, pro-oxidant, cytotoxicity, pro-apoptotic, and anticarcinogenic activities [4,5,6,7].
Some reports from the genus Cladonia including C. metacorallifera var. reagens KoLRI002260 as a rare lichen from Korean. They have been studied as mycobiont producing red naphthoquinonic pigments as cristazarin, and 6-methylcristazarin in culture media with 1% fructose and/or light [25]. Besides, these two compounds are produced by Cladonia cristatella mycobiont, but not in lichen thalli [26]. From Cladonia cariosa fumarprotocetraric acid was isolated, which showed 50 µM decrease on the β sheet content, demonstrated through Thioflavin T assays. In addition, the oligomers formed in that study with fumarprotocetraric acid were not toxic in N2a neuroblastoma cells [27]. A study based on UHPLC/QTOF/HRMS/MS of Algerian lichen Cladonia rangiformis detected the presence of the following thirteen metabolites highlighting ethyl orsellinate, squamatic acid, atranorin, evernic acid, usnic acid, roccellic acid, jackinic acid, norrangiformic acid, isorangiformic acid, and rangiformic acid [28]. In this context, our study detected twenty five metabolites using UHPLC/Q/Orbitrap/MS/MS.
According to our search on SciFinder, the compounds identified with peaks 5 and 7 are reported here for the first time. As mentioned above, fumarprotocetraric acid display considerable pharmacological activity. Therefore, these two compounds could be isolated to evaluate their potential as pharmacological agent.
4. Conclusions
Our results demonstrate that the identification for lichen metabolites using UHPLC-Q/Orbitrap/ESI/MS/MS is a fast and accurate methodology. Based on this hyphenated technique, we identified twenty-five compounds; two of them being reported for the first time, based on careful high resolution mass spectrometry analysis and biosynthetic considerations (peak 5 and peak 7). Besides, ten phenolic compounds known as orsellinic acid (peak 1), ethyl 4-carboxyorsellinate (peak 2), psoromic acid isomer (peak 4), connorstictic acid (peak 6), siphulellic acid (peak 8), lecanoric acid, (peak 9), succinprotocetraric (peak 10), cryptostictic acid (peak 15), gyrophoric acid (peak 18), and lobaric acid (peak 21) are reported for the first time in the Cladonia genus. Finally, six lipids were tentatively identified as peaks 11, 12, 14, 16, 20 and 25. Further prospection in metabolomics based on LC/MS/MS should be considered from Antarctic lichens.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/separations9020041/s1, Figure S1: UHPLC-ESI-MS-MS Chromatogram of methanolic extract of C. metacorallifera.
Author Contributions
Conceptualization, C.A.; methodology, C.A., B.S. and A.C.; formal analysis, D.B.-P. and J.C.; investigation, C.A., B.S. and A.C.; writing—original draft preparation, C.A.; writing—review and editing, D.B.-P. and J.C.; supervision, C.A.; funding acquisition, C.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by INACH RT 18-19; INACH RT 13-13 and Fondecyt Regular 1190314. D.B.-P. and J.C. thank the support of Technology Agency of the Czech Republic (NCK grant TN010000048/03, J.C. and D.B.-P.) and the National Programme of Sustainability I of the Ministry of Education, Youth and Sports of the Czech Republic (ID: LO1416, J.C.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Daniela Bárcenas-Pérez gratefully acknowledges the research supervision of José Cheel (Centre Algatech–Czech Academy of Sciences) as well as the University of South Bohemia for a doctoral scholarship and the grant GAJU 017/2019/P.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Boustie, J.; Grube, M. Lichens—A promising source of bioactive secondary metabolites. Plant Genet. Resour. 2005, 3, 273–287. [Google Scholar] [CrossRef] [Green Version]
- Shukla, V.; Joshi, G.P.; Rawat, M.S.M. Lichens as a potential natural source of bioactive compounds: A review. Phytochem. Rev. 2010, 9, 303–314. [Google Scholar] [CrossRef]
- Molnár, K.; Farkas, E. Current Results on Biological Activities of Lichen Secondary Metabolites: A Review. Z. Naturforsch. C. J. Biosci. 2010, 65, 157–173. [Google Scholar] [CrossRef] [PubMed]
- Boustie, J.; Tomasi, S.; Grube, M. Bioactive lichen metabolites: Alpine habitats as an untapped source. Phytochem. Rev. 2011, 10, 287–307. [Google Scholar] [CrossRef]
- Shrestha, G.; Clair, L.L.S. Lichens: A promising source of antibiotic and anticancer drugs. Phytochem. Rev. 2013, 12, 229–244. [Google Scholar] [CrossRef]
- White, P.A.S.; Oliveira, R.C.M.; Oliveira, A.P.; Serafini, M.R.; Araújo, A.A.S.; Gelain, D.P.; Moreira, J.C.F.; Almeida, J.R.G.S.; Quintans, J.; Quintans-Junior, L.J.; et al. Antioxidant Activity and Mechanisms of Action of Natural Compounds Isolated from Lichens: A Systematic Review. Molecules 2014, 19, 14496–14527. [Google Scholar] [CrossRef]
- Zambare, V.P.; Christopher, L.P. Biopharmaceutical potential of lichens. Pharm. Biol. 2012, 50, 778–798. [Google Scholar] [CrossRef]
- Calla-Quispe, E.; Robles, J.; Areche, C.; Sepulveda, B. Are Ionic Liquids Better Extracting Agents Than Toxic Volatile Organic Solvents? A Combination of Ionic Liquids, Microwave and LC/MS/MS, Applied to the Lichen Stereocaulon glareosum. Front. Chem. 2020, 8, 450. [Google Scholar] [CrossRef]
- Parrot, D.; Jan, S.; Baert, N.; Guyot, S.; Tomasi, S. Comparative metabolite profiling and chemical study of Ramalina siliquosa complex using LC–ESI-MS/MS approach. Phytochemistry 2013, 89, 114–124. [Google Scholar] [CrossRef]
- Musharraf, S.G.; Kanwal, N.; Thadhani, V.M.; Choudhary, M.I. Rapid identification of lichen compounds based on the structure–fragmentation relationship using ESI-MS/MS analysis. Anal. Methods 2015, 7, 6066–6076. [Google Scholar] [CrossRef]
- Le Pogam, P.; Schinkovitz, A.; Legouin, B.; Le Lamer, A.-C.; Boustie, J.; Richomme, P. Matrix-Free UV-Laser Desorption Ionization Mass Spectrometry as a Versatile Approach for Accelerating Dereplication Studies on Lichens. Anal. Chem. 2015, 87, 10421–10428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cornejo, A.; Salgado, F.; Caballero, J.; Vargas, R.; Simirgiotis, M.; Areche, C. Secondary Metabolites in Ramalina terebrata Detected by UHPLC/ESI/MS/MS and Identification of Parietin as Tau Protein Inhibitor. Int. J. Mol. Sci. 2016, 17, 1303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kind, T.; Tsugawa, H.; Cajka, T.; Ma, Y.; Lai, Z.; Mehta, S.S.; Wohlgemuth, G.; Barupal, D.K.; Showalter, M.R.; Arita, M.; et al. Identification of small molecules using accurate mass MS/MS search. Mass Spectrom. Rev. 2018, 37, 513–532. [Google Scholar] [CrossRef] [PubMed]
- Vaniya, A.; Fiehn, O. Using fragmentation trees and mass spectral trees for identifying unknown compounds in metabolomics. Trends Analyt. Chem. 2015, 69, 52–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro, O.N.; Benites, J.; Rodilla, J.M.L.; Santiago, J.C.; Simirgiotis, M.; Sepulveda, B.; Areche, C. Metabolomic Analysis of the Lichen Everniopsis trulla Using Ultra High Performance Liquid Chromatography-Quadrupole-Orbitrap Mass Spectrometry (UHPLC-Q-OT-MS). Chromatographia 2017, 80, 967–973. [Google Scholar] [CrossRef]
- Li, Z.H.; Wang, Q.; Lia, J.J.; Ruan, X.; Pan, C.D.; Jiang, D.A.; Luo, C.C. Elementary identification of potential autotoxins from Picea schrenkiana litters. Chin. J. Anal. Chem. 2009, 6, 888–892. [Google Scholar]
- Huneck, S.; Yoshimura, I. Data of lichen substances. In Identification of Lichen Substances; Springer: Berlin/Heidelberg, Germany, 1996; Volume 3, pp. 125–446. [Google Scholar]
- Osyczka, P. The lichen genus Cladonia (Cladoniaceae, lichenized Ascomycota) from Spitsbergen. Pol. Polar Res. 2006, 27, 207–242. [Google Scholar]
- Studzińska-Sroka, E.; Hołderna-Kędzia, E.; Galanty, A.; Bylka, W.; Kacprzak, K.; Ćwiklińska, K. In Vitro antimicrobial activity of extracts and compounds isolated from Cladonia uncialis. Nat. Prod. Res. 2015, 29, 2302–2307. [Google Scholar] [CrossRef]
- Ranković, B.; Kosanić, M. Lichens as a potential source of bioactive secondary metabolites. In Lichen Secondary Metabolites, 1st ed.; Ranković, B., Ed.; Springer: Cham, Switzerland, 2015; pp. 1–26. [Google Scholar]
- Xu, M.; Heidmarsson, S.; Thorsteinsdottir, M.; Kreuzer, M.; Hawkins, J.; Omarsdottir, S.; Olafsdottir, E.S. Authentication of Iceland Moss (Cetraria islandica) by UPLC-QToF-MS chemical profiling and DNA barcoding. Food Chem. 2018, 245, 989–996. [Google Scholar] [CrossRef]
- Salgado, F.; Albornoz, L.; Cortéz, C.; Stashenko, E.; Urrea-Vallejo, K.; Nagles, E.; Galicia-Virviescas, C.; Cornejo, A.; Ardiles, A.; Simirgiotis, M.; et al. Secondary Metabolite Profiling of Species of the Genus Usnea by UHPLC-ESI-OT-MS-MS. Molecules 2018, 23, 54. [Google Scholar] [CrossRef] [Green Version]
- Oettl, S.K.; Gerstmeier, J.; Khan, S.Y.; Wiechmann, K.; Bauer, J.; Atanasov, A.G.; Malainer, C.; Awad, E.M.; Uhrin, P.; Heiss, E.H.; et al. Imbricaric Acid and Perlatolic Acid: Multi-Targeting Anti-Inflammatory Depsides from Cetrelia monachorum. PLoS ONE 2013, 8, e76929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araújo, A.A.S.; de Melo, M.G.D.; Rabelo, T.K.; Nunes, P.S.; Santos, S.L.; Serafini, M.R.; Santos, M.R.V.; Quintans, L.; Gelain, D.P. Review of the biological properties and toxicity of usnic acid. Nat. Prod. Res. 2015, 29, 2167–2180. [Google Scholar] [CrossRef] [PubMed]
- Jeong, M.-H.; Park, C.-H.; Kim, J.; Choi, E.; Kim, S.; Hur, J.-S.; Park, S.-Y. Production and Activity of Cristazarin in the Lichen-Forming Fungus Cladonia metacorallifera. J. Fungi 2021, 7, 601. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Matsubara, H.; Kinoshita, Y.; Kinoshita, K.; Koyama, K.; Takahashi, K.; Ahmadjiam, V.; Kurokawa, T.; Yoshimura, I. Naphthazarin derivatives from cultures of the lichen Cladonia cristatella. Phytochemistry 1996, 43, 1239–1242. [Google Scholar] [CrossRef]
- González, C.; Cartagena, C.; Caballero, L.; Melo, F.; Areche, C.; Cornejo, A. The Fumarprotocetraric Acid Inhibits Tau Covalently, Avoiding Cytotoxicity of Aggregates in Cells. Molecules 2021, 26, 3760. [Google Scholar] [CrossRef]
- Brakni, R.; Ahmed, M.A.; Burger, P.; Schwing, A.; Michel, G.; Pomares, C.; Hasseine, L.; Boyer, L.; Fernandez, X.; Landreau, A.; et al. UHPLC-HRMS/MS Based Profiling of Algerian Lichens and Their Antimicrobial Activities. Chem. Biodivers. 2018, 15, e1800031. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).