Essential Oils of Taxodium distichum Winter Leaves Obtained by Supercritical Carbon Dioxide Extraction Method and Hydrodistillation
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material
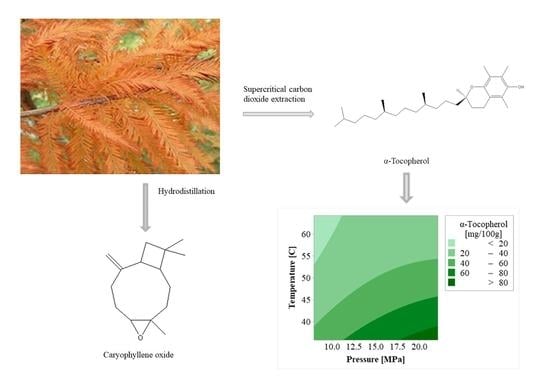
2.3. Extractions
2.4. GC-MS Analysis
3. Results
4. Discussion
5. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Farjon, A. The Kew Review: Conifers of the World. Kew Bull. 2018, 73, 8. [Google Scholar] [CrossRef]
- Debreczy, Z.; Racz, I. Conifers Around the World; DendroPress Ltd.: Budapest, Hungary, 2011. [Google Scholar]
- Christenhusz, M.J.M.; Reveal, J.L.; Fajron, A.; Gardner, M.F.; Mill, R.R.; Chase, M.W. A new classification and linear sequence of extant gymnosperms. Phytotaxa 2011, 19, 55–70. [Google Scholar] [CrossRef]
- Biffin, E.; Brodribb, T.J.; Hill, R.S.; Thomas, P.; Lowe, A.J. Leaf evolution in Southern Hemisphere conifers tracks the angiosperm ecological radiation. Proc. R. Soc. B Biol. Sci. 2012, 279, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Kunzmann, L.; Kvacek, Z.; Hans Mai, D.; Walther, H. The genus Taxodium (Cupresseaceae) in the Palaeogene and Neogene of Central Europe. Rev. Palaeobot. Palynol. 2009, 153, 153–183. [Google Scholar] [CrossRef]
- Li, C.X.; Zhong, Z.C.; Geng, Y.H.; Schneider, R. Comparative studies on physiological and biochemical adaptation of Taxodium distichum and Taxodium ascendens seedlings to different soil water regimes. Plant Soil 2010, 329, 481–494. [Google Scholar] [CrossRef]
- Burton, T.M. Swamps–Wooded Wetlands. In Encyclopedia of Inland Waters; Likens, G.E., Ed.; Academic Press: Amsterdam, The Netherlands, 2009; pp. 549–557. [Google Scholar] [CrossRef]
- Howard, A.L. Deciduous Cypress (Taxodium distichum). Nature 1944, 154, 775–776. [Google Scholar] [CrossRef]
- Pezeshki, S.R.; Santos, M.I. Relationships among rhizosphere oxygen deficiency, root restriction, photosynthesis, and growth in bald cypress (Taxodium distichum L.) seedlings. Photosynthetica 1998, 35, 381–390. [Google Scholar] [CrossRef]
- Sijacic-Nikolic, M.; Vilotic, D.; Veselinovic, M.; Mitrovic, S.; Jokanovic, D. Bald cypress (Taxodium distichum (L.) Rich.) in the protected ares „Veliko ratno ostrvo”. Bull. Fac. For. 2010, 103, 173–184. [Google Scholar] [CrossRef]
- Ninić-Todorović, J.; Ocokoljić, M. Ekofiziološke karakteristike taksodijuma (Taxodium distichum L. Rich.) u parkovima Novog Sada. In Proceedings of the Environmental Protection of Urban and Suburban Settlement, Eko-Konferencija, Novi Sad, Serbia, 26–29 September 2001. [Google Scholar]
- Ninic-Todorovic, J.; Ocokoljic, M. Varijabilnost populacija taksodijuma (Taxodium distichum (L.) Rich.) u parkovima Novog Sada. In Proceedings of the 7th Symposium on Flora of Southeastern Serbia and Neighbouring Regions, Dimitrovgrad, Yugoslavia, 6–9 June 2002. [Google Scholar]
- Padalia, R.C.; Verma, R.S.; Chauhan, A.; Goswami, P.; Chanotiya, C.S. Compositional and enantiomeric analysis of the essential oil of Taxodium distichum from India. Nat. Prod. Commun. 2016, 11, 419–422. [Google Scholar] [CrossRef]
- Flamini, G.; Cioni, P.L.; Morelli, I. Investigation of the essential oil of feminine cones, leaves and branches of Taxodium distichum from Italy. J. Essent. Oil Res. 2000, 12, 310–312. [Google Scholar] [CrossRef]
- Adams, R.P.; Arnold, M.A.; King, A.R.; Denny, G.C. Seasonal variation in the leaf essential oil of Taxodium distichum (Cupressaceae). Phytologia 2012, 94, 91–102. [Google Scholar]
- Muzika, R.-M.; Campbell, C.L.; Hanover, J.W.; Smith, A.L. Comparison of techniques for extracting volatile compounds from conifer needles. J. Chem. Ecol. 1990, 16, 2713–2722. [Google Scholar] [CrossRef]
- Reverchon, E. Supercritical fluid extraction and fractionation of essential oils and related products. J. Supercrit Fluids 1997, 10, 1–37. [Google Scholar] [CrossRef]
- Trubetskaya, A.; Budarin, V.; Arshadi, M.; Magajhaes, D.; Kazanc, F.; Hunt, A.J. Supercritical extraction of biomass as an effective pretreatment step for the char yield control in pyrolysis. Renew. Energy 2021, 170, 107–117. [Google Scholar] [CrossRef]
- Trubetskaya, A.; Hunt, A.J.; Budarin, V.L.; Umeki, K. Supercritical extraction and microwave activation of wood wastes for enhanced syngas production and generation of fullerene-like soot particles. Fuel Process Technol. 2020, 212, 106633. [Google Scholar] [CrossRef]
- Orav, A.; Kailas, T.; Koel, M. Simultaneous distillation, extraction and supercritical fluid extraction for isolating volatiles and other materials from conifer needles. J. Essent. Oil Res. 1998, 10, 387–393. [Google Scholar] [CrossRef]
- Alaydi, H.; Downey, P.; McKeon-Bennett, M.; Beletskaya, T. Supercritical-CO2 extraction, identification and quantification of polyprenol as a bioactive ingredient from Irish trees species. Sci. Rep. 2021, 11, 7461. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2000. [Google Scholar]
- Vesilind, P.A. The Rosin-Rammler particle size distribution. Resour. Recov. Conserv. 1980, 5, 275–277. [Google Scholar] [CrossRef]
- Djapic, N. Parrotia persica Yellow and Amber Leaves’ Lipophilic Phytochemicals Obtained by Supercritical Carbon Dioxide Extracton. Molecules 2022, 27, 5237. [Google Scholar] [CrossRef]
- Martinez, J.L.; Vance, S.W. Supercritical extraction plants. In Supercritical Fluid Extraction of Nutraceuticals and Bioactive Compounds, 1st ed.; Martinez, J.L., Ed.; CRC Press: Boca Raton, FL, USA, 2007; pp. 25–28. [Google Scholar] [CrossRef]
- Bas, D.; Boyaci, I.H. Modeling and optimization I: Usability of response surface methodology. J. Food Eng. 2007, 78, 836–845. [Google Scholar] [CrossRef]
- Pharmacopoea Iugoslavica, Editio Quarta (Ph. Iug. IV); Federal Institute of Public Health: Belgrade, Yougoslavia, 1984; Volume 2.
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corp.: Carol Stream, IL, USA, 2007. [Google Scholar]
- Adams, R. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing: Carol Stream, IL, USA, 1995. [Google Scholar]
- de Lucas, A.; Martinez de la Ossa, E.J.; Rincon, J.; Blanco, M.A.; Gracia, I. Supercritical fluid extraction of tocopherol concentrates from olive tree leaves. J. Super Fluids 2002, 22, 221–228. [Google Scholar] [CrossRef]
- Barzotto, I.L.M.; Santos, K.A.; da Silva, E.A.; Sene, A.C.; da Silva, N.S.; Vieira, L. Supercritical extraction of Eugenia involucrate leaves: Influence of operating conditions on yield and α-tocopherol content. J. Super Fluids 2019, 143, 55–63. [Google Scholar] [CrossRef]
- vom Dorp, K.; Hoelzl, G.; Plohmann, C.; Eisenhut, M.; Abraham, M.; Weber, A.P.; Hanson, A.D.; Doermann, P. Remobilization of phytol from chlorophyll degradation is essential for tocopherol synthesis and growth of Arabidopsis. Plant Cell 2015, 27, 2846–2859. [Google Scholar] [CrossRef] [PubMed]
- Mach, J. Phytol from degradation of chlorophyll feeds biosynthesis of tocopherols. Plant Cell 2015, 27, 2676. [Google Scholar] [CrossRef]


| Run | Pressure [MPa], X1 | Temperature [°C], X2 | Extraction Yield [%] |
|---|---|---|---|
| 1. | 22.07 | 50 | 3.86 |
| 2. | 15 | 64 | 4.08 |
| 3. | 7.93 | 50 | 1.93 |
| 4. | 10 | 40 | 2.84 |
| 5. | 15 | 50 | 3.97 |
| 6. | 15 | 50 | 3.91 |
| 7. | 20 | 60 | 3.92 |
| 8. | 10 | 60 | 2.98 |
| 9. | 15 | 36 | 3.64 |
| 10. | 15 | 50 | 4.02 |
| 11. | 20 | 40 | 3.80 |
| 12. | 15 | 50 | 3.86 |
| 13. | 15 | 50 | 3.98 |
| Term | Coefficient | Standard Error Coefficient | T-Value | p-Value |
|---|---|---|---|---|
| Intercept | 3.948 | 0.058 | 67.88 | 0.000 |
| X1 | 0.578 | 0.046 | 12.59 | 0.000 |
| X2 | 0.110 | 0.046 | 2.40 | 0.048 |
| X1·X1 | −0.525 | 0.049 | −10.64 | 0.000 |
| X2·X2 | −0.042 | 0.049 | −0.85 | 0.421 |
| X1·X2 | −0.005 | 0.065 | −0.08 | 0.941 |
| R2 = 0.9754 |
| No. | Compound | Run 1 | Run 2 | Run 3 | Run 4 | Run 5 | Run 6 | Run 7 | Run 8 | Run 9 | Run 10 | Run 11 | Run 12 | Run 13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Caryophyllene oxide | - | 0.92 | - | - | - | - | 1.00 | - | - | 0.94 | - | 1.01 | 1.05 |
| 2. | Hexahydrofarnesyl acetone | 0.91 | 0.86 | 0.31 | - | 1.32 | 0.42 | 0.88 | 0.86 | - | 0.82 | - | 0.90 | 0.92 |
| 3. | Neophytadiene | 39.64 | 75.93 | 84.71 | 17.64 | 28.72 | 20.58 | 75.82 | 23.50 | 35.80 | 76.38 | 67.34 | 77.03 | 76.27 |
| 4. | Eicosane | 7.81 | 13.08 | 4.36 | 14.27 | 1.68 | - | 13.71 | - | - | 12.91 | - | 13.56 | 14.02 |
| 5. | Stearyl aldehyde | 18.93 | 18.39 | 21.06 | 5.78 | 15.41 | 9.62 | 18.62 | 22.36 | 8.64 | 17.83 | 11.53 | 18.54 | 18.67 |
| 6. | 1-Octadecanol | 3.37 | 2.56 | 4.18 | 1.83 | - | - | 2.71 | 1.62 | 0.84 | 2.77 | - | 2.92 | 2.68 |
| 7. | Phytol | 45.06 | 26.78 | 27.30 | 11.19 | 41.12 | 48.72 | 24.98 | 36.34 | 11.17 | 25.06 | 38.16 | 25.93 | 26.11 |
| 8. | Sandaracopimaradiene | 3.26 | 2.93 | 6.13 | 5.32 | 4.87 | 3.96 | 2.76 | 4.82 | 2.68 | 3.14 | 2.93 | 2.85 | 3.05 |
| 9. | Geranylgeraniol | 2.36 | 4.96 | 3.54 | 26.92 | 2.77 | - | 4.85 | 3.92 | 3.39 | 5.18 | 10.76 | 5.02 | 5.34 |
| 10. | Ferruginol | 4.91 | 1.04 | 4.39 | - | 4.60 | - | 0.91 | - | - | 1.09 | 5.86 | 0.99 | 1.19 |
| 11. | m-Pentadecylphenol | 10.76 | 7.46 | 21.86 | 6.91 | 8.07 | 5.80 | 7.02 | 6.61 | 16.39 | 7.23 | 7.36 | 7.68 | 7.63 |
| 12. | α-Tocopherol | 32.85 | 39.83 | 20.96 | 68.03 | 22.98 | 50.08 | 40.54 | 77.36 | 19.07 | 39.94 | 45.87 | 40.68 | 40.03 |
| 13. | β-Sitosterol | - | 2.21 | 3.60 | - | - | - | 1.83 | 4.12 | - | 1.96 | 6.58 | 2.07 | 2.71 |
| Term | Coefficient | Standard Error Coefficient | T-Value | p-Value |
|---|---|---|---|---|
| Constant | 40.20 | 1.78 | 22.53 | 0.000 |
| X1 | 9.38 | 1.41 | 6.65 | 0.000 |
| X2 | −17.27 | 1.41 | −12.24 | 0.000 |
| X1·X1 | −2.03 | 1.51 | −1.34 | 0.221 |
| X2·X2 | 3.98 | 1.51 | 2.63 | 0.034 |
| X1·X2 | −4.35 | 1.99 | −2.18 | 0.065 |
| R2 = 0.9676 |
| No. | Compound | RI | % |
|---|---|---|---|
| 1. | n-nonanal | 1100 | 0.36 |
| 2. | α-Campholenal | 1122 | 0.19 |
| 3. | trans-Pinocarveol | 1135 | 0.22 |
| 4. | Borneol | 1165 | 1.57 |
| 5. | p-Mentha-1,5-dien-8-ol | 1166 | 0.09 |
| 6. | Terpinen-4-ol | 1174 | 0.05 |
| 7. | α-Terpineol | 1186 | 0.31 |
| 8. | Myrtenol | 1194 | 0.73 |
| 9. | Verbenone | 1204 | 0.88 |
| 10. | trans-Carveol | 1215 | 0.14 |
| 11. | Bornyl acetate | 1287 | 11.36 |
| 12. | trans-Pinocarvyl acetate | 1298 | 0.44 |
| 13. | Myrtenyl acetate | 1324 | 0.31 |
| 14. | trans-Carvyl acetate | 1339 | 0.28 |
| 15. | α-Terpinyl acetate | 1346 | 0.34 |
| 16. | Silphiperfol-4,7(14)-diene | 1358 | 0.03 |
| 17. | Ethyl decanoate | 1395 | 0.11 |
| 18. | trans-β-Caryophyllene | 1417 | 0.20 |
| 19. | trans-α-Ionone | 1428 | 0.19 |
| 20. | α-Humulene | 1452 | 0.11 |
| 21. | Geranyl acetone | 1453 | 0.41 |
| 22. | 2-Isopropenyl-4,8-dimethyl octahydronaphthalene | 1473 | 0.10 |
| 23. | ar-Curcumene | 1479 | 0.29 |
| 24. | trans-β-Ionone | 1487 | 0.09 |
| 25. | α-Selinene | 1498 | 0.08 |
| 26. | α-Muurolene | 1500 | 0.09 |
| 27. | β -Bisabolene | 1505 | 0.16 |
| 28. | γ-Cadinene | 1513 | 0.25 |
| 29. | trans-Calamenene | 1521 | 0.23 |
| 30. | α -Cadinene | 1537 | 0.11 |
| 31. | α -Calacorene | 1544 | 0.06 |
| 32. | Italicene epoxide | 1549 | 1.46 |
| 33. | Salviadienol | 1549 | 0.55 |
| 34. | trans-Nerolidol | 1561 | 0.23 |
| 35. | Caryophyllene oxide | 1582 | 55.56 |
| 36. | 4(14)-Salvialene-1-one | 1592 | 0.57 |
| 37. | Humulene epoxide I | 1593 | 0.84 |
| 38. | Humulene epoxide II | 1608 | 5.71 |
| 39. | Isoaromadendrene epoxide | 1612 | 0.33 |
| 40. | Humulene epoxide III | 1626 | 0.22 |
| 41. | allo-Aromadendrene epoxide | 1639 | 0.60 |
| 42. | Caryophylla-4(12),8(13)-dien-5-α-ol | 1639 | 2.28 |
| 43. | α-Muurolol (=Torreyol) | 1644 | 0.32 |
| 44. | β-Eudesmol | 1649 | 0.12 |
| 45. | α-Cadinol | 1652 | 0.38 |
| 46. | 14-Hydroxy-(Z)-caryophyllene | 1666 | 2.41 |
| 47. | 4-Hydroxy-9-epi-(E)-caryophyllene | 1668 | 3.65 |
| 48. | Germacra-4(15),5,10(14)-trien-1-α-ol | 1680 | 0.48 |
| 49. | α-Costol | 1765 | 0.27 |
| 50. | 14-Hydroxy-α-muurolene | 1779 | 0.06 |
| 51. | 8-Cedren-13-ol acetate | 1788 | 0.49 |
| 52. | 2-α-Acetoxy-amorpha-4,7(11)-diene | 1805 | 0.09 |
| 53. | Khusinol acetate | 1823 | 0.11 |
| 54. | Hexahydrofarnesyl acetone | 1838 | 0.14 |
| 55. | (5E,9E)-Farnesyl acetone | 1913 | 0.08 |
| 56. | Pimaradiene | 1948 | 0.07 |
| 57. | Ethyl hexadecanoate | 1992 | 0.10 |
| 58. | Abietatriene | 2055 | 0.02 |
| 59. | Phytol | 2111 | 0.07 |
| 60. | Ethyl linoleate | 2151 | 0.04 |
| 61. | Ethyl oleate | 2171 | 0.11 |
| 62. | Ethyl octadecanoate | 2196 | 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djapic, N. Essential Oils of Taxodium distichum Winter Leaves Obtained by Supercritical Carbon Dioxide Extraction Method and Hydrodistillation. Separations 2022, 9, 436. https://doi.org/10.3390/separations9120436
Djapic N. Essential Oils of Taxodium distichum Winter Leaves Obtained by Supercritical Carbon Dioxide Extraction Method and Hydrodistillation. Separations. 2022; 9(12):436. https://doi.org/10.3390/separations9120436
Chicago/Turabian StyleDjapic, Nina. 2022. "Essential Oils of Taxodium distichum Winter Leaves Obtained by Supercritical Carbon Dioxide Extraction Method and Hydrodistillation" Separations 9, no. 12: 436. https://doi.org/10.3390/separations9120436
APA StyleDjapic, N. (2022). Essential Oils of Taxodium distichum Winter Leaves Obtained by Supercritical Carbon Dioxide Extraction Method and Hydrodistillation. Separations, 9(12), 436. https://doi.org/10.3390/separations9120436