Effect of Alkali Cation on Performance of Alkali-Activated Slag Mortar in Cold Environments
Abstract
1. Introduction
2. Materials and Methods
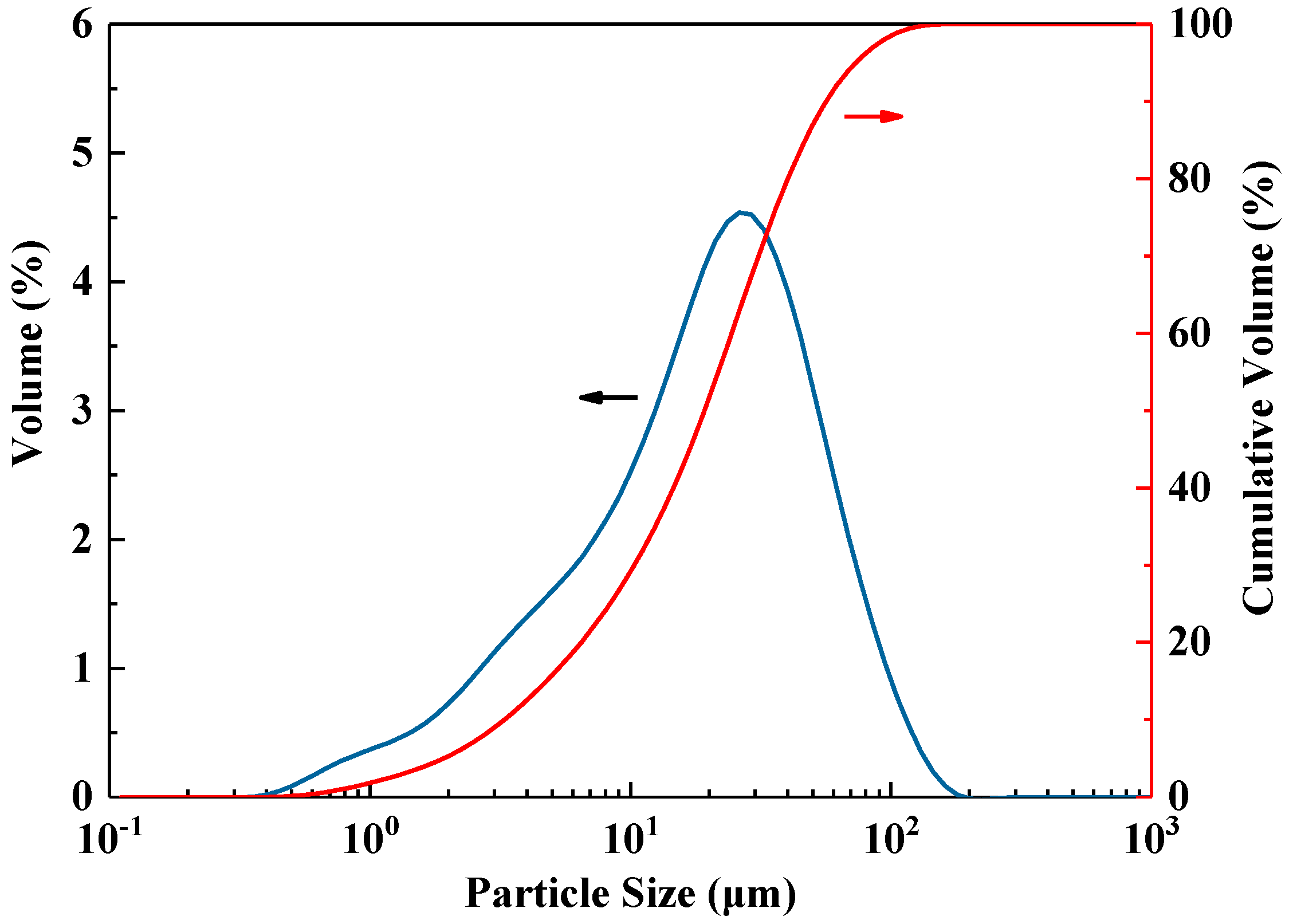
2.1. Raw Materials and Mixing Propottion
2.2. Methods
3. Results and Discussion
3.1. Setting Time
3.2. Mechanical Properties
3.3. Hydration Heat
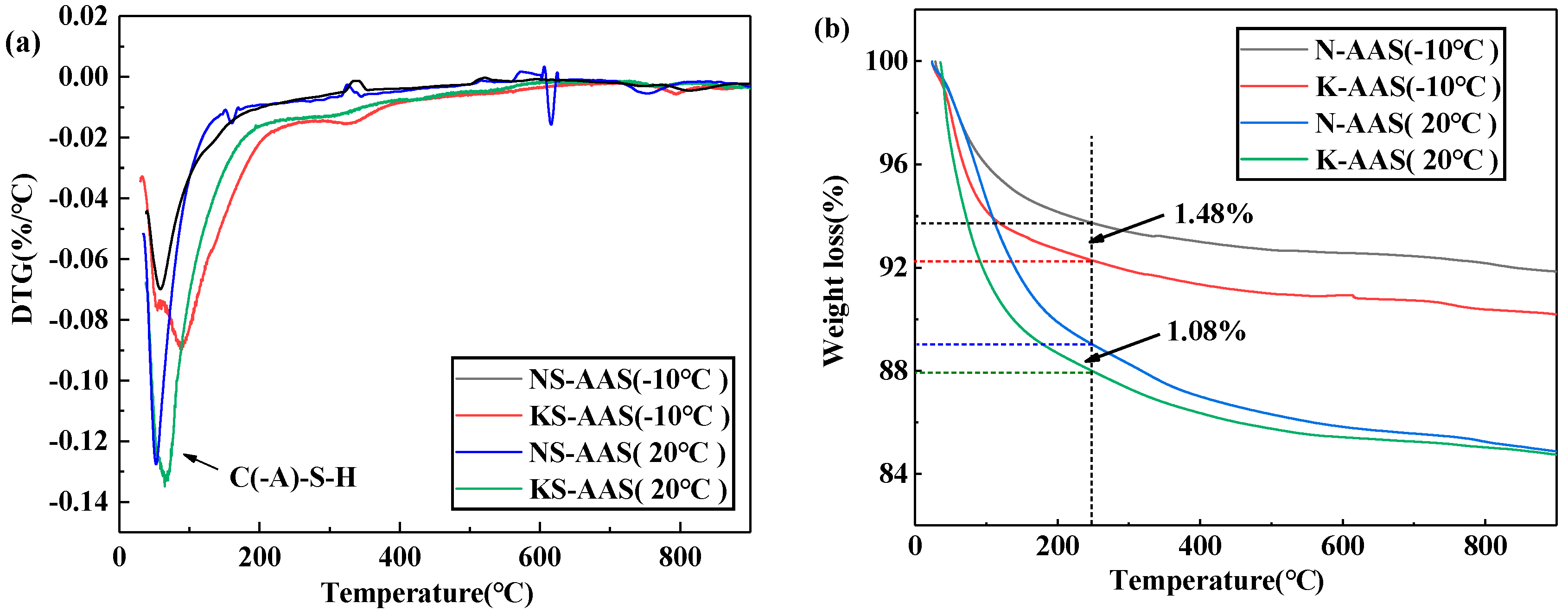
3.4. Thermal Analysis
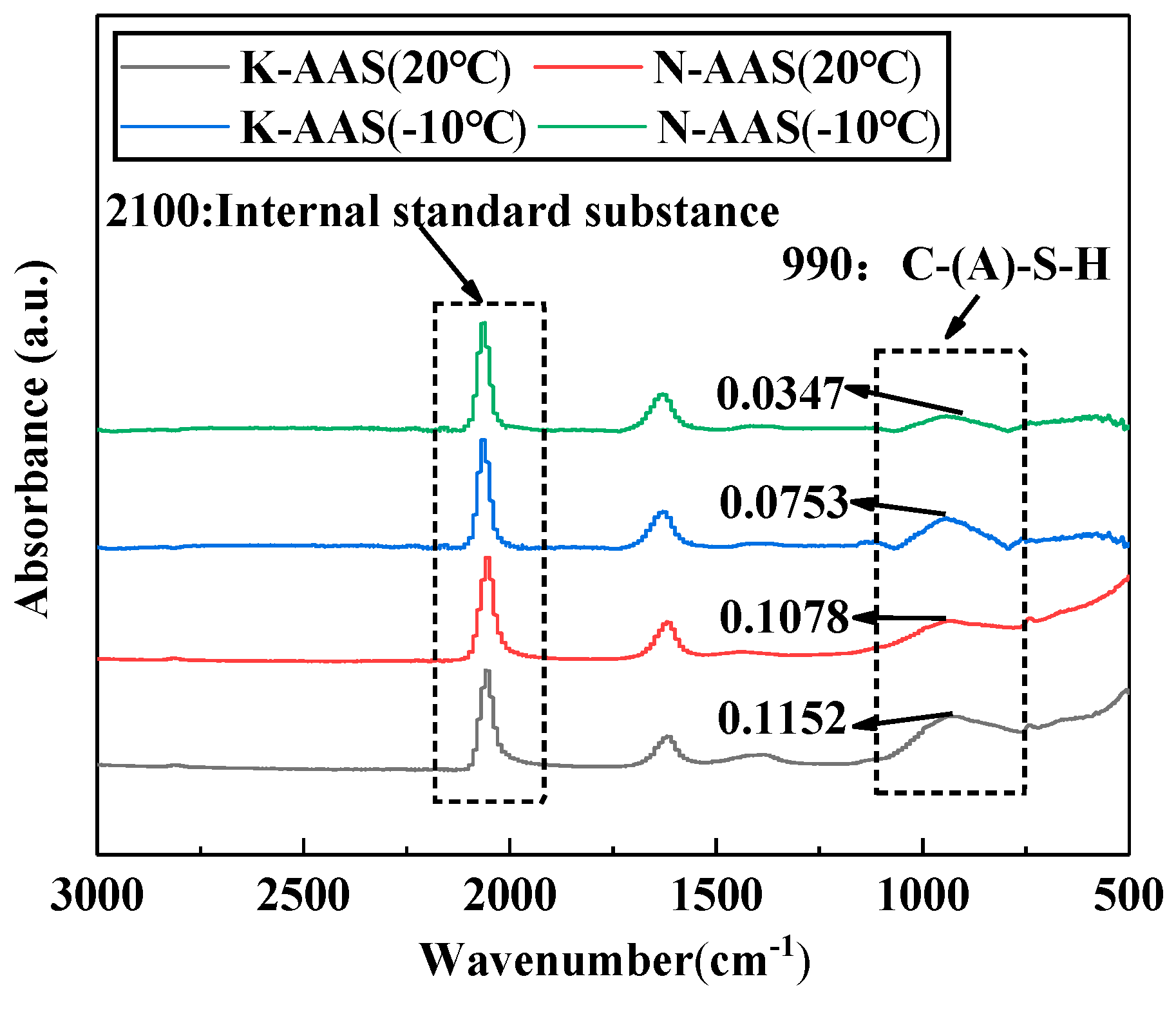
3.5. ATR-FTIR Spectrum
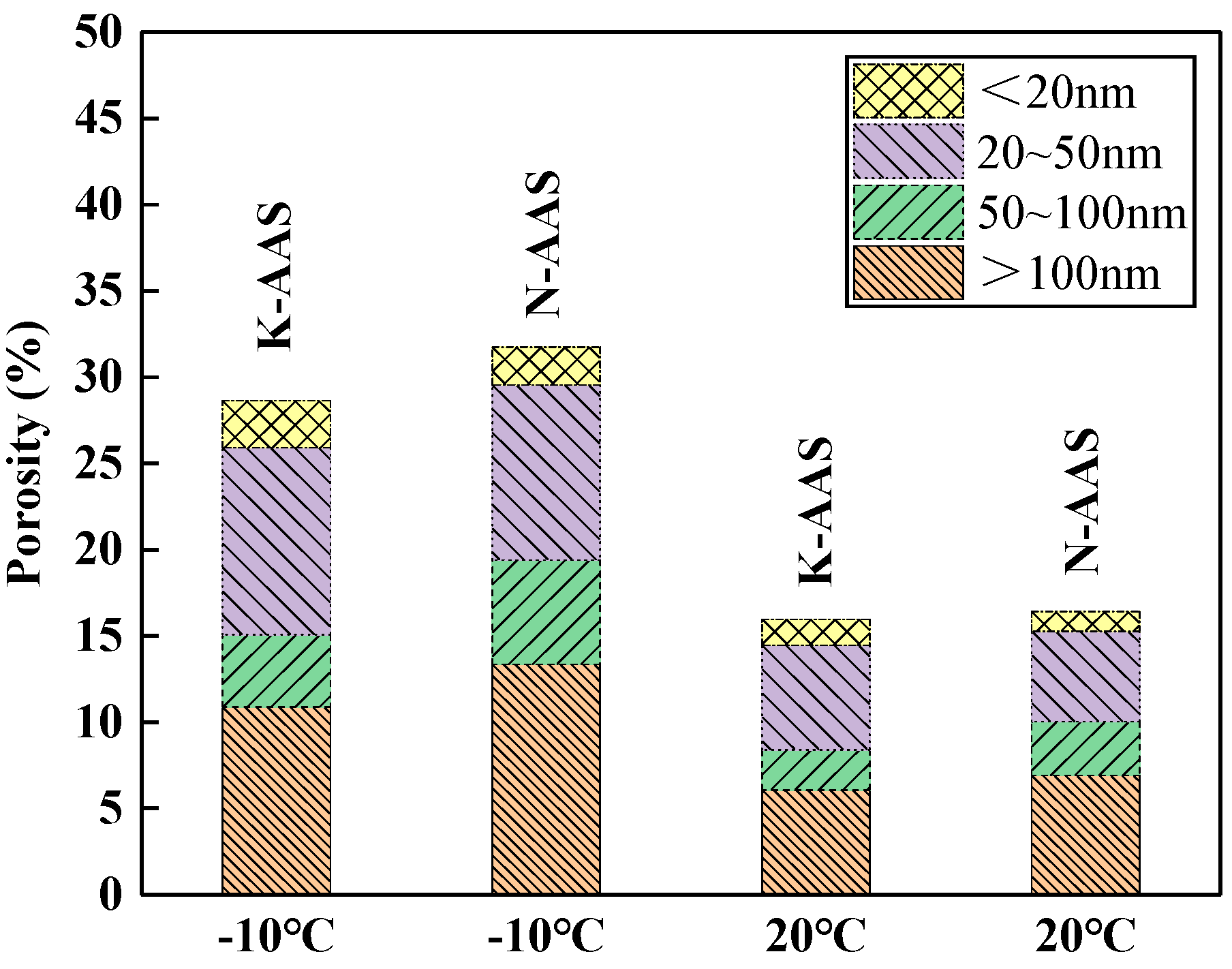
3.6. Pore Structure
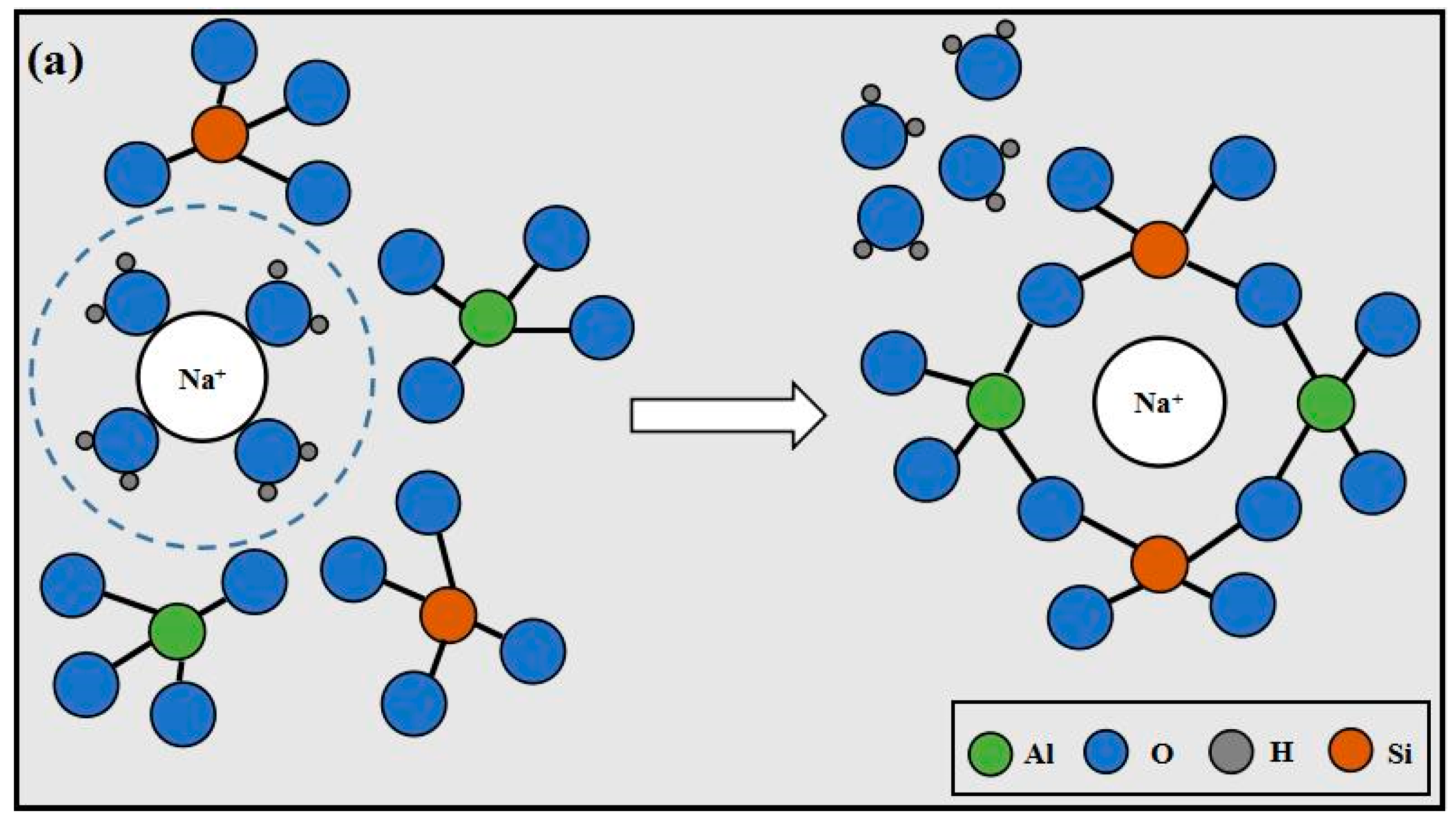
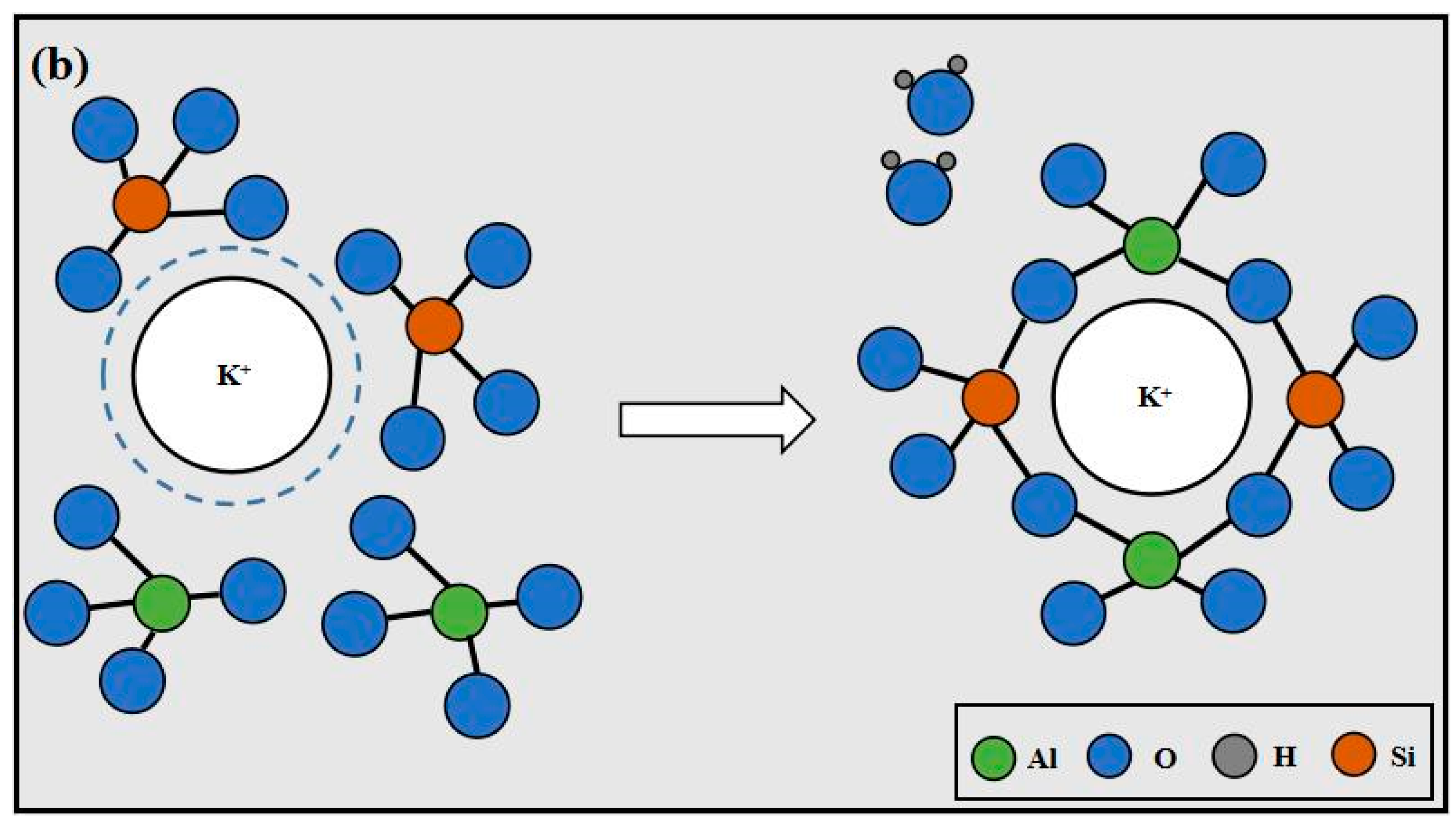
3.7. Mechanisms
3.8. Economic Evaluation
4. Conclusions and Future Prospects
- (1)
- The setting time of K-AAS is shorter than that of N-AAS at sub-zero temperature. The early compressive and flexural strengths (1 day) of K-AAS mortar are 130.4% and 72.3% higher than those of N-AAS, the 7-day compressive and flexural strengths of K-AAS mortar increased by 75.6% and 51.6%, and the 28-day compressive and flexural strengths of K-AAS mortar, respectively, increased by 49% and 33.7% compared with N-AAS mortar at a temperature of −10 °C. Therefore, K-AAS mortar shows a more excellent increase of strength compared with N-AAS mortar at sub-zero temperature, indicating that K-AAS mortar has a greater application potential in weather construction.
- (2)
- The hydration heat flow and cumulative hydration heat release of K-AAS is higher than that of N-AAS, indicating that K-AAS has a higher reaction degree compared with N-AAS. DTG/TG and ATR-FTIR spectra show that K-AAS generate more gel product C(-A)-S-H compared with N-AAS at same curing temperature, and as the temperature decreases, the gap is more remarkable. The results show that K+ is more beneficial to mechanical strength than Na+.
- (3)
- The microstructure of K-AAS is denser than N-AAS at the same curing temperature because potassium silicate activator has stronger excitation ability and more products of C(-A)-S-H are formed. As the temperature decreases, the excitation effect of potassium silicate is more remarkable than sodium silicate. K+ is more beneficial to the refinement of pore structure of AAS at sub-zero temperature.
- (4)
- The existence form of K+ and Na+ is hydrated ions in the system, which is surrounded by a layer of water film, and the water film of Na+ is much thicker than that of K+. In order to participate in the reaction, K+ and Na+ must take off the water film by absorbing energy. Under the same energy applied, a greater quantity of K+ can take off the water film, and more alkali metal cations can participate in the reaction. This accelerates the polymerization reaction to enhance strength by generating more polymerization products.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, J.; Li, Y.; Ouyang, P.; Yang, Y. Hydration of the silica fume-Portland cement binary system at lower temperature. Constr. Build. Mater. 2015, 93, 919–925. [Google Scholar] [CrossRef]
- Çullu, M.; Arslan, M. The effects of antifreeze use on physical and mechanical properties of concrete produced in cold weather. Compos. Part B 2013, 50, 202–209. [Google Scholar] [CrossRef]
- Krylov, B.A. Cold weather concreting. Book review. J. Cold Reg. Eng. 1999, 3, 213–216. [Google Scholar]
- Yu, K.; Jia, M.; Yang, Y.; Liu, Y. A clean strategy of concrete curing in cold climate: Solar thermal energy storage based on phase change material. Appl. Energy 2023, 331, 120375. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, F.; Yu, K.; Yang, Y. Experimental and numerical research on development of synthetic heat storage form incorporating phase change materials to protect concrete in cold weather. Renew. Energy 2020, 149, 1424–1433. [Google Scholar] [CrossRef]
- Bakharev, T.; Sanjayan, J.G.; Cheng, Y. Alkali activation of Australian slag cements. Cem. Concr. Res. 1999, 29, 113–120. [Google Scholar] [CrossRef]
- Gruskovnjak, A.; Lothenbach, B.; Holzer, L.; Figi, R.; Winnefeld, F. Hydration of alkali-activated slag: Comparison with ordinary Portland cement. Adv. Cem. Res. 2006, 18, 119–128. [Google Scholar] [CrossRef]
- FernándezJiménez, A.; Puertas, F.; Palomo, J.G. Alkali-activated slag mortars: Mechanical strength behaviour. Cem. Concr. Res. 1999, 29, 1313–1321. [Google Scholar] [CrossRef]
- Sun, Q.; Li, T.; Liang, B. Preparation of a New Type of Cemented Paste Backfill with an Alkali-Activated Silica Fume and Slag Composite Binder. Materials 2020, 13, 372. [Google Scholar] [CrossRef]
- Mater, Y.; Kamel, M.; Karam, A.; Bakhoum, E. ANN-Python prediction model for the compressive strength of green concrete. Constr. Innov. 2021, 8, 145. [Google Scholar] [CrossRef]
- Jiayuan, Y.; Zhang Wensheng, Z.; Di, S.; Wang, H.; Wang, Z. Effect of Low-temperature Curing and Water Addition Method on Strength of Geopolymers Synthesized from Ore dressing Tailing of Bauxite. Bull. Chin. Ceram. Soc. 2013, 32, 537–541. [Google Scholar]
- Zhang, G.; Yang, H.; Ju, C.; Yang, Y. Novel selection of environment-friendly cementitious materials for winter construction: Alkali-activated slag/Portland cement. J. Clean. Prod. 2000, 258, 120592. [Google Scholar] [CrossRef]
- Ju, C.; Liu, Y.; Jia, M.; Yu, K.; Yu, Z.; Yang, Y. Effect of calcium oxide on mechanical properties and microstructure of alkali-activated slag composites at sub-zero temperature. J. Build. Eng. 2020, 32, 101561. [Google Scholar] [CrossRef]
- Xiao, X.; Ming, X.; Guang, X.; Jingting, L. The effect of different conditions on the strength of alkali slag concrete in low temperature. Constr. Des. Proj. 2017, 1007–9467, 10-0071-03. [Google Scholar]
- Wang, K. Reaction Mechanism and Applications of Alkali-Based Geopolymers at Low Temperature and Vacuum Conditions. Ph.D. Thesis, Guangxi University, Nanning, China, 2016. [Google Scholar]
- Lizcano, M.; Kim, H.S.M.; Basu, S.; Radovic, M. Mechanical properties of sodium and potassium activated metakaolin-based geopolymers. J. Mater. Sci. 2012, 47, 2607–2616. [Google Scholar] [CrossRef]
- Król, M.; Rożek, P.; Chlebda, D.; Mozgawa, W. Influence of alkali metal cations/type of activator on the structure of alkali-activated fly ash-ATR-FTIR studies. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 198, 33–37. [Google Scholar] [CrossRef]
- Kinrade, S.D.; Pole, D.L. Effect of alkali-metal cations on the chemistry of aqueous silicate solutions. Inorg. Chem. 1992, 31, 4558–4563. [Google Scholar] [CrossRef]
- Palomo, A.; Grutzeck, M.W.; Blancoa, M.T. Alkali-activated fly ashes: A cement for the future. Cem. Concr. Res. 1999, 29, 1323–1329. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A.; Criado, M. Alkali-activated fly ash binders. A comparative study between sodium and potassium activators. Mater. Constr. 2006, 56, 51–55. [Google Scholar]
- Jiao, Z.; Wang, Y.; Zheng, W.; Huang, W. Effect of the activator on the performance of alkali-activated slag mortars with pottery sand as fine aggregate. Constr. Build. Mater. 2019, 197, 83–90. [Google Scholar] [CrossRef]
- Shao, Q.F.; Ming, D.; Chen, S.H.; Chao, W.; Xie, X.H. Study on quantitative analysis of cross linked copolymer by IR. Spectrosc. Spectr. Anal. 2008, 27, 2445–2447. [Google Scholar]
- Sindhunata, J.S.J.; van Deventer, G.; Lukey, C.; Xu, H. Effect of curing temperature and silicate concentration on fly-ash-based geopolymerization. Ind. Eng. Chem. Res. 2006, 45, 3559–3568. [Google Scholar] [CrossRef]
- Chang, J. A study on the setting characteristics of sodium silicate-activated slag pastes. Cem. Concr. Res. 2003, 33, 1005–1011. [Google Scholar] [CrossRef]
- Aydın, S.; Baradan, B. Effect of activator type and content on properties of alkali-activated slag mortars. Compos. Part B Eng. 2014, 57, 166–172. [Google Scholar] [CrossRef]
- Abdalqader, A.F.; Jin, F.; Al-Tabbaa, A. Characterisation of reactive magnesia and sodium carbonate-activated fly ash/slag paste blends. Constr. Build. Mater. 2015, 93, 506–513. [Google Scholar] [CrossRef]
- Gu, K.; Jin, F.; Al-Tabbaa, A.; Shi, B.; Liu, J. Mechanical and hydration properties of ground granulated blastfurnace slag pastes activated with MgO-CaO mixtures. Constr. Build. Mater. 2014, 69, 101–108. [Google Scholar] [CrossRef]
- Jin, F.; Gu, K.; Al-Tabbaa, A. Strength and hydration properties of reactive MgO-activated ground granulated blastfurnace slag paste. Cem. Concr. Compos. 2015, 57, 8–16. [Google Scholar] [CrossRef]
- Yu, P.; Kirkpatrick, R.J.; Poe, B.; Mcmillan, P.F.; Cong, X. Structure of Calcium Silicate Hydrate (C-S-H): Near-, Mid-, and Far-Infrared Spectroscopy. J. Am. Ceram. Soc. 2010, 82, 742–748. [Google Scholar] [CrossRef]
- Li, N.; Gu, H.; Zhao, H. Refractory; Metallurgical Industry Press: Beijing, China, 2010; pp. 305–309. [Google Scholar]
- Song, P. To from hydrated ion and to have an influence on the nature of inorganic salt. J. Qinghai Jr. Teach. Coll. 1998, 4, 62–64. [Google Scholar]
- Krivenko, P.V. Alkaline Cements. In Proceedings of the 9th International Congress on the Chemistry of Cements, New Delhi, India, 23 November–28 November 1992; Volume 4, pp. 482–494. [Google Scholar]
- Glukhovskii, V.D.; Pashkov, I.A. Starchevskaya E A, et al, Soil silicate concrete for hydraulic and irrigation structures. Hydrotech. Constr. 1967, 12, 120–124. [Google Scholar] [CrossRef]
- Arbin, K.; Palomo, A.; Jiménez, A.F. Alkali-activated blends of calcium aluminate cement and slag/diatomite. Ceram. Int. 2013, 39, 9237–9245. [Google Scholar] [CrossRef]
- Rovnaník, P. Effect of curing temperature on the development of hard structure of metakaolin-based geopolymer. Constr. Build. Mater. 2010, 24, 1176–1183. [Google Scholar] [CrossRef]
- Collins, K.D. Ions from the Hofmeister series and osmolytes: Effects on proteins in solution and in the crystallization process. Methods 2004, 34, 300–311. [Google Scholar] [CrossRef]
- Takano, M.; Ogata, K.; Kawauchi, S.; Satoh, M.; Komiyama, J. Ion-specific swelling behavior of poly (N-vinyl-2-pyrrolidone) gel: Correlations with water hydrogen bond and non-freezable water. Polym. Gels Netw. 1998, 6, 217–232. [Google Scholar] [CrossRef]
- Nightingale, E.R. Phenomenological theory of ion solvation, Effective radii of hydrated ions. J. Chem. Phys. 1959, 63, 1381–1387. [Google Scholar] [CrossRef]
- Jang, E.S.; Kamcev, J.; Kobayashi, K.; Yan, N.; Sujanani, R.; Dilenschneider, T.J.; Park, H.B.; Paul, D.R.; Freeman, B.D. Influence of water content on alkali metal chloride transport in cross-linked Poly(ethylene glycol) Diacrylate.1. Ion sorption. Polymer 2019, 178, 1–10. [Google Scholar] [CrossRef]
- Binder, H.; Zschornig, O. The effect of metal cations on the phase behavior and hydration characteristics of phospholipid membranes. Chem. Phys. Lipids 2002, 115, 39–61. [Google Scholar] [CrossRef]
- Gregory, J.K.; Clary, D.C.; Liu, K.; Brown, M.G.; Saykally, R.J. The water dipole moment in water clusters. Science 1997, 275, 814–817. [Google Scholar] [CrossRef]



| Chemical Composition | SiO2 | CaO | Al2O3 | Fe2O3 | MgO | SO3 | FeO | TiO2 | Loss |
|---|---|---|---|---|---|---|---|---|---|
| Mass fraction | 31.85 | 35.5 | 16.69 | 0.16 | 9.52 | — | 0.76 | 0.64 | 0.25 |
| Samples | GGBS | KOH | Potassium Silicate Solution | NaOH | Sodium Silicate Solution | Fine Sand | Water |
|---|---|---|---|---|---|---|---|
| K-AAS | 600 | 31.92 | 157.28 | 0 | 0 | 1200 | 184.96 |
| N-AAS | 600 | 0 | 0 | 20.16 | 197.24 | 1200 | 135.46 |
| Properties | Na+ | K+ |
|---|---|---|
| Ionic radius, (nm) [38] | 9.5 | 13.3 |
| Surface charge densit, (mC/cm2) [39] | 0.141 | 0.072 |
| Hydrated ionic radius, (nm) [38] | 35.8 | 33.1 |
| Hydration energy, (kJ/mol) [40] | −365 | −271 |
| Raw Materials | Unit Price (CNY/kg) | Usage Cost (CNY/kg) | |
|---|---|---|---|
| K-AAS mortar | N-AAS mortar | ||
| Potassium silicate solution | 15 | 1.52 | 1.4 |
| Sodium silicate solution | 12 | ||
| KOH | 18 | ||
| NaOH | 14 | ||
| GGBS | 0.5 | ||
| Fine sand | 0.05 | ||
| Water | 0.004 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ju, C.; Ye, R.; Wu, Y.; Sun, P.; Liu, Y.; Yang, Y. Effect of Alkali Cation on Performance of Alkali-Activated Slag Mortar in Cold Environments. Separations 2022, 9, 450. https://doi.org/10.3390/separations9120450
Ju C, Ye R, Wu Y, Sun P, Liu Y, Yang Y. Effect of Alkali Cation on Performance of Alkali-Activated Slag Mortar in Cold Environments. Separations. 2022; 9(12):450. https://doi.org/10.3390/separations9120450
Chicago/Turabian StyleJu, Cheng, Rongrong Ye, Yunfei Wu, Pengfei Sun, Yushi Liu, and Yingzi Yang. 2022. "Effect of Alkali Cation on Performance of Alkali-Activated Slag Mortar in Cold Environments" Separations 9, no. 12: 450. https://doi.org/10.3390/separations9120450
APA StyleJu, C., Ye, R., Wu, Y., Sun, P., Liu, Y., & Yang, Y. (2022). Effect of Alkali Cation on Performance of Alkali-Activated Slag Mortar in Cold Environments. Separations, 9(12), 450. https://doi.org/10.3390/separations9120450







