Abstract
The present work aims to provide an insight on the chemical constituents of essential oils obtained from six aromatic plants of the Langtang National Park (LNP), Nepal. LNP harbors an enriched biodiversity of medicinal and aromatic plants (MAPs). The composition of essential oils obtained from Rhododendron anthopogon D. Don, Artemisia dubia Wall. ex Besser, Boenninghausenia albiflora (Hook.) Rchb. ex Meisn., Elsholtzia fruticosa (D. Don) Rehder, Juniperus recurva Buch.-Ham. ex D. Don and Rhododendron setosum D. Don, were analyzed by Gas Chromatography-Mass Spectrometry (GC-MS). The essential oils were extracted via the hydrodistillation method using the Clevenger apparatus. GC-MS analysis showed that E-caryophyllene, α-pinene, γ-terpinene, β-pinene and δ-cadinene in Rhododendron anthopogon; santolina-triene, β-cubebene and sabinene in Artemisia dubia; β-myrcene, β-cubebene, E-β-ocimene and bicyclogermacrene in Boenninghausenia albiflora; perillene, eucalyptol and β-pinene in Elsholtzia fruticosa; δ-3-carene, cadina-1(6),4-diene and δ-cadinene in Juniperus recurva; trans-sabinyl acetate, sabinene, α-elemol and germacrene D in Rhododendron setosum are the principal components. The major compounds in the essential oil were monoterpenes and sesquiterpenes, representing almost 80% to 90% of the total constituents of the essential oil. In comparison to the previous studies, the results showed a significant difference in the qualitative composition of the essential oil. This is also the first report on the study of chemical constituents from the essential oil of R. setosum. Despite hosting a plethora of MAPs, only a limited number of studies have been carried out to identify their chemical and biological properties. Hence, further investigations on the MAPs of the Langtang region are highly essential to identify the major chemical constituents and explore their biological activities.
1. Introduction
LNP is considered one of the first mountain parks in Nepal. It covers a total area of 1710 km2, with an additional 420 km2 added as a buffer zone. Its territory expands over three districts of Nepal, viz., Rasuwa (56%), Nuwakot (6%) and Sindupalchowk (38%) district [1]. LNP has an enriched biodiversity, sheltering a wide range of MAPs, thus making this region one of the key protected areas of Nepal [2]. MAPs have a high potential to serve as the source of medicinal products [3], and primarily, they are used in cosmetics, the food industry and for extracting essential oils [4]. They can contribute to revenue collection and have the potential to uplift the socio-economic status of the Nepali people [5].
Aromatic plants are considered the storehouse of essential oils that are biosynthesized in specialized cells, such as osmophores, ducts, cavities and glandular trichomes [6]. They have garnered huge attention in traditional medicine [7], and their use in the pharmaceutical, food, agronomic, cosmetic, and perfume industries is burgeoning [8,9]. Essential oil mostly consists of volatile and low-molecular-weight organic compounds. The vast majority of these volatile compounds comprise of a complex mixture of terpenes (hydrocarbon and oxygenated terpenes): mono-, sesqui- and di-terpenes that are biogenerated by mevalonate and mevalonate-independent pathways [9,10]. Moreover, they also consist of phenolic compounds (eugenol and cinnamaldehyde), non-terpenoid components of essential oil that are produced via the shikimate pathway, along with ketones, aldehydes, alkanes and aliphatic alcohols [11,12].
Terpenes are one of the largest groups of natural products that consist of structurally diverse chemical components produced by living organisms to carry out a specific set of physiological roles, such as providing characteristic aroma, color and flavor to the plant species [13]. The classification of these naturally occurring chemical components depends on the number of isoprene units, an unsaturated C5H8 moiety that serves as the building block of terpenes [13]. The functions of terpenes are far-reaching, and they can serve as anticancer, antiplasmodial, antiviral, antioxidant, anti-diabetic and antidepressant agents [14,15].
By this means, a great deal of effort has been invested in studying the chemical constituents of essential oils. Conventionally, infrared spectroscopy (IR) in combination with nuclear magnetic resonance (NMR) for assigning the stereochemistry and ultraviolet spectroscopy (UV) were considered as a convenient technique for the identification of those components [16]. However, these days, GC-MS has been extensively used to study the chemical constituents of aromatic plant species from a myriad of plant families [17,18]. GC-MS is one of the most effective techniques to unveil the underlying chemical blueprint of an essential oil [19]. Specifically, using the integrated technique of gas chromatography and mass spectrometry, the qualitative composition of the essential oil is analyzed by comparing the mass spectra with the library and further confirmed by their retention indices and fragmentation patterns.
Most of the highly valued MAPs are found in the forests and grasslands at high altitudes in northern Nepal. The conditions are extreme at higher altitudes, and the plants growing in these habitats adapt themselves to different mechanisms of metabolite synthesis [20]. They are able to bear the adverse climatic conditions through novel biosynthetic pathways, leading to the development of new molecular structure. As a result, the chemical components found in such plants are unique, multifarious, and may differ from the ones that are found in lower regions [21]. Hence, an in-depth study of these plant species is of paramount importance, as it may lead to the identification of indispensable chemical entities. However, only a limited number of studies have been carried out to explore the aromatic plants and essential oils of Nepal [22,23,24,25,26,27].
As a result, the present work aims to explore and identify the chemical entities of essential oils from the aromatic plants, especially those at higher altitudes, in Nepal. The Langtang region serves as a habitat for 172 highly valued plant species, in which almost 100 species are used for medicinal purposes [28]. Nonetheless, the accounts of aromatic plants from this region, especially that may serve as a precursor for the extraction of essential oil, has been overlooked. In this aspect, six aromatic plant species, Rhododendron anthopogon D. Don, Artemisia dubia Wall. ex Besser, Boenninghausenia albiflora (Hook.) Rchb. ex Meisn., Elsholtzia fruticosa (D. Don) Rehder, Juniperus recurva Buch.-Ham. ex D. Don and Rhododendron setosum D. Don, have been collected from a small part of the LNP in the Rasuwa district, Nepal. To the best of our knowledge, this is the first report on the study of chemical constituents of essential oil, extracted from R. setosum, and an attempt to enrich the library of chemical components that are found in the essential oil of aromatic plants from the Langtang region in Nepal.
2. Materials and Methods
2.1. Plant Collection
Aerial parts of the plant species (wild), Rhododendron anthopogon D. Don, Artemisia dubia Wall. ex Besser, Boenninghausenia albiflora (Hook.) Rchb. ex Meisn., Elsholtzia fruticosa (D. Don) Rehder, Juniperus recurva Buch.-Ham. ex D. Don and Rhododendron setosum D. Don, were collected from the Langtang region of the Rasuwa district, Nepal, in the month of September, at an altitude ranging from 2600 m to 4300 m. The collected plant species were shade-dried for two weeks. The collection of plant species, as listed in Table 1, was identified by the National Herbarium and Plant Laboratories (KATH), Nepal.

Table 1.
List of collected plant species.
2.2. Reagents
All chemicals were commercially available and used as received without further purification. Anhydrous sodium carbonate (analytical grade) and dichloromethane (HPLC grade) were obtained from Fisher Scientific (Mumbai, India) and Merck (Mumbai, India), respectively. Eugenol, δ-3-carene, α-phellandrene, D-limonene, E-caryophyllene, p-cymene, α-pinene, β-pinene and eucalyptol were purchased from Sigma Aldrich (St. Louis, MO, USA).
2.3. Extraction of Essential Oil
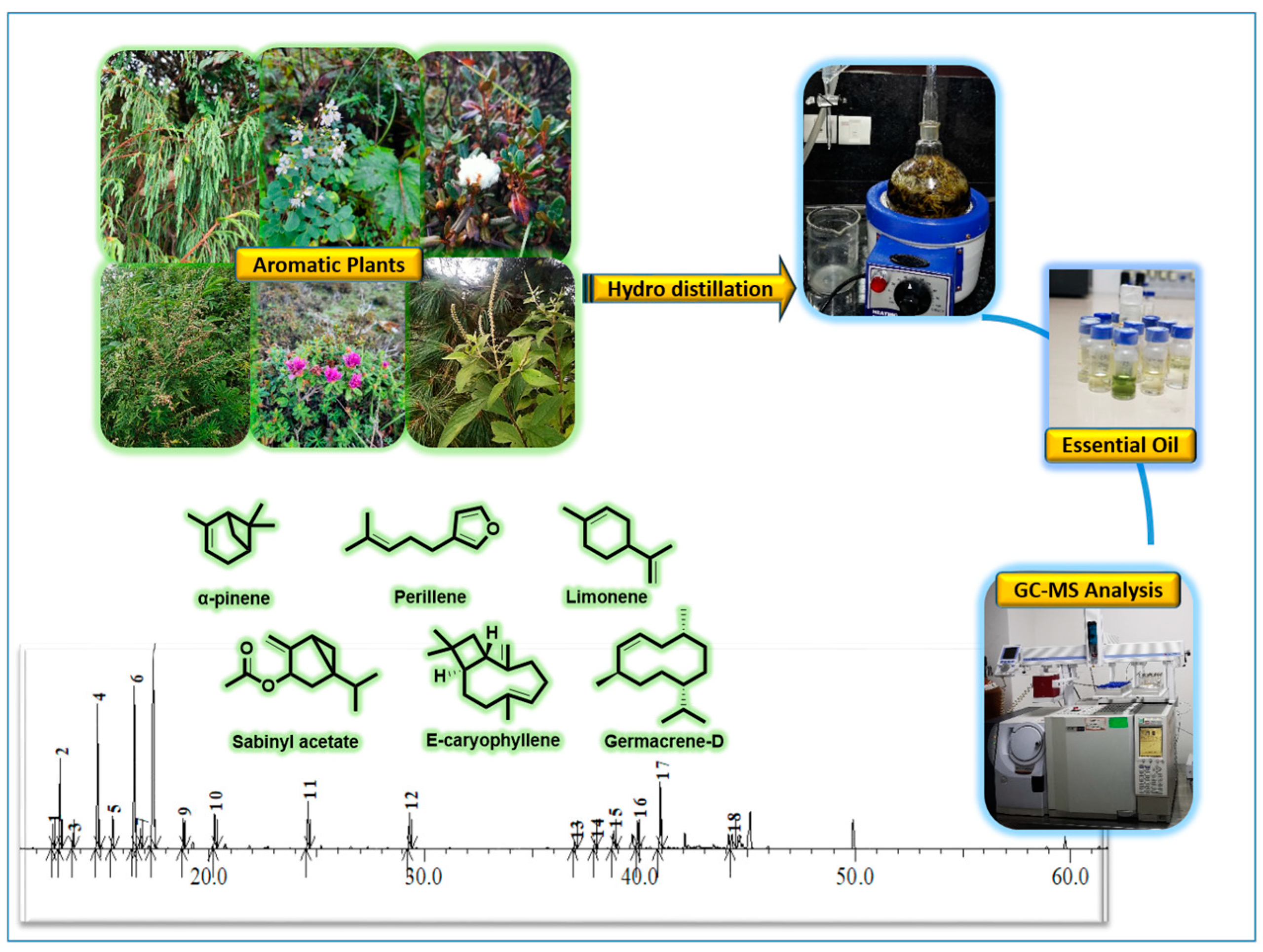
Essential oil from the shade-dried plant species was extracted by means of the hydro-distillation method. About 30–40 g of the plant parts were kept in a 500-mL round bottom flask and fitted to a Clevenger apparatus [29]. The oil was extracted for 4 h and collected in a glass vial. The moisture present in the essential oil was removed by using anhydrous sodium carbonate and stored under refrigerated conditions at 4°C. The experimental design for this study has been shown in Figure 1.
Figure 1.
Experimental design for the GC-MS analysis of essential oils.
2.4. GC-MS Analysis
The essential oils were analyzed by GC-MS. The GC-MS analysis was performed on a Shimadzu GC-MS-QP2010 Plus available at the Instrument Section of the Department of Plant Resources. The capillary column used for the analysis was SH-RTX-5MS (60 m × 0.32 mm × 0.25 µm) with a crossbond of 5% diphenyl/95% dimethyl polysiloxane as the stationary phase. The GC analysis was performed under the following conditions: column oven temperature, 50 °C; injection temperature, 250 °C; ion source temperature, 250 °C; interface temperature, 200 °C; split injection mode with a split ratio of 80; Helium with a pressure of 53.8 kPa; total gas flow, 112.3 mL/min; column flow, 1.35 mL/min. The GC-MS system starts with an initial oven temperature of 50 °C for 1 min, then increases to 230 °C at a rate of 3 °C for 9 min. Mass spectral detection was carried out in electron ionization mode by scanning at 40 to 350 m/z. The total time required for analyzing a single sample was 70 min.
The chemical components of the essential oils were identified by comparing their mass spectral fragmentation patterns with those in the National Institute of Standard Technology Library (NIST) 2017 and Flavor and Fragrance Natural and Synthetic Compounds (FFNSC) 4.0 library and also by comparing the retention times of the components with those of the reference compounds. The percentage of each component (Area %) is reported as raw percentages based on the total ion chromatogram (TIC) without standardization.
3. Results
The composition of essential oils obtained via hydrodistillation of leaves of Rhododendron anthopogon (AN), Artemisia dubia (AR), Boenninghausenia albiflora (BE), Elsholtzia fruticosa (EL), Juniperus recurva and Rhododendron setosum (SE) was determined by GC-MS. Figure 2, Figure 3, Figure 4, Figure 5, Figure 6 and Figure 7 show the TIC of the essential oils with peak numbers (A), extracted chromatograms of the prominent peaks with the names of the constituents (B, C), and the mass spectrum of the major constituent (C) of each essential oil. The composition of the essential oil (see the Supplementary Materials) as per the GC-MS analysis confirmed the presence of terpenoid components, mostly monoterpenes and sesquiterpenes.
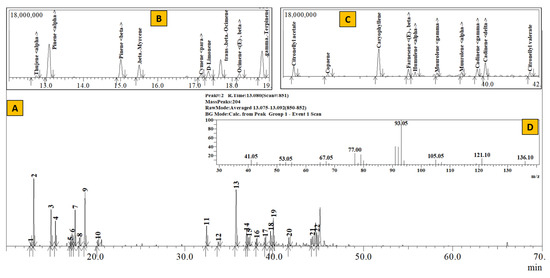
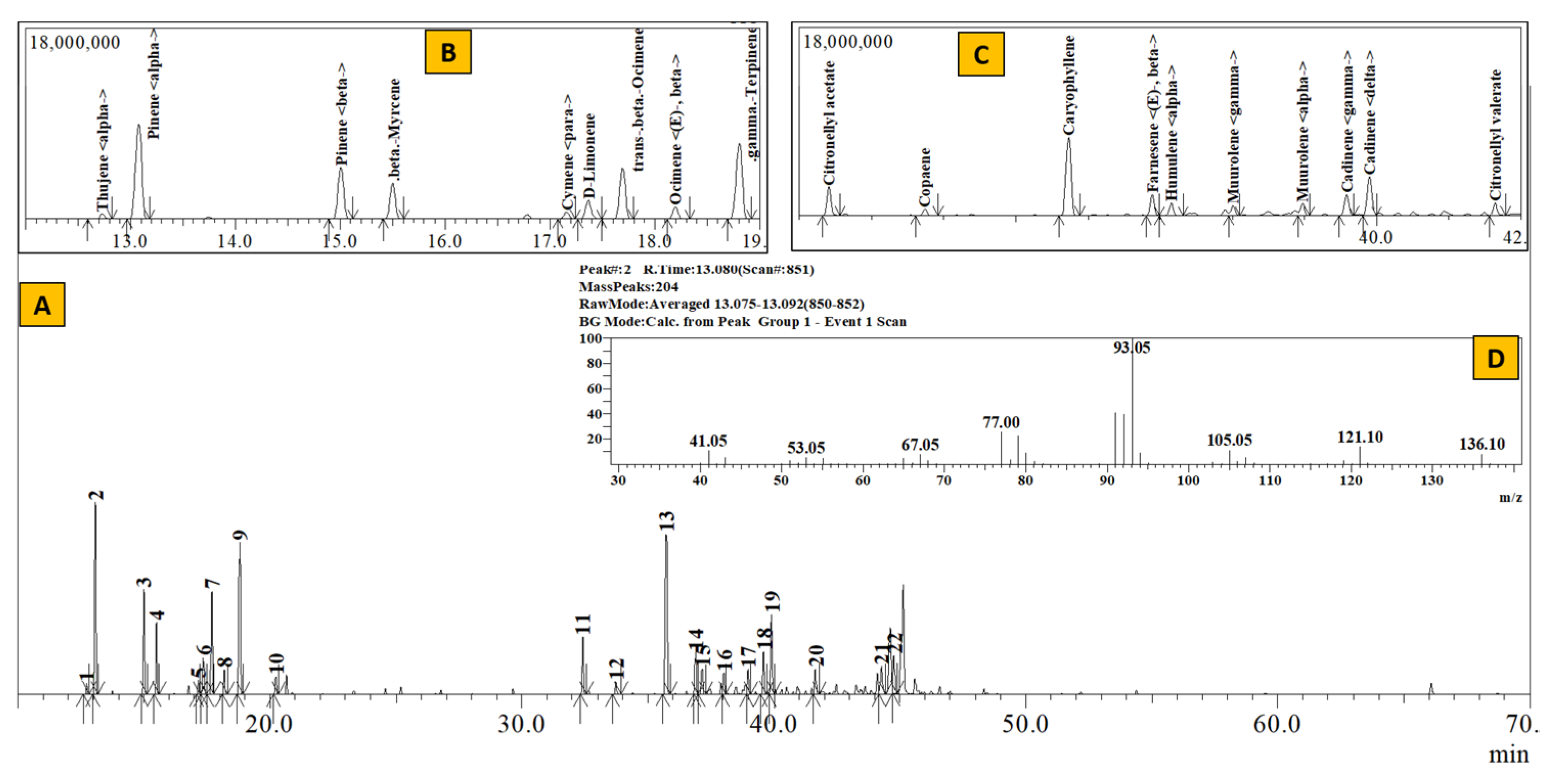
Figure 2.
TIC of essential oil from Rhododendron anthopogon (A), Inset: Extracted chromatogram from RT 12–20 min (B) and 32–42 min (C) and mass spectrum of Peak #2 (α–pinene) (D).
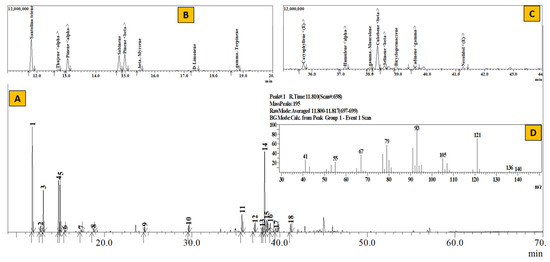
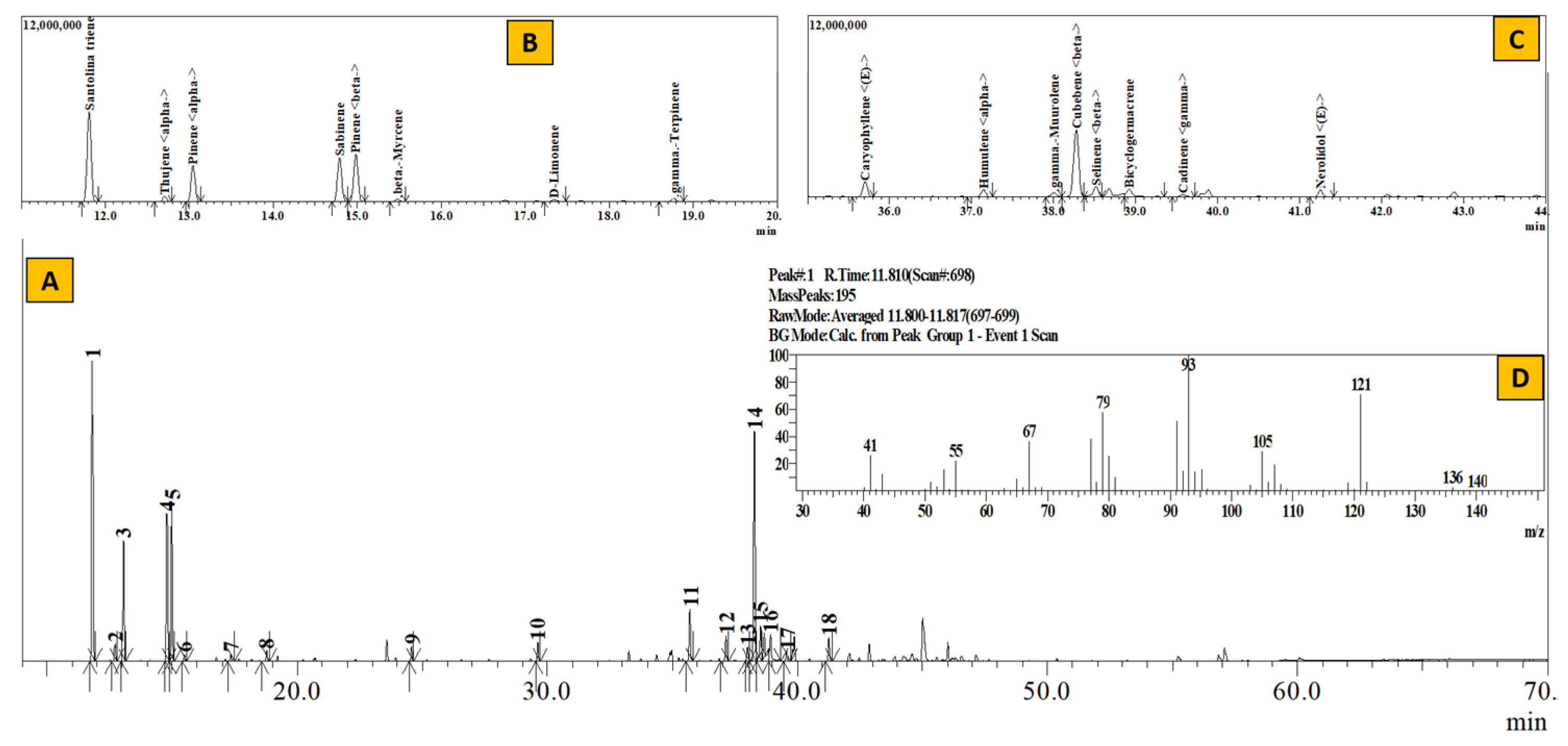
Figure 3.
TIC of essential oil from Artemisia dubia (A), Inset: Extracted chromatogram from RT 11–20 min (B) and 35–44 min (C) and mass spectrum of Peak #1 (santolina-triene) (D).
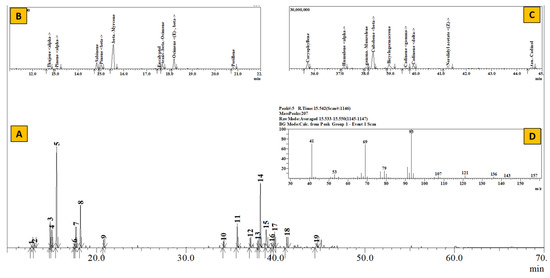
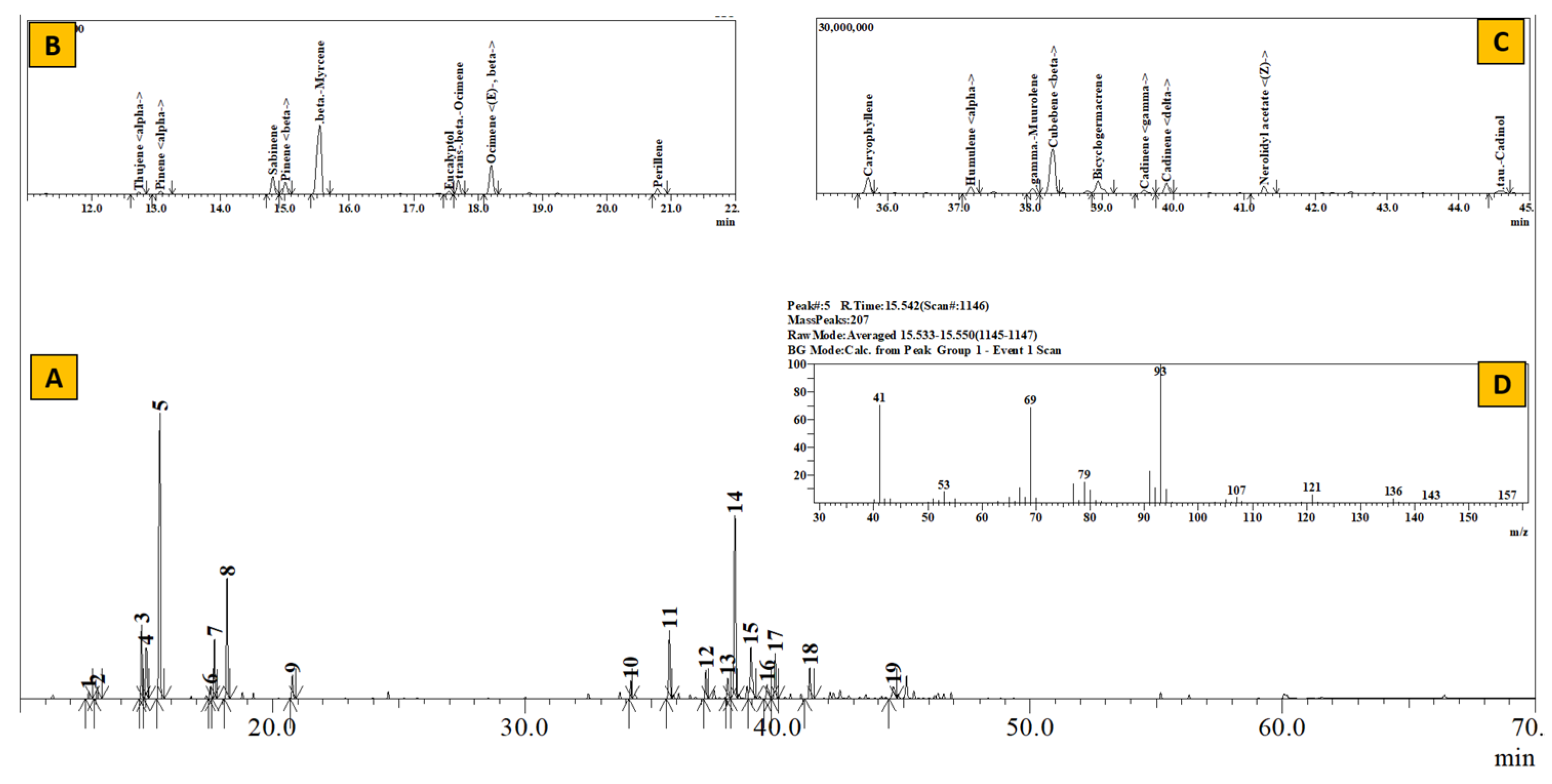
Figure 4.
TIC of essential oil from Boenninghausenia albiflora (A), Inset: Extracted chromatogram from RT 11–22 min (B) and 35–45 min (C) and mass spectrum of Peak #5 (β-myrcene) (D).
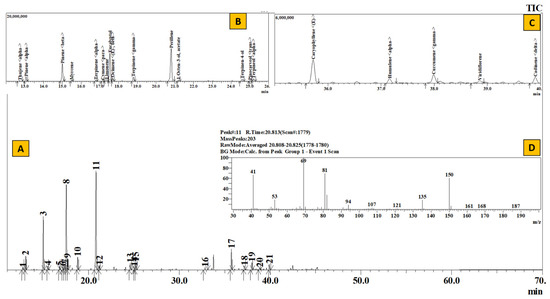
Figure 5.
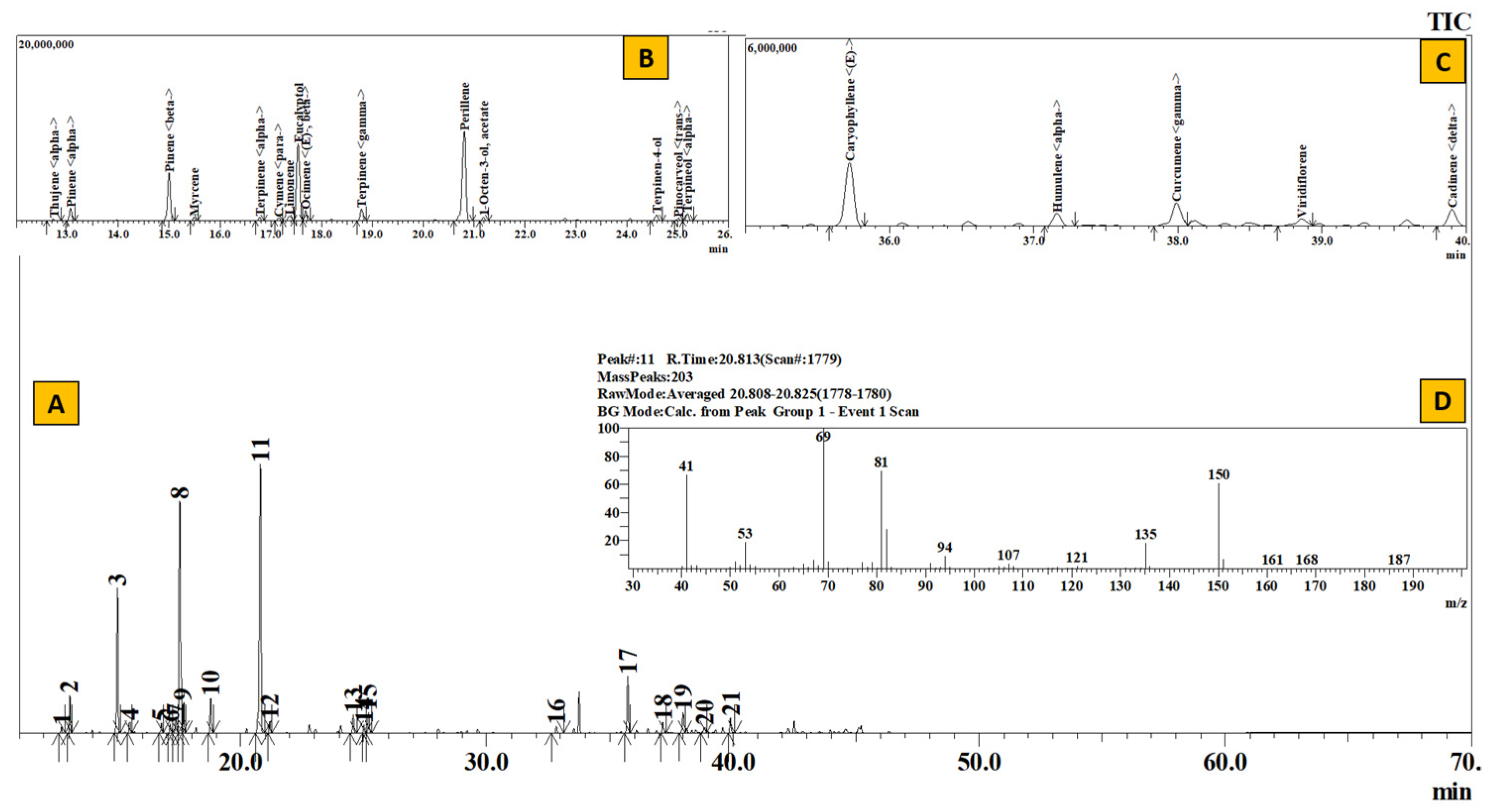
TIC of essential oil from Elsholtzia fruticosa (A), Inset: Extracted chromatogram from RT 12–26 min (B) and 35–40 min (C) and mass spectrum of Peak #11 (Perillene) (D).
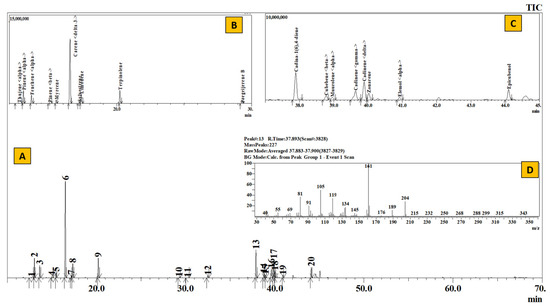
Figure 6.
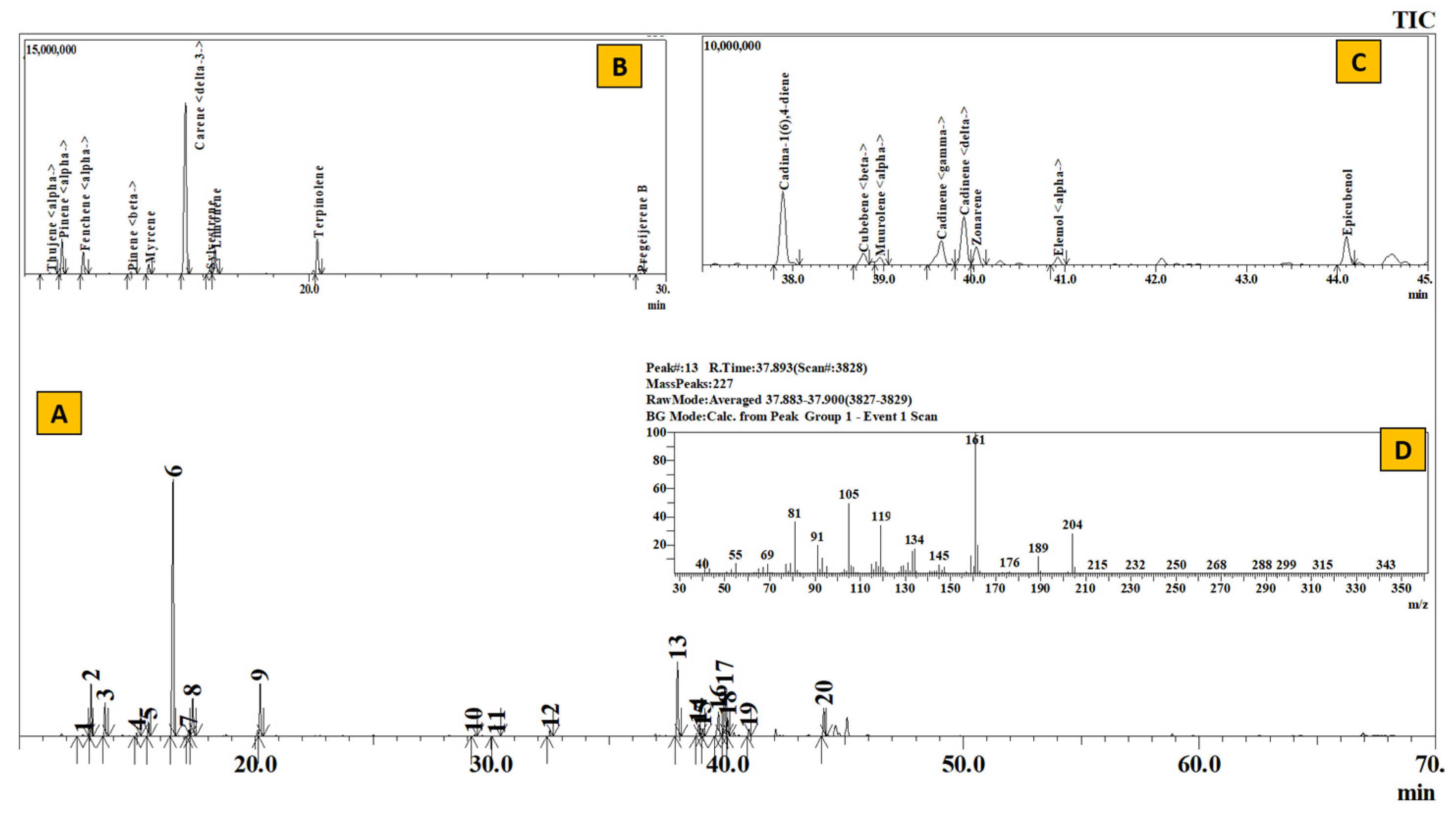
TIC of essential oil from leaves of Juniperus recurva (A), Inset: Extracted chromatogram from RT 11–30 min (B) and 37–45 min (C) and mass spectrum of Peak #13 (cadina-1(6),4-diene (D).
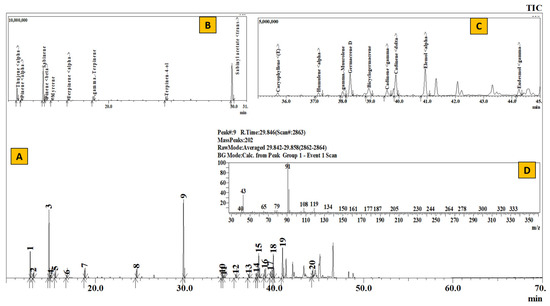
Figure 7.
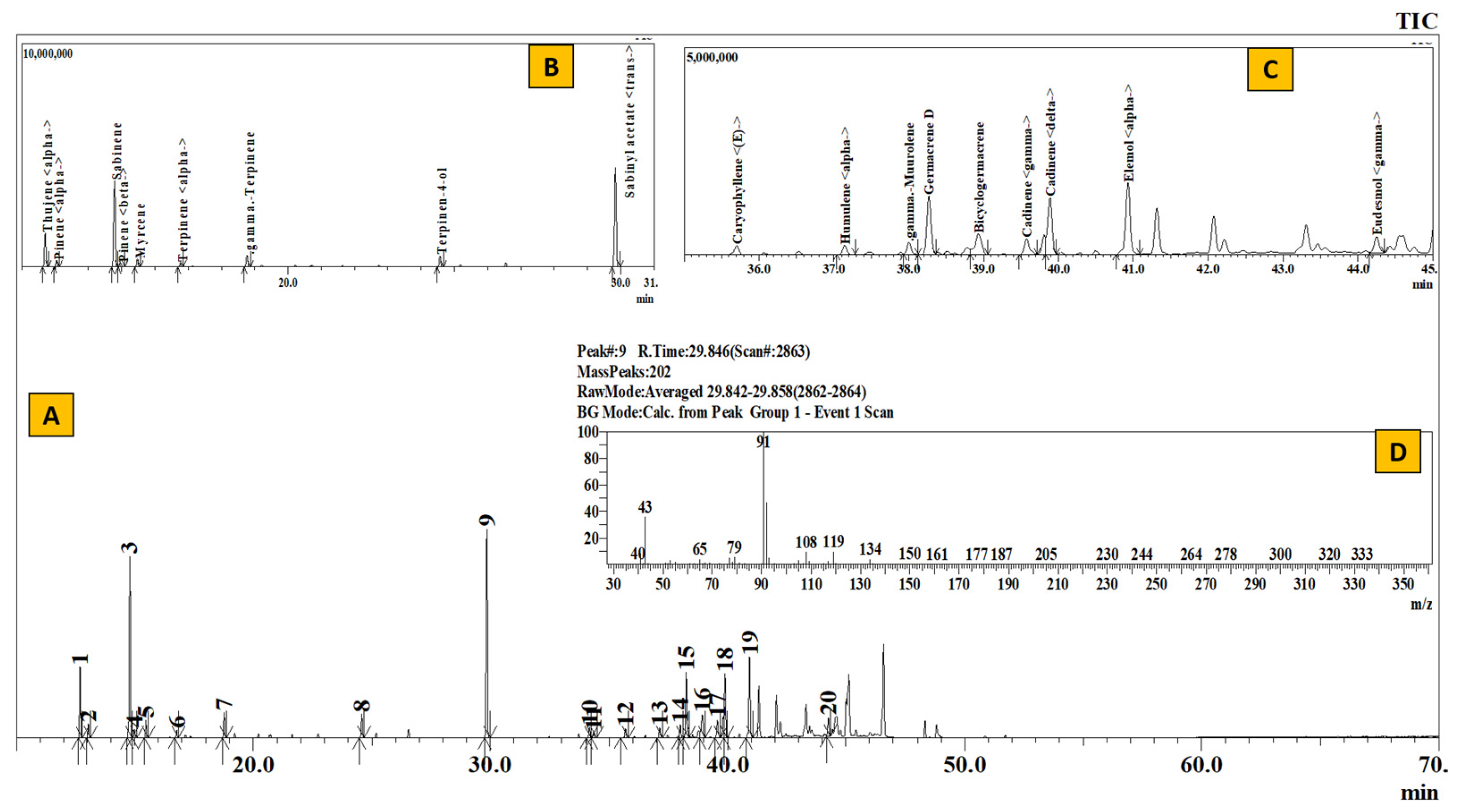
TIC of essential oil from Rhododendron setosum (A), Inset: Extracted chromatogram from RT 11–31 min (B) and 35–45 min (C) and mass spectrum of Peak #9 (trans-sabinyl acetate) (D).
Table 2, Table 3, Table 4 and Table 5 delineate the chemical constituents present in the essential oils of the plant species. Monoterpenes and sesquiterpenes formed the majority, almost 90%, of the identified components. GC-MS analysis of these oils showed that E-caryophyllene, α-pinene, γ-terpinene, β-pinene and δ-cadinene in Rhododendron anthopogon; santolina-triene, β-cubebene and sabinene in Artemisia dubia; β-myrcene, β-cubebene, E-β-ocimene and bicyclogermacrene in Boenninghausenia albiflora; perillene, eucalyptol and β-pinene in Elsholtzia fruticosa; δ-3-carene, cadina-1(6),4-diene and δ-cadinene in Juniperus recurva; trans-sabinyl acetate, sabinene, α-elemol and germacrene D in Rhododendron setosum are the principal components.

Table 2.
Qualitative composition (area %) of monoterpenes in the essential oils from Anthopogon (AN), Artemisia (AR), Boenninghausenia (BE), Elsholtzia (EL), Juniperus (JL) and Setosum (SE).

Table 3.
Qualitative composition (area %) of esters, alcohols, pregeijerene B and phenolic compounds in the essential oils from Anthopogon (AN), Artemisia (AR), Boenninghausenia (BE), Elsholtzia (EL), Juniperus (JL) and Setosum (SE).

Table 4.
Qualitative composition (area %) of Sesquiterpenes in the essential oils from Anthopogon (AN), Artemisia (AR), Boenninghausenia (BE), Elsholtzia (EL), Juniperus (JL) and Setosum (SE).

Table 5.
Qualitative composition (area %) of sesquiterpene esters and alcohols and diterpene in the essential oils from Anthopogon (AN), Artemisia (AR), Boenninghausenia (BE), Elsholtzia (EL), Juniperus (JL) and Setosum (SE).
Table 2 represents the qualitative composition of monoterpenes present in the essential oil. The number of monoterpenes dominated the constituents present in the essential oil. Where 82.55% from Elsholtzia (EL), 71.01% from Juniperus (JL), 57.56% from Artemisia (AR), 53.65% from Anthopogon (AN) and 53.22% from Boenninghausenia (BE) constituted only the monoterpene hydrocarbons, except in R. setosum (SE), where the monoterpenes comprised 32.7% of the whole essential oil with sabinene (18.67%) as the major component. In a similar manner, α–pinene (14.95%) and γ-terpinene (12.16%) in Anthopogon, santolina-triene (21.82%) and β-pinene (12.30%) in Artemisia, β-myrcene (27.04%) in Boenninghausenia, perillene (31.74%) and eucalyptol (23.27%) in Elsholtzia and δ-3-carene (43.88%) in Juniperus served as the most abundant monoterpenoid components.
The qualitative composition of monoterpenoid esters, alcohols and pregeijerene B has been given in Table 3. Compared to monoterpene hydrocarbons, the amount of oxygenated monoterpenes is remarkably low. The most common alcoholic component is terpinen-4-ol, ranging from 0.58% in Boenninghausenia to 2.58% in Setosum. The highest number of monoterpene alcohols were present in Elsholtzia (5.36%), consisting of 0.97% 1-octen-3-ol acetate, 1.74% terpinen-4-ol, 0.75% trans-pinocarveol and 1.90% α –terpineol. Moreover, it also contained 0.78% eugenol. Likewise, 24.11% trans-sabinyl acetate in Setosum, 4.18% citronellyl acetate in Anthopogon, 1.48% lavandulyl acetate in Artemisia constituted the monoterpenoid ester. Similarly, the hydrocarbon pregeijerene B was found only in Juniperus oils.
Table 4 depicts the compositional variation of sesquiterpenes present in the essential oils. E-caryophyllene, α-humulene, γ-muurolene, γ-cadinene and δ-cadinene were some of the common sesquiterpenes present in the essential oil. The highest number of sesquiterpenes was present in Boenninghausenia (41.38%), consisting mainly of β-cubebene (18.26%), bicyclogermacrene (6.17%) and E-caryophyllene (5.79%). Likewise, 37.67%, 34.07%, 28.2% and 23.52% of sesquiterpenes constituted the essential oils of Artemisia, Anthopogon, Setosum and Juniperus, respectively. Wherein, β-cubebene (21.77%) in Artemisia, E-caryophyllene (14.63%) in Anthopogon, germacrene D (7.67%) in Setosum and cadina-1(6),4-diene (11.41%) in Juniperus formed the major sesquiterpenes.
Correspondingly, Table 5 enumerates the oxygenated sesquiterpenes and a diterpene, m-camphorene, obtained via GC-MS analysis of the essential oils. The composition of sesquiterpene esters and alcohol, such as oxygenated monoterpenes, was considerably low. α-elemol, E-nerolidol, citronellyl valerate, epicubenol, γ-eudesmol, τ-cadinol and α-muurolol are some of the components identified in the extracted essential oils. Combinedly, the amount of these compounds varied from 2.04% in Artemisia, 4.36% in Boenninghausenia, 5.2% in Juniperus, 8.12% in Anthopogon, and 12.42% in Setosum. α-elemol formed the most abundant sesquiterpene alcohol, ranging from 1.01% in Juniperus to 10.01% in Setosum. In a similar manner, 0.45% m-camphorene, a diterpene hydrocarbon, was also identified in the essential oil of Boenninghausenia.
4. Discussion
GC-MS analysis of the essential oils from the six aromatic plant species showed that these oils were abundant in monoterpenes and sesquiterpenes. However, the qualitative composition of the essential oil from the current study has shown that there are significant differences from the previously published reports. As shown in Table 2–Table 5, a total of 22 compounds were identified from the leaves of R. anthopogon (AN) essential oils. The oil is mainly composed of E-caryophyllene (14.63%), α-pinene (14.25%), γ-terpinene (12.16%), β-pinene (7.44%), Z-β-ocimene (7.25%), δ-cadinene (6.70%), γ-cadinene (3.32%), α–muurolol (3.17%), γ-eudesmol (3.00%), β-farnesene (3.01%), β-myrcene (4.65%), citronellyl acetate (4.18%), D-limonene (2.68%), α-humulene (1.97%), citronellyl valerate (1.95%) and α-muurolene (1.90%) with other minor constituents (<1.9%). On the contrary, two separate studies with plant materials collected from the Dolakha district [22] and the Dhankuta district [23] of Nepal reported the oils to be highly rich in α-pinene. Innocenti et al. [22] had reported 17 compounds from the oil of aerial parts (leaves and flowers) of R. anthopogon with α-pinene (37.4%), β-pinene (16.1%), limonene (13.3%) and δ-cadinene (9.1%) as the major constituents. Likewise, Dosoky et al. [23] had identified 70 volatile components from the leaves essential oil of R. anthopogon, and the major constituents in their study were α-pinene (21.5%), δ-cadinene (13.8%) and β-pinene (9.5%). Similarly, E-caryophyllene (11.6%), limonene (11.3%), and α-humulene (7.2%) were relatively abundant in the oil extracted from the leaves of R. anthopogon, India [30], further confirming the variation in the composition of the essential oil.
Furthermore, the composition of the essential oil from R. anthopogon is also different from the leaf essential oil of Rhododendron lepidotum collected from the Makwanpur district, Nepal [24] and Rhododendron setosum (presented in the current work). Joshi et al. [24] have reported 21 components with α-pinene (39.35%), β-pinene (13.82%), E-caryophyllene (9.79%) and δ-cadinene (9.40%) as the chief components from the essential oil of R. lepidotum. From the leaf essential oil of R. setosum (SE, Table 2–Table 5), 20 components have been identified. Setosum oil is mainly composed of trans-sabinyl acetate (24.11%), sabinene (18.67%), α-elemol (10.01%), germacrene D (7.67%), δ-cadinene (7.51%), α-thujene (6.62%) and bicyclogermacrene (4.16%), with other minor components (<3.0%). This is the first attempt to study the chemical composition of essential oil from R. setosum, and the constituents are remarkably different from the essential oil of other reported Rhododendron spp. [31,32,33,34,35,36,37].
Similar variations in the chemical composition were also observed in the essential oil obtained from Artemisia dubia. In the current study, a total of 18 components were identified in the essential oil of the leaves of Artemisia dubia (AR, Table 2–Table 5). The oil is mainly composed of santolina-triene (21.82%), β-cubebene (21.77%), β-pinene (12.30%), sabinene (11.13%), α-pinene (8.90%), E-caryophyllene (4.65%), bicyclogermacrene (3.15%), α-humulene (2.23%), E-nerolidol (2.04%), lavandulyl acetate (1.48%) and other minor constituents (<1.40%). These components are notably different from the earlier reports. Satyal et al. [26] reported chrysanthenone (29.0%), coumarin (18.3%), and camphor (16.4%) as the chief constituents from the leaf essential oil of A. dubia collected from Kathmandu, Nepal. Kim [38] identified fifty-eight components from the leaf oil (Seoul, Korea), and the oil was found to be rich in camphor (17.18%) and germacrene-D (15.70%). Likewise, Liang et al. [39] reported terpinolene (19.02%), limonene (17.40%), 2,5-etheno [4.2.2]propella3,7,9-triene (11.29%) and isoelemicin (11.05%) from the leaf essential oil (China), and Shameem et al. [40], from the flower essential oil (Kashmir, India), identified nerylisovalerate (9.79%), 1,8-cineole (8.32%), neryl-2-methyl-butanoate (7.32%) and chamazulene (5.92%) as the major constituents.
Twenty-four components were identified from the leaf essential oil of Boenninghausenia albiflora (BE, Table 2–Table 5). β-myrcene (27.04%), β-cubebene (18.26%), E-β-ocimene (8.95%), bicyclogermacrene (6.17%), E-caryophyllene (5.79%), Z-β-ocimene (4.05%) and δ-cadinene (3.62%) were the major constituents. A similar result, the presence of β-myrcene and E-caryophyllene, was also reported in the analysis of essential oils from leaves and aerial parts collected from India [41,42]. Padalia et al. [41] identified eighty-one components from the leaf and root essential oils, which are mainly composed of β-myrcene (23.9%), (Z)-β-guaiene (12.1%), (Z)-β-ocimene (10.1%), E-caryophyllene (7.9%) in the leaf and bicyclogermacrene (25.2%), α-terpinyl acetate (19.9%), and geijerene (7.9%) in the root. Similarly, the same group has also been reported to contain β-myrcene (2.09–26.11%), β-pinene (8.36–13.77%), germacrene D (4.18–18.21%), epi-α-cadinol (0.14–16.25%), β -caryophyllene (4.62–13.14%), globulol (0.26–9.22%), and β-copaene-4 α-ol (0.14–7.52%) as the main components in the essential oil of aerial parts grown at various stages [42].
Likewise, the qualitative composition of the essential oil of Elsholtzia fruticosa (EL, Table 2, Table 3, Table 4 and Table 5) was also found to be slightly similar to a couple of previous works where perillene and eucalyptol formed the major components [43,44]. In our study, 21 components have been identified, which are mainly composed of perillene (32.29%), eucalyptol (23.67%), β-pinene (13.59%) and E-caryophyllene (5.48%). Thappa et al. [43] have also reported perillene (20.5%) as the prime component along with 1,8-cineole (18.0%) and terpinen-4-ol (12.6%) from the oil of the aerial parts of E. fruticosa (Himalayan region). However, in a separate study carried out by Liang et al., perillene was not found to be present in the oil of the aerial parts of E. fruticosa [44]. Rather, the oil was highly rich in eucalyptol (40.1%), γ-terpinene (15.8%), limonene (9.3%) and β-pinene (5.2%). In their recent studies, Fusani et al. [45] have shown that the essential oils obtained from the cultivated plant species were highly abundant with 1,8-cineole (50.06%) and γ-terpinene (14.11%), showing the variation in composition from the wild plant species. To the best of our knowledge, there have not been any previous reports on the studies of the essential oils of B. albiflora and E. fruticosa from Nepal. This is the first attempt to analyze the essential oil from this region.
The composition of essential oil from the leaves of Juniperus recurva has also shown some similarities with the previous report [46]. Adams et al. [46] had compared the components in the oil of Juniperus recurva from Nepal to Juniperus recurva var. squamata and Juniperus recurva from India. The Nepalese variety was rich in δ-3-carene (23.7%), limonene (18.4%), sabinene (13.4%) and α-pinene (6.9%), and had a similar composition to Juniperus recurva var. squamata than to Juniperus recurva from India. On a similar note, the essential oil from our present study (JL, Table 2–Table 5) was also significantly rich in δ-3-carene (43.88%). However, on the contrary, sabinene and limonene were present in the trace to slight amounts. Instead, the oil in the present study was composed of β-pinene (13.59%), cadina-1(6),4-diene (11.41%), δ-cadinene (7.57%) and α-terpinolene (7.24%) to greater extent. Hence, the differences in the chemical composition of the essential oils might have been due to the difference in time period of collection, geographical location and altitude, parts of the plant used for extraction and nature of the plant species (wild or cultivated).
5. Conclusions
In summary, six aromatic plants were collected from the Langtang region. The essential oils were extracted from the leaves of these plant species by the hydrodistillation method. GC-MS analysis of these oils suggested that they were rich in mono- and sesqui-terpene hydrocarbons. The essential oil contents and compositions were significantly different from the earlier reports. This might be due to the difference in time period of collection, geographical location and altitude, parts of the plant used for extraction and nature of the plant species. This is the first report on the chemical composition of R. setosum essential oil, which is mainly composed of trans-sabinyl acetate, sabinene, α-elemol, germacrene D, δ-cadinene, α-thujene and bicyclogermacrene. The constituents are remarkably different from the essential oils of other reported Rhododendron spp. and should be screened further for their biological properties. Langtang region harbors a plethora of MAPs, and hence it is highly essential to conduct extensive studies for identifying their chemical and biological properties.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/separations10010052/s1.
Author Contributions
S.P. collected the plant materials from Langtang region, dried them, extracted and analyzed the essential oil, conducted literature survey and prepared the manuscript; H.R.P. identified the collected plant materials; R.M. helped in extracting the essential oil and reviewing the manuscript; K.S. supervised, reviewed and finalized the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank Radha Wagle, Director General and Saroj Kumar Chaudhary, Deputy Director General, Department of Plant Resources for their unequivocal support, Krishna Ram Bhattarai, Jeevan Pandey, Paras Mani Yadav and Poonam Thapa for their co-operation during plant collection, herbarium preparation, and field support and guidance. The authors would also like to thank the Department of Plant Resources for providing the opportunity to conduct the research work and for the financial support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Karki, J. Status Paper of Langtang National Park. In Proceedings of the Grassland Ecology and Management in Protected Areas of Nepal, Kathmandu, Nepal, 15–19 March 1999. [Google Scholar] [CrossRef]
- Humagain, K.; Shrestha, K.K. Medicinal plants in Rasuwa district, central Nepal: Trade and livelihood. Bot. Orient. J. Plant Sci. 2010, 6, 39–46. [Google Scholar] [CrossRef]
- Shafi, A.; Zahoor, I. Chapter 12-Metabolomics of Medicinal and Aromatic Plants: Goldmines of Secondary Metabolites for Herbal Medicine Research. In Medicinal and Aromatic Plants; Aftab, T., Hakeem, K.R., Eds.; Academic Press: New York, NY, USA, 2021; pp. 261–287. [Google Scholar] [CrossRef]
- Novais, C.; Pereira, C.; Molina, A.K.; Liberal, N.; Dias, M.I.; Añibarro-Ortega, M.; Alves, M.J.; Calhelha, R.C.; Ferreira, I.C.; Barros, L. Bioactive and nutritional potential of medicinal and aromatic plant (MAP) seasoning mixtures. Molecules 2021, 26, 1587. [Google Scholar] [CrossRef]
- Mckenna, J.M. Strategic Segmentation Analysis: Nepal: Medicinal and Aromatic Plants; World Bank Group: Washington, DC, USA, 2018. [Google Scholar]
- Rehman, R.; Asif Hanif, M. Biosynthetic factories of essential oils: The aromatic plants. Nat. Prod. Chem. Res. 2016, 4, 227. [Google Scholar] [CrossRef]
- Schnaubelt, K. Essential oil therapy according to traditional Chinese medical concepts. Int. J. Aromather. 2005, 15, 98–105. [Google Scholar] [CrossRef]
- Mothana, R.A.; Al-Said, M.S.; Al-Yahya, M.A.; Al-Rehaily, A.J.; Khaled, J.M. GC and GC/MS analysis of essential oil composition of the endemic Soqotraen Leucas virgata Balf.f. and its antimicrobial and antioxidant activities. Int. J. Mol. Sci. 2013, 14, 23129–23139. [Google Scholar] [CrossRef]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential oils’ chemical characterization and investigation of some biological activities: A critical review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef]
- Daferera, D.J.; Ziogas, B.N.; Polissiou, M.G. GC-MS analysis of essential oils from some Greek aromatic plants and their fungitoxicity on Penicillium digitatum. J. Agric. Food Chem. 2000, 48, 2576–2581. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. The 1-Deoxy-D-xylulose-5-phosphate pathway of isoprenoid biosynthesis in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 47–65. [Google Scholar] [CrossRef]
- Dewick, P.M. The biosynthesis of C5–C25 terpenoid compounds. Nat. Prod. Rep. 2002, 19, 181–222. [Google Scholar] [CrossRef]
- Gershenzon, J.; Dudareva, N. The function of terpene natural products in the natural world. Nat. Chem. Biol. 2007, 3, 408–414. [Google Scholar] [CrossRef]
- Modzelewska, A.; Sur, S.; Kumar, S.; Khan, S. Sesquiterpenes: Natural products that decrease cancer growth. Curr. Med. Chem.-Anti-Cancer Agents 2005, 5, 477–499. [Google Scholar] [CrossRef] [PubMed]
- Cox-Georgian, D.; Ramadoss, N.; Dona, C.; Basu, C. Therapeutic and Medicinal Uses of Terpenes. In Medicinal Plants: From Farm to Pharmacy; Joshee, N., Dhekney, S.A., Parajuli, P., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 333–359. ISBN 978-3-030-31269-5. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Adams, R.P., Ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; p. 804. [Google Scholar]
- Angioni, A.; Barra, A.; Russo, M.T.; Coroneo, V.; Dessí, S.; Cabras, P. Chemical composition of the essential oils of Juniperus from ripe and unripe berries and leaves and their antimicrobial activity. J. Agric. Food Chem. 2003, 51, 3073–3078. [Google Scholar] [CrossRef] [PubMed]
- Stefanakis, M.K.; Papaioannou, C.; Lianopoulou, V.; Philotheou-Panou, E.; Giannakoula, A.E.; Lazari, D.M. Seasonal variation of aromatic plants under cultivation conditions. Plants 2022, 11, 2083. [Google Scholar] [CrossRef] [PubMed]
- Marriott, P.J.; Shellie, R.; Cornwell, C. Gas chromatographic technologies for the analysis of essential oils. J. Chromatogr. A 2001, 936, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Gale, J. Plants and altitude—Revisited. Ann. Bot. 2004, 94, 199. [Google Scholar] [CrossRef] [PubMed]
- Kour, J.; Balgotra, S.; Rajput, P.; Kour, H.; Verma, P.K.; Sawant, S.D. Medicinal Value of High-Altitude Plants of Indian Himalaya. In Botanical Leads for Drug Discovery, 1st ed.; Singh, B., Ed.; Springer Nature: Singapore, 2020; pp. 295–324. [Google Scholar] [CrossRef]
- Innocenti, G.; Dall’Acqua, S.; Scialino, G.; Banfi, E.; Sosa, S.; Gurung, K.; Barbera, M.; Carrara, M. Chemical composition and biological properties of Rhododendron anthopogon essential oil. Molecules 2010, 15, 2326–2338. [Google Scholar] [CrossRef]
- Dosoky, N.S.; Satyal, P.; Pokharel, S.; Setzer, W.N. Chemical composition, enantiomeric distribution, and biological activities of Rhododendron anthopogon leaf essential oil from Nepal. Nat. Prod. Commun. 2016, 11, 1934578X1601101230. [Google Scholar] [CrossRef]
- Joshi, S.; Thapa, P. Chemical composition and antioxidant activity of Rhododendron lepidotum Wall. ex D. Don, essential oil from Nepal. Int. J. Adv. Res. Chem. Sci. 2018, 5, 12–18. [Google Scholar] [CrossRef]
- Satyal, P.; Dosoky, N.S.; Kincer, B.L.; Setzer, W.N. Chemical compositions and biological activities of Amomum subulatum essential oils from Nepal. Nat. Prod. Commun. 2012, 7, 1934578X1200700935. [Google Scholar] [CrossRef]
- Satyal, P.; Paudel, P.; Kafle, A.; Pokharel, S.K.; Lamichhane, B.; Dosoky, N.S.; Moriarity, D.M.; Setzer, W.N. Bioactivities of volatile components from Nepalese. Artemisia Species. Nat. Prod. Commun. 2012, 7, 1934578X1200701228. [Google Scholar] [CrossRef]
- Satyal, P.; Paudel, P.; Poudel, A.; Dosoky, N.S.; Moriarity, D.M.; Vogler, B.; Setzer, W.N. Chemical compositions, phytotoxicity, and biological activities of Acorus Calamus essential oils from Nepal. Nat. Prod. Commun. 2013, 8, 1934578X1300800839. [Google Scholar] [CrossRef]
- Shrestha, N.; Shrestha, K.K. Threatened Medicinal Plants in Langtang National Park, Nepal. In Medicinal Plants in Nepal: An Anthology of Contemporary Research; Jha, P.K., Karmacharya, S.B., Chettri, M.K., Thapa, C.B., Shrestha, B.B., Eds.; Ecological Society (ECOS): Kathmandu, Nepal, 2008; pp. 246–251. [Google Scholar]
- Clevenger, J. Apparatus for the determination of volatile oil*. J. Am. Pharm. Assoc. 1928, 17, 345–349. [Google Scholar] [CrossRef]
- Guleria, S.; Jaitak, V.; Saini, R.; Kaul, V.K.; Lal, B.; Babu, G.K.; Singh, B.; Singh, R. Comparative studies of volatile oil composition of Rhododendron anthopogon by hydrodistillation, supercritical carbon dioxide extraction and head space analysis. Nat. Prod. Res. 2011, 25, 1271–1277. [Google Scholar] [CrossRef] [PubMed]
- Jesionek, A.; Kokotkiewicz, A.; Mikosik-Roczynska, A.; Ciesielska-Figlon, K.; Luczkiewicz, P.; Bucinski, A.; Daca, A.; Witkowski, J.M.; Bryl, E.; Zabiegala, B.; et al. Chemical variability of Rhododendron tomentosum (Ledum palustre) essential oils and their pro-apoptotic effect on lymphocytes and rheumatoid arthritis synoviocytes. Fitoterapia 2019, 139, 104402. [Google Scholar] [CrossRef]
- He, J.; Shang, X.; Dai, L.; Yang, X.; Li, B.; Wei, Y.; Zhang, J.; Pan, H. Chemical constituents, antibacterial, acaricidal and anti-inflammatory activities of the essential oils from four Rhododendron species. Front. Vet. Sci. 2022, 9, 2060. [Google Scholar] [CrossRef]
- Bai, L.; Jiao, M.L.; Zang, H.Y.; Guo, S.S.; Wang, Y.; Sang, Y.L.; Du, S.S. Chemical composition of essential oils from four Rhododendron species and their repellent activity against three stored-product insects. Environ. Sci. Pollut. Res. 2019, 26, 23198–23205. [Google Scholar] [CrossRef] [PubMed]
- Judzentiene, A.; Budiene, J.; Svediene, J.; Garjonyte, R. Toxic, radical scavenging, and antifungal activity of Rhododendron tomentosum H. essential oils. Molecules 2020, 25, 1676. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Özek, G.; Özek, T.; Kirpotina, L.N.; Khlebnikov, A.I.; Quinn, M.T. Chemical composition and immunomodulatory activity of essential oils from Rhododendron albiflorum. Molecules 2021, 26, 3652. [Google Scholar] [CrossRef]
- Chen, X.Y.; Wu, Y.X.; Li, Y.; Zhang, J.N.; Bi, S.F. Chemical composition and biological activity of the essential oil from Rhododendron anwheiense flowers. Chem. Nat. Compd. 2022, 58, 947–950. [Google Scholar] [CrossRef]
- Belousova, N.I.; Domrachev, D.V.; Fursa, N.S.; Belousov, M.V. Composition of essential oil from Rhododendron caucasicum. Chem. Nat. Compd. 2017, 53, 574–575. [Google Scholar] [CrossRef]
- Kim, J.I. Anti-porcine epidemic diarrhea virus (PEDV) activity and antimicrobial activities of Artemisia dubia essential oil. Korean J. Microbiol. Biotechnol. 2012, 40, 396–402. [Google Scholar] [CrossRef]
- Liang, J.Y.; Guo, S.S.; Zhang, W.J.; Geng, Z.F.; Deng, Z.W.; Du, S.S.; Zhang, J. Fumigant and repellent activities of essential oil extracted from Artemisia dubia and its main compounds against two stored product pests. Nat. Prod. Res. 2017, 32, 1234–1238. [Google Scholar] [CrossRef] [PubMed]
- Shameem, S.A.; Khan, K.Z.; Waza, A.A.; Banday, A.H.; Ramzan, A.; Shah, A.H.; Ganai, B.A. Chemical profile and biological activities of essential oil from flowers of Artemisia dubia Wall. ex Bess. growing wild in Western Himalaya, India. Asian J. Chem. 2019, 31, 1762–1766. [Google Scholar] [CrossRef]
- Padalia, R.C.; Verma, R.S.; Chauhan, A.; Chanotiya, C.S. Chemical composition of leaf and root essential oils of Boenninghausenia albiflora Reichb. from northern India. Nat. Prod. Res. 2011, 26, 2040–2044. [Google Scholar] [CrossRef] [PubMed]
- Padalia, R.C.; Verma, R.S.; Chauhan, A. Compositional Variations in Volatile Constituents of Boenninghausenia albiflora Reichb. from Western Himalaya. Natl. Acad. Sci. Lett. 2013, 36, 635–640. [Google Scholar] [CrossRef]
- Thappa, R.K.; Agarwal, S.G.; Kapahl, B.K.; Srivastava, T.N. Chemosystematics of the Himalayan Elsholtzia. J. Essent. Oil Res. 1999, 11, 97–103. [Google Scholar] [CrossRef]
- Liang, J.Y.; Ning, A.Q.; Lu, P.Y.; Shao, Y.Z.; Xu, J.; Yang, Y.Y.; Wang, H.L. Chemical composition and biological activity of essential oil extracted from the aerial part of Elsholtzia fruticosa against Ditylenchus destructor. J. Essent. Oil Bear. Plants 2020, 23, 575–582. [Google Scholar] [CrossRef]
- Fusani, P.; Ronga, D.; Carminati, D.; Mandrioli, M.; Manicardi, G.C.; Giannì, S.; Tava, A. Composition and biological activity of essential oils from Artemisia roxburghiana Besser and Elsholtzia fruticosa Rehder cultivated in Italy. Ind. Crops Prod. 2022, 187, 115317. [Google Scholar] [CrossRef]
- Adams, R.P.; Thappa, R.K.; Agarwal, S.G.; Kapahi, B.K.; Srivastava, T.N.; Chaudhary, R.P. The leaf essential oil of Juniperus recurva Buch.-Ham. ex D. Don from India and Nepal compared with J. recurva var. squamata (D. Don) Parl. J. Essent. Oil Res. 1998, 10, 21–24. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).