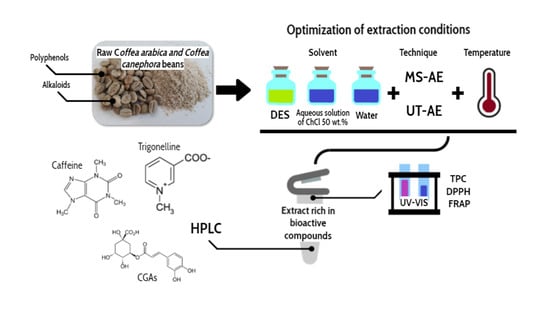
Valorization of Raw Coffee Beans (Coffea arabica and Coffea canephora) through Solvent Development and Extraction of Bioactive Compounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Coffee Samples
2.2. Chemicals
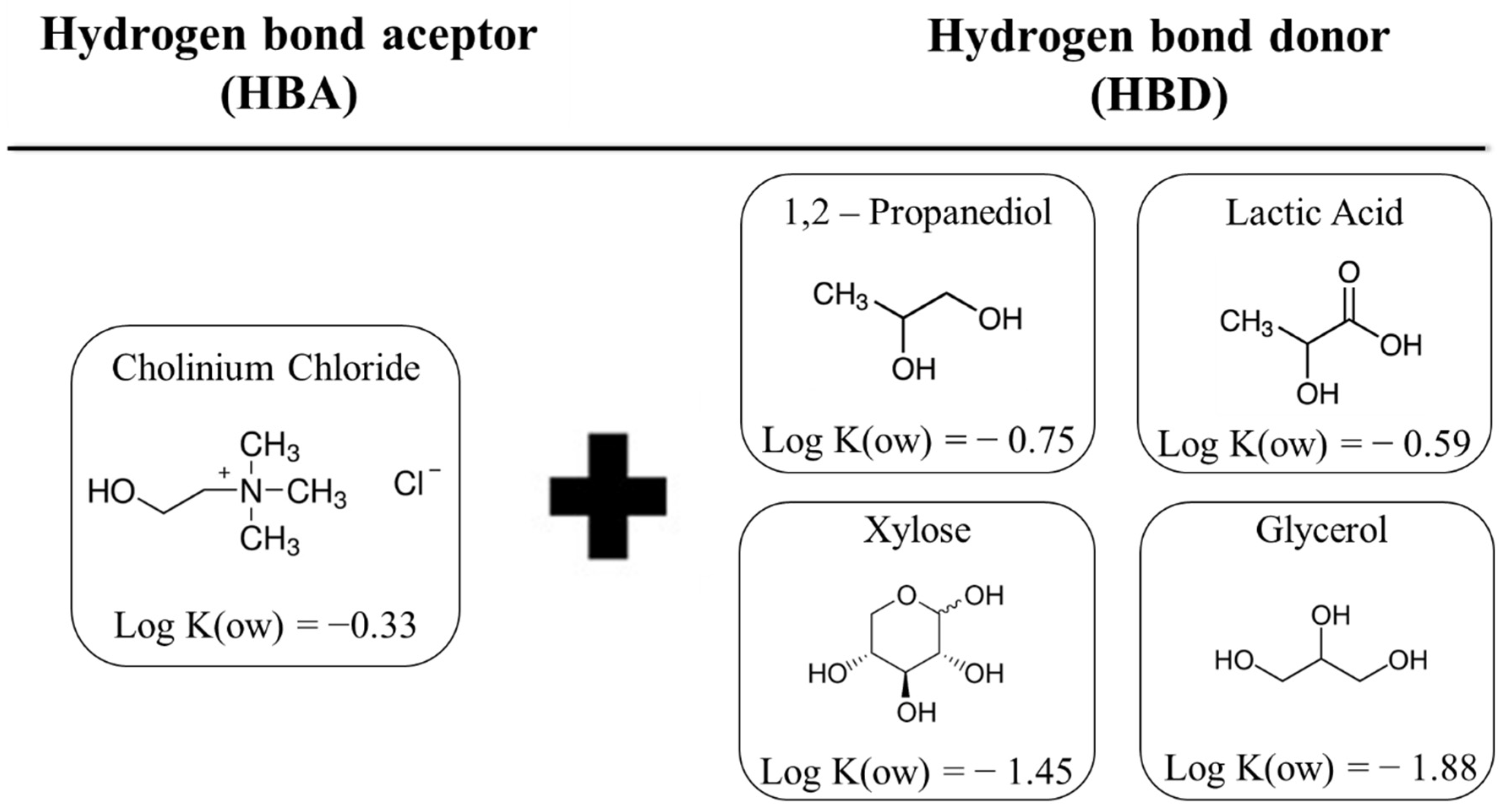
2.3. DES Preparation
2.4. Optimization of Extraction Conditions by Experimental Design
2.5. Solid–Liquid Extraction (SLE)
2.6. Evaluation of Raw Coffee Bean Extracts
2.7. TPC and Chemical Antioxidant Activity of the Extracts
2.8. Extract Composition Profiles Determined by High-Performance Liquid Chromatography (HPLC)
2.9. Statistical Analysis
3. Results and Discussion
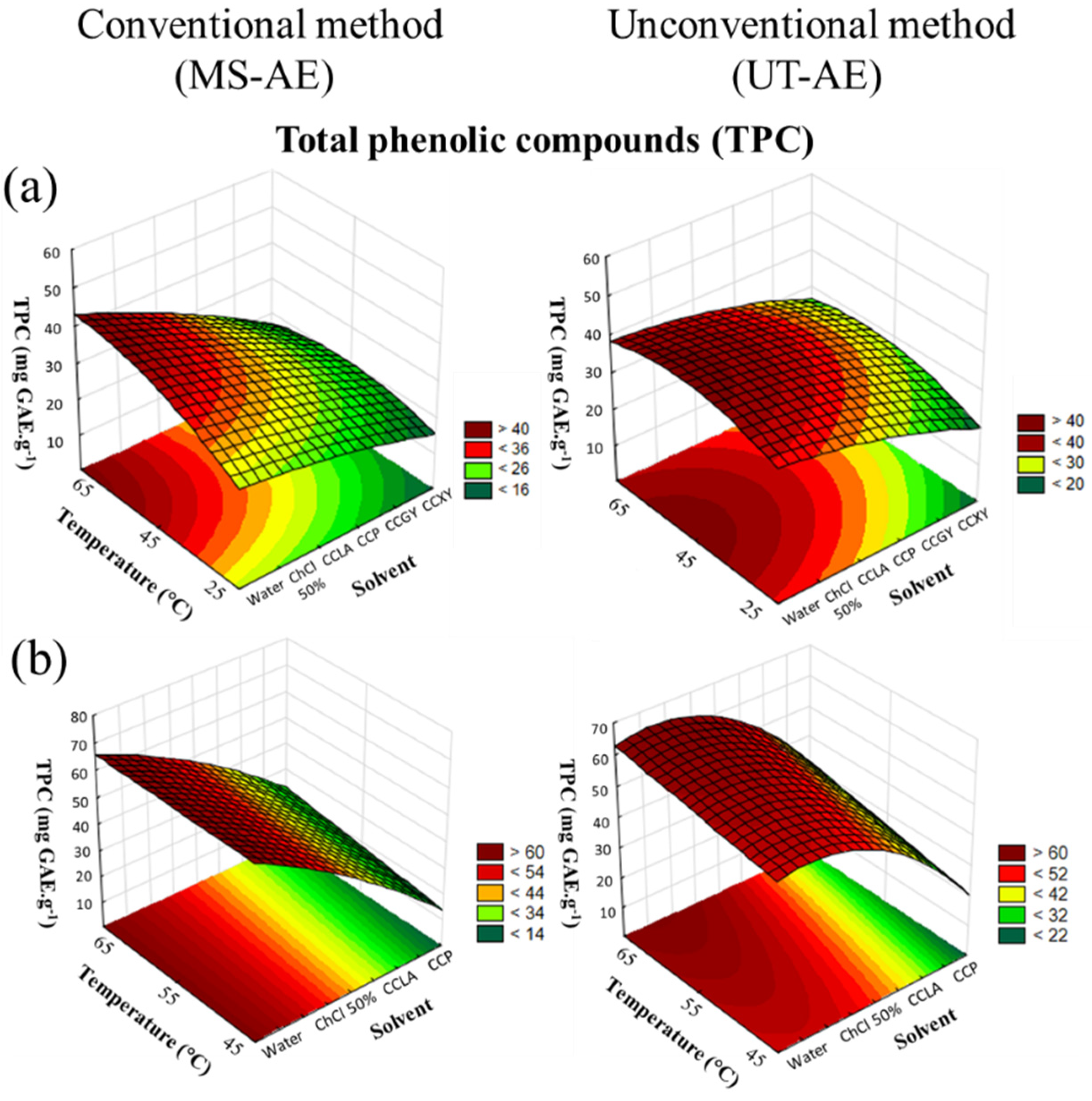
3.1. Optimization of Extraction Conditions by Experimental Design
3.2. TPC Content
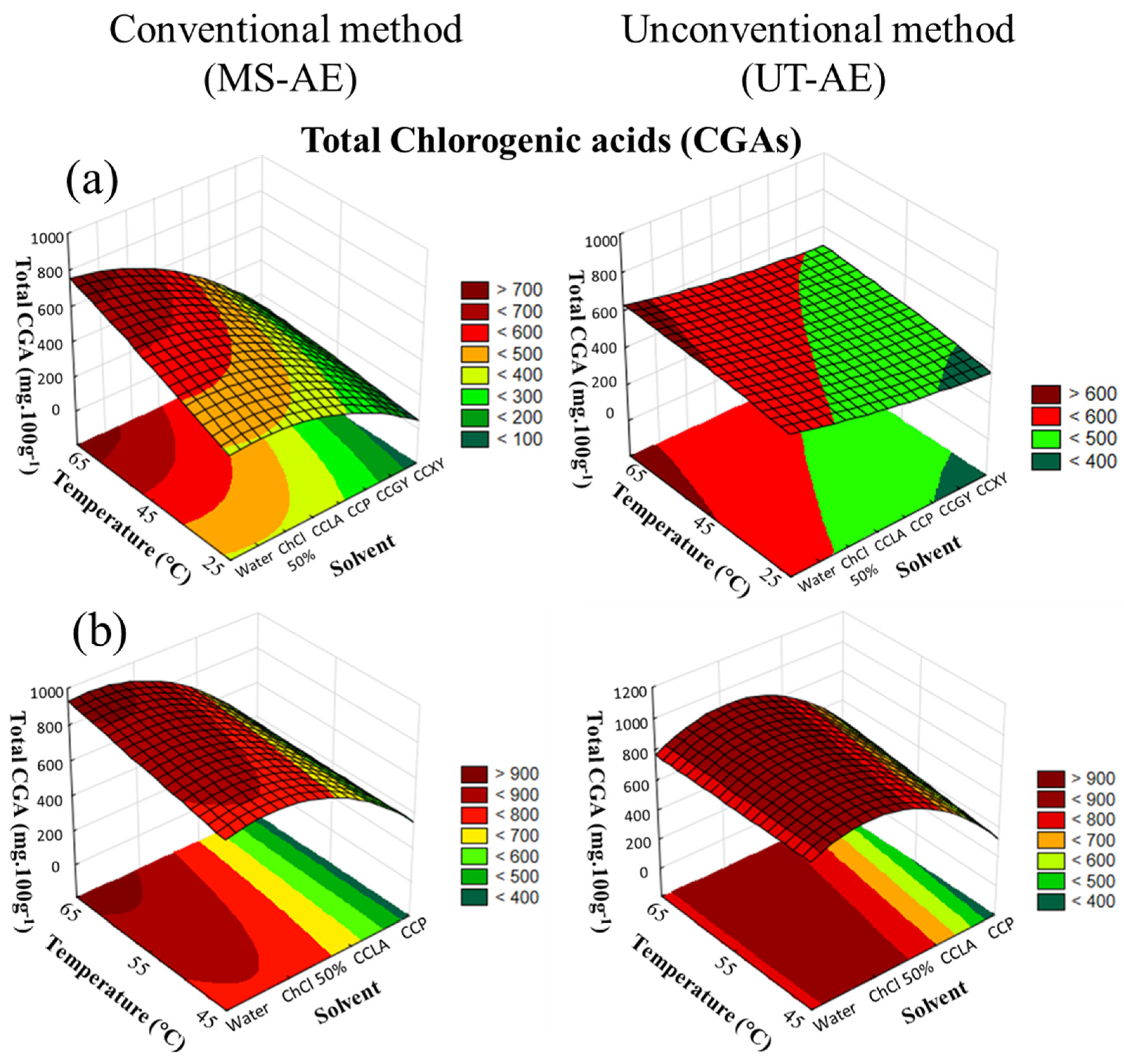
3.3. Chlorogenic Acids, Caffeine, and Trigonelline Contents
3.3.1. Chlorogenic Acids
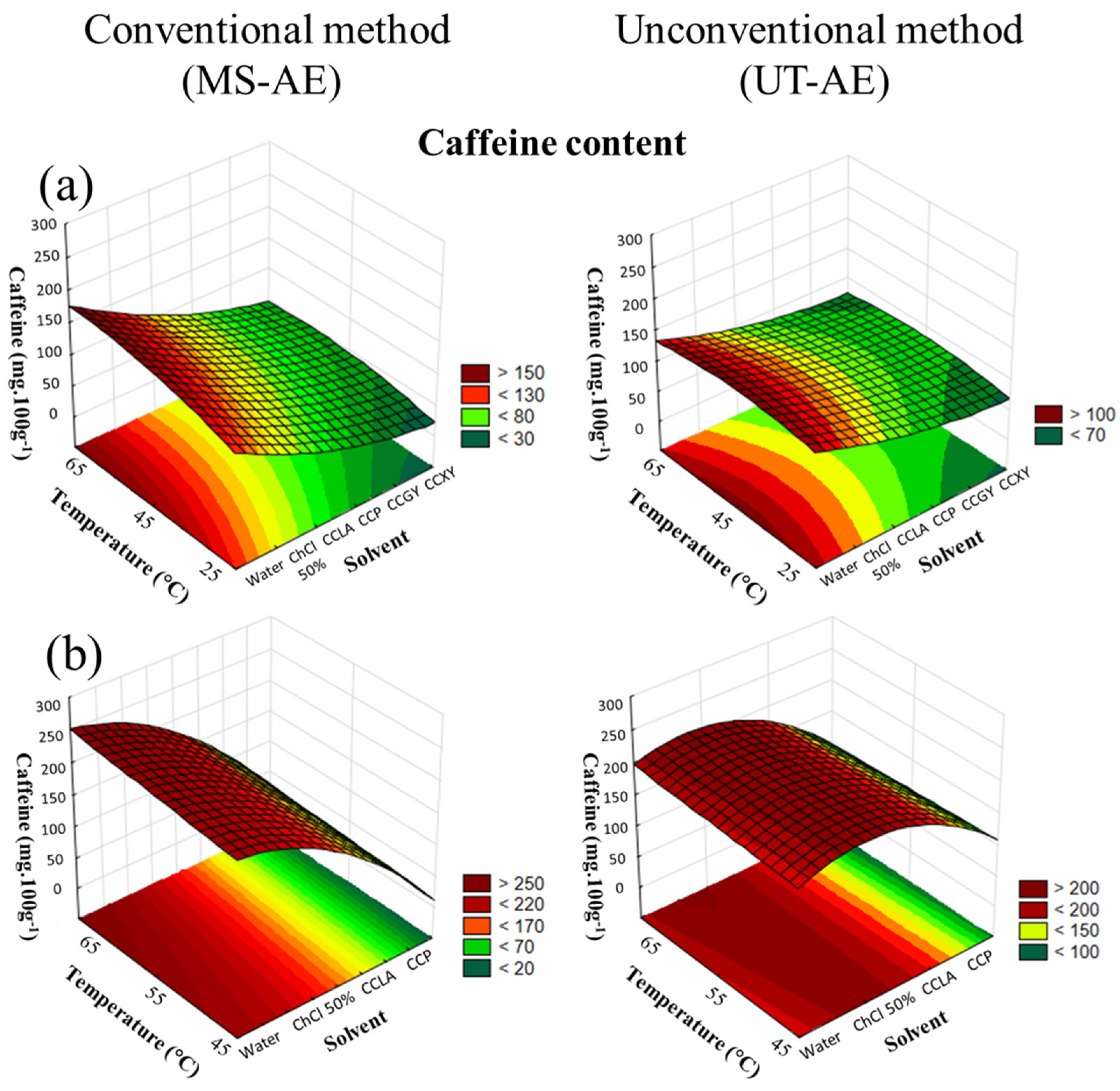
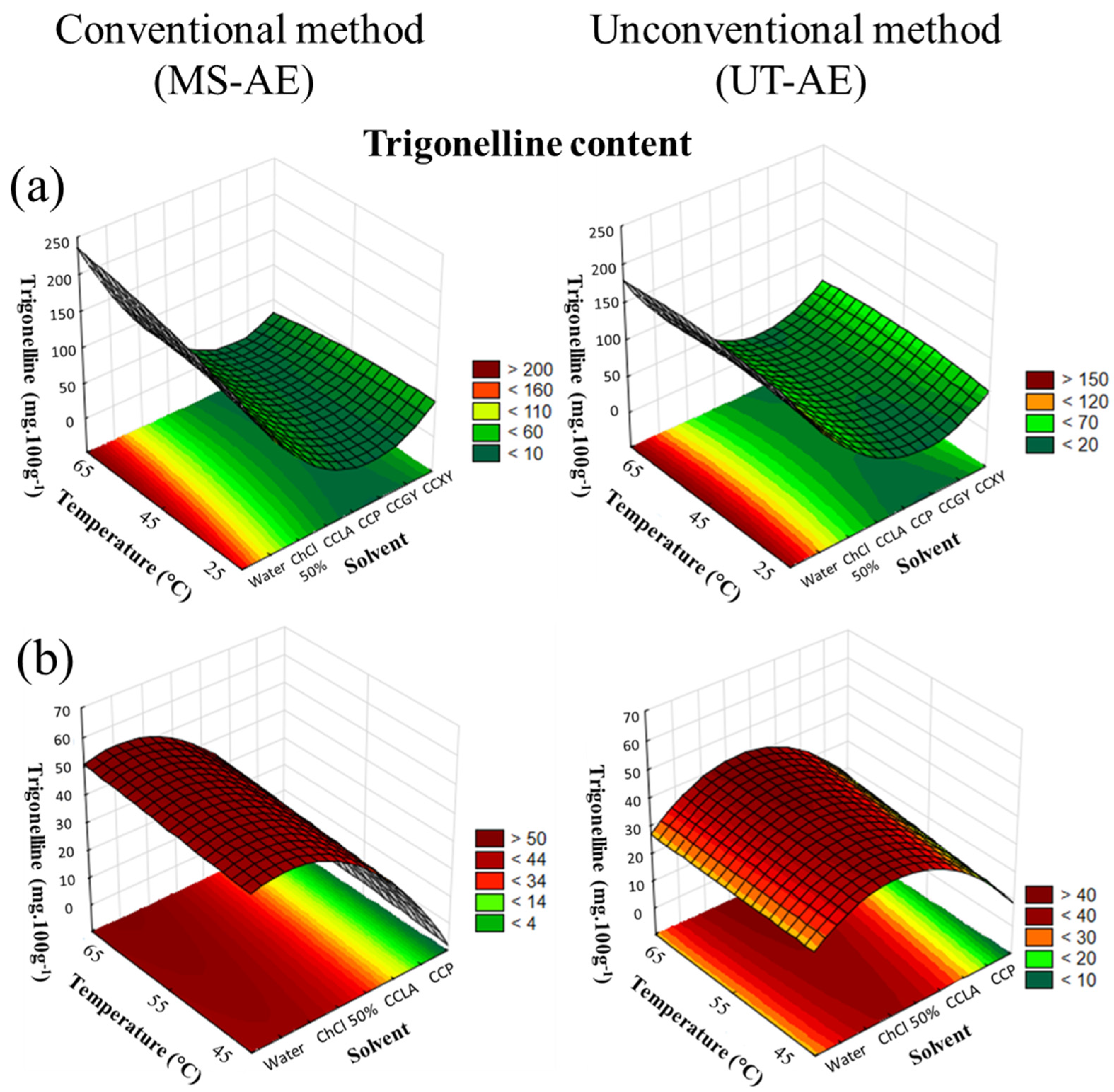
3.3.2. Alkaloids: Caffeine and Trigonelline
3.4. Antioxidant Activity (DPHH and FRAP)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Getachew, A.T.; Chun, B.S. Influence of hydrothermal process on bioactive compounds extraction from green coffee bean. Innov. Food Sci. Emerg. Technol. 2016, 38, 24–31. [Google Scholar] [CrossRef]
- Sarraguça, M.C.; Pascoa, R.N.M.J.; Lopo, M.; Sarraguça, J.M.G.; Lopes, J.A. Bioactive Compounds in Coffee as Health Promotors. In Natural Bioactive Compounds from Fruits and Vegetables as Health Promoters Part II; Sarraguca, M.C., Pascoa, R., Lopo, M., Sarraguca, J., Lopes, J.A., Eds.; Bentham Science Publishers: Sharjah, United Arab Emirates, 2016; pp. 180–220. [Google Scholar]
- Babova, O.; Occhipinti, A.; Maffei, M.E. Chemical partitioning and antioxidant capacity of green coffee (Coffea arabica and Coffea canephora) of different geographical origin. Phytochemistry 2016, 123, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Quadra, G.R.; Paranaíba, J.R.; Vilas-Boas, J.; Roland, F.; Amado, A.M.; Barros, N.; Dias, R.J.P.; Cardoso, S.J. A global trend of caffeine consumption over time and related-environmental impacts. Environ. Pollut. 2020, 256, 2016–2021. [Google Scholar] [CrossRef] [PubMed]
- McLellan, T.M.; Caldwell, J.A.; Lieberman, H.R. A review of caffeine’s effects on cognitive, physical and occupational performance. Neurosci. Biobehav. Rev. 2016, 71, 294–312. [Google Scholar] [CrossRef] [PubMed]
- Arai, K.; Terashima, H.; Aizawa, S.I.; Taga, A.; Yamamoto, A.; Tsutsumiuchi, K.; Kodama, S. Simultaneous determination of trigonelline, caffeine, chlorogenic acid and their related compounds in instant coffee samples by HPLC using an acidic mobile phase containing octanesulfonate. Anal. Sci. 2015, 31, 831–835. [Google Scholar] [CrossRef]
- Yisak, H.; Redi-abshiro, M.; Chandravanshi, B.S. Selective determination of caffeine and trigonelline in aqueous extract of green coffee beans by FT-MIR-ATR spectroscopy. Vib. Spectrosc. 2018, 97, 33–38. [Google Scholar] [CrossRef]
- Ky, C.-L.; Louarn, J.; Dussert, S.; Guyot, B.; Hamon, S.; Noirot, M. Caffeine, trigonelline, chlorogenic acids and sucrose diversity in wild Coffea arabica L. and C. canephora P. accessions. Food Chem. 2001, 75, 223–230. [Google Scholar] [CrossRef]
- Pellegrini, N.; Serafini, M.; Colombi, B.; Del Rio, D.; Salvatore, S.; Bianchi, M.; Brighenti, F. Total Antioxidant Capacity of Plant Foods, Beverages and Oils Consumed in Italy Assessed by Three Different In Vitro Assays. J. Nutr. 2003, 133, 2812–2819. [Google Scholar] [CrossRef]
- Monteiro, Â.; Colomban, S.; Azinheira, H.G.; Guerra-Guimarães, L.; Silva, M.D.C.; Navarini, L.; Resmini, M. Dietary antioxidants in coffee leaves: Impact of botanical origin and maturity on chlorogenic acids and xanthones. Antioxidants 2020, 9, 6. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Barbieri, J.B.; Goltz, C.; Batistão Cavalheiro, F.; Theodoro Toci, A.; Igarashi-mafra, L.; Mafra, M.R.; Batistão, F.; Theodoro, A.; Igarashi-mafra, L.; Mafra, M.R. Deep eutectic solvents applied in the extraction and stabilization of rosemary (Rosmarinus officinalis L.) phenolic compounds. Ind. Crops Prod. 2020, 144, 112049. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Yoo, D.E.; Jeong, K.M.; Han, S.Y.; Kim, E.M.; Jin, Y.; Lee, J. Deep eutectic solvent-based valorization of spent co ff ee grounds. Food Chem. 2018, 255, 357–364. [Google Scholar] [CrossRef]
- Martins, M.A.R.R.; Pinho, S.P.; Coutinho, J.A.P.P. Insights into the Nature of Eutectic and Deep Eutectic Mixtures. J. Solut. Chem. 2019, 48, 962–982. [Google Scholar] [CrossRef]
- Xu, W.; Zhai, J.; Cui, Q.; Liu, J.; Luo, M.; Fu, Y.; Zu, Y. Ultra-turrax based ultrasound-assisted extraction of five organic acids from honeysuckle (Lonicera japonica Thunb.) and optimization of extraction process. Sep. Purif. Technol. 2016, 166, 73–82. [Google Scholar] [CrossRef]
- Zhou, P.; Wang, X.; Liu, P.; Huang, J.; Wang, C.; Pan, M.; Kuang, Z. Enhanced phenolic compounds extraction from Morus alba L. leaves by deep eutectic solvents combined with ultrasonic-assisted extraction. Ind. Crops Prod. 2018, 120, 147–154. [Google Scholar] [CrossRef]
- Dai, Y.; Van Spronsen, J.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef]
- Ueda, K.M.; Ronko, L.Z.; Toci, A.T.; Igarashi-Mafra, L.; Mafra, M.R.; Farias, F.O. Green Designer Solvents for Boosting the Extraction of Biocompounds from Eugenia pyriformis Cambess Leaves. ACS Food Sci. Technol. 2022, 2, 532–540. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A., Jr. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Toci, A.; Farah, A.; Trugo, L.C. Efeito do processo de descafeinação com diclorometano sobre a composição química dos cafés arábica e robusta antes e após a torração. Quim. Nova 2006, 29, 965–971. [Google Scholar] [CrossRef]
- Trugo, L.C.; Macrae, R. Chlorogenic acid composition of instant coffees. Analyst 1984, 109, 263. [Google Scholar] [CrossRef] [PubMed]
- Sari, F.; Velioglu, Y.S. Effects of particle size, extraction time and temperature, and derivatization time on determination of theanine in tea. J. Food Compos. Anal. 2011, 24, 1130–1135. [Google Scholar] [CrossRef]
- Toazza, C.E.B.; Leal, F.C.; Marques, C.; Oliveira, G.; Farias, F.O.; Belan, A.L.D.; Leite, N.F.; Mafra, M.R.; Igarashi-Mafra, L.; Masson, M.L. Bioactive compounds extraction from different lemongrass species: Strategies and deep eutectic solvents evaluation. J. Food Process Eng. 2022, 1–14. [Google Scholar] [CrossRef]
- Xu, H.; Wang, W.; Liu, X.; Yuan, F.; Gao, Y. Antioxidative phenolics obtained from spent coffee grounds (Coffea arabica L.) by subcritical water extraction. Ind. Crops Prod. 2015, 76, 946–954. [Google Scholar] [CrossRef]
- Wei, Z.; Qi, X.; Li, T.; Luo, M.; Wang, W.; Zu, Y.; Fu, Y. Application of natural deep eutectic solvents for extraction and determination of phenolics in Cajanus cajan leaves by ultra performance liquid chromatography. Sep. Purif. Technol. 2015, 149, 237–244. [Google Scholar] [CrossRef]
- Ruesgas-Ramón, M.; Figueroa-Espinoza, M.C.; Durand, E. Application of Deep Eutectic Solvents (DES) for Phenolic Compounds Extraction: Overview, Challenges, and Opportunities. J. Agric. Food Chem. 2017, 65, 3591–3601. [Google Scholar] [CrossRef]
- Oliveira, G.; Farias, F.O.; Sosa, F.H.B.; Mafra, M.R. Green solvents to tune the biomolecules’ solubilization in aqueous media: An experimental and in silico approach by COSMO-RS. J. Mol. Liq. 2021, 341, 117314. [Google Scholar] [CrossRef]
- Cheong, M.W.; Tong, K.H.; Ong, J.J.M.; Liu, S.Q.; Curran, P.; Yu, B. Volatile composition and antioxidant capacity of Arabica coffee. Food Res. Int. 2013, 51, 388–396. [Google Scholar] [CrossRef]
- Hečimović, I.; Belščak-Cvitanović, A.; Horžić, D.; Komes, D. Comparative study of polyphenols and caffeine in different coffee varieties affected by the degree of roasting. Food Chem. 2011, 129, 991–1000. [Google Scholar] [CrossRef]
- Sato, T.; Takahata, T.; Honma, T.; Watanabe, M.; Wagatsuma, M.; Matsuda, S.; Smith, R.L.; Itoh, N. Hydrothermal Extraction of Antioxidant Compounds from Green Coffee Beans and Decomposition Kinetics of 3- o-Caffeoylquinic Acid. Ind. Eng. Chem. Res. 2018, 57, 7624–7632. [Google Scholar] [CrossRef]
- Syakfanaya, A.M.; Saputri, F.C.; Munim, A. Simultaneously Extraction of Caffeine and Chlorogenic Acid from Coffea canephora Bean using Natural Deep Eutectic Solvent-Based Ultrasonic Assisted Extraction. Pharmacogn. J. 2019, 11, 267–271. [Google Scholar] [CrossRef]
- Alonso-Salces, R.M.; Serra, F.; Reniero, F.; HÉberger, K. Botanical and Geographical Characterization of Green Coffee (Coffea arabica and Coffea canephora): Chemometric Evaluation of Phenolic and Methylxanthine Contents. J. Agric. Food Chem. 2009, 57, 4224–4235. [Google Scholar] [CrossRef]
- Shalmashi, A.; Abedi, M.; Golmohammad, F.; Eikani, M.H. Isolation of caffeine from tea waste using subcritical water extraction. J. Food Process Eng. 2009, 33, 701–711. [Google Scholar] [CrossRef]
- Narita, Y.; Inouye, K. High antioxidant activity of coffee silverskin extracts obtained by the treatment of coffee silverskin with subcritical water. Food Chem. 2012, 135, 943–949. [Google Scholar] [CrossRef]
- Dai, Y.; Rozema, E.; Verpoorte, R.; Choi, Y.H. Application of natural deep eutectic solvents to the extraction of anthocyanins from Catharanthus roseus with high extractability and stability replacing conventional organic solvents. J. Chromatogr. A 2016, 1434, 50–56. [Google Scholar] [CrossRef]

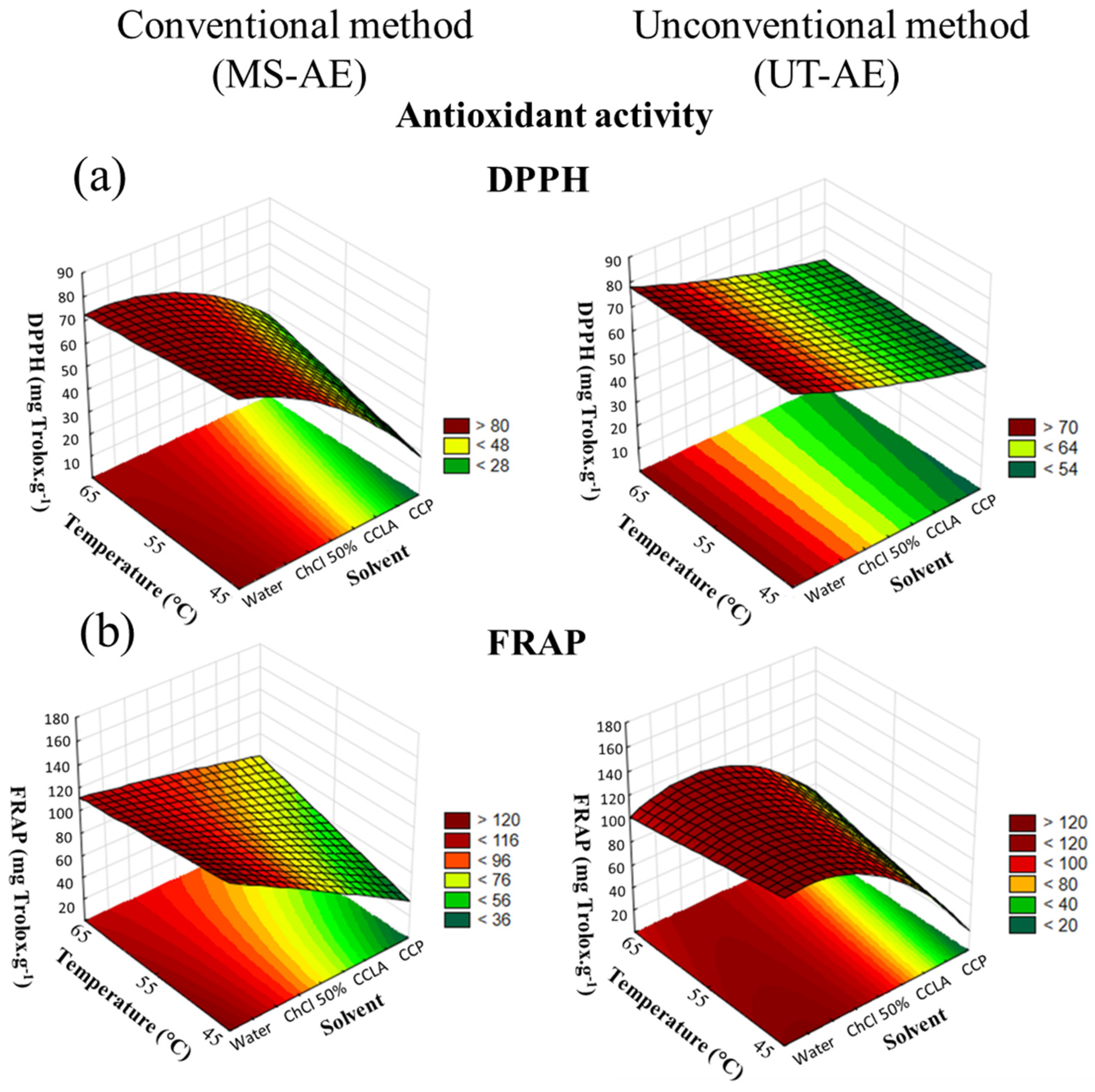
| Explanatory Variables | TPC (mgGAE g−1) * | DPPH (mgtrollox g−1) * | FRAP (mgtrollox g−1) * | ||
|---|---|---|---|---|---|
| Solvent | T (°C) | Technique | |||
| Water | 25 | MS-AE | 22.3 ± 5.8 db | 36.5 ± 4.5 eb | 51.7 ± 1.1 cb |
| ChCl 50% wt. | 36.4 ± 2.9 aa | 49.6 ± 3.6 ba | 63.9 ± 4.4 ba | ||
| CCLA | 22.8 ± 0.6 db | 26.3 ± 0.6 hc | 44.2 ± 6.3 cb | ||
| CCP | 18.1 ± 1.2 eb | 32.2 ± 3.1 fc | 45.2 ± 6.9 cb | ||
| CCGY | 20.8 ± 5.9 eb | 27.1 ± 3.3 hc | 42.8 ± 4.5 cb | ||
| CCXY | 12.2 ± 0.6 gb | 29.7 ± 1.1 gc | 31.2 ± 4.5 cc | ||
| Water | UT-AE | 33.9 ± 0.6 bb | 44.8 ± 1.6 cb | 70.6 ± 2.4 bb | |
| ChCl 50 wt.% | 36.3 ± 1.2 aa | 53.6 ± 1.7 aa | 99.3 ± 2.2 aa | ||
| CCLA | 25.5 ± 1.8 dc | 42 ± 2 dc | 59.7 ± 5.2 bb | ||
| CCP | 30.8 ± 2.2 cb | 39 ± 2 ed | 66.4 ± 6.2 bb | ||
| CCGY | 28.9 ± 2.7 cb | 37.1 ± 2.1 ed | 55.4 ± 0.9 cc | ||
| CCXY | 22.9 ± 4.5 fd | 31.7 ± 0.1 fe | 35.1 ± 0.5 c | ||
| Water | 45 | MS-AE | 31.5 ± 5.1 cb | 39.4 ± 6.7 bb | 54.6 ± 3.9 bb |
| ChCl 50 wt.% | 45.7 ± 6.3 ba | 61.5 ± 2.7 aa | 68.6 ± 0.9 ba | ||
| CCLA | 25.3 ± 2.3 eb | 34.3 ± 0.2 bb | 66.4 ± 0.4 ba | ||
| CCP | 25.9 ± 2.6 eb | 34.6 ± 2.2 bb | 62.3 ± 6.7 ba | ||
| CCGY | 29.1 ± 1.3 eb | 35.3 ± 6.8 bb | 43.2 ± 5.9 cb | ||
| CCXY | 24.0 ± 2.8 fc | 25.7 ± 1.1 cb | 46.5 ± 5.9 bb | ||
| Water | UT-AE | 39.3 ± 0.2 cb | 47.7 ± 4.9 bb | 77.5 ± 1.5 bb | |
| ChCl 50 wt.% | 47.5 ± 1.3 aa | 62.4 ± 1.7 aa | 102 ± 5 aa | ||
| CCLA | 36.2 ± 2.9 cc | 38.3 ± 0.8 bb | 71.7 ± 0.8 bb | ||
| CCP | 34.3 ± 0.7 cd | 44.5 ± 6.8 bb | 65.2 ± 1.5 bb | ||
| CCGY | 30.5 ± 1.7 de | 44.1 ± 6.4 bb | 61.3 ± 4.6 bb | ||
| CCXY | 26.1 ± 0.4 ef | 30.69 ± 0.04 bc | 54.3 ± 6.9 bc | ||
| Water | 65 | MS-AE | 40.3 ± 6.7 bb | 59.7 ± 6.8 bb | 87.9 ± 6.2 bb |
| ChCl 50 wt.% | 54.6 ± 1.4 aa | 75.4 ± 2.2 aa | 112.1 ± 2.7 fa | ||
| CCLA | 22.4 ± 2.1 dc | 35.2 ± 4.7 dd | 69.9 ± 2.4 cb | ||
| CCP | 27.5 ± 2.3 bc | 47.1 ± 0.5 dc | 87.1 ± 6.6 bb | ||
| CCGY | 23.6 ± 0.9 dc | 37.1 ± 0.8 dd | 65.6 ± 7.9 cb | ||
| CCXY | 17.3 ± 0.3 ec | 24.1 ± 3.6 ee | 53 ± 4 cc | ||
| Water | UT-AE | 36.94 ± 0.04 cb | 39.6 ± 5.4 dc | 76 ± 6 bb | |
| ChCl 50 wt.% | 46.5 ± 2.2 ba | 56.3 ± 0.8 ba | 90.1 ± 3.2 ca | ||
| CCLA | 27.6 ± 1.1 db | 43.6 ± 0.4 db | 68.6 ± 2.2 cb | ||
| CCP | 37.4 ± 6.3 cb | 49.9 ± 1.3 cb | 60.1 ± 5.7 cb | ||
| CCGY | 27.7 ± 2.9 db | 49.8 ± 0.4 cb | 72.2 ± 5.1 bb | ||
| CCXY | 29.3 ± 1.8 db | 28.4 ± 2.3 ed | 50.4 ± 1.1 cc | ||
| Explanatory Variables | TPC (mgGAE g−1) * | DPPH (mgtrolox g−1) * | FRAP (mgtrolox g−1) * | ||
|---|---|---|---|---|---|
| Solvent | T (°C) | Technique | |||
| Water | 45 | MS-AE | 49.7 ± 2.1 db | 71.7 ± 0.1 ba | 104.9 ± 0.2 cb |
| ChCl 50 wt.% | 58.1 ± 1.3 ba | 72.1 ± 2.4 ba | 115.4 ± 2.1 ba | ||
| CCLA | 31.1 ± 2.6 gc | 44.99 ± 0.05 eb | 55.9 ± 2.4 gc | ||
| CCP | 22.8 ± 2.2 hd | 31 ± 4 fc | 48.3 ± 1.2 hd | ||
| Water | UT-AE | 52.4 ± 1.2 cb | 71.8 ± 1.1 b | 98.5 ± 3.7 db | |
| ChCl 50 wt.% | 62,1 ± 0.4 aa | 73.2 ± 0.2 ba | 128.7 ± 2.1 aa | ||
| CCLA | 36.2 ± 0.4 ec | 51.2 ± 0.4 dc | 69.7 ± 2.8 fd | ||
| CCP | 32.4 ± 3.1 fd | 58.9 ± 0.2 ed | 52.5 ± 0.2 ec | ||
| Water | 65 | MS-AE | 61.4 ± 0.5 ca | 75.5 ± 0.9 aa | 106.9 ± 0.5 db |
| ChCl 50 wt.% | 57.7 ± 0.2 db | 72.4 ± 1.6 cb | 118.1 ± 0.6 ca | ||
| CCLA | 34.6 ± 3.3 gc | 54.7 ± 0.9 fc | 68.5 ± 2.1 hd | ||
| CCP | 33.1 ± 0.7 gc | 48.8 ± 1.5 gd | 94.6 ± 0.4 ec | ||
| Water | UT-AE | 62.4 ± 0.4 bb | 73.7 ± 2.7 ba | 122.6 ± 3.1 bb | |
| ChCl 50 wt.% | 68.9 ± 0.9 aa | 73.2 ± 0.2 ba | 142.6 ± 1.1 aa | ||
| CCLA | 47.8 ± 1.4 ec | 59.3 ± 0.4 db | 77.7 ± 2.8 gd | ||
| CCP | 43.1 ± 1.4 fd | 58.1 ± 0.2 ec | 79.2 ± 0.2 fc | ||
| Explanatory Variables | Chlorogenic Acids (mg 100g−1) | Alkaloids (g 100g−1) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Solvent | T (°C) | Technique | 4.5diCQA | 3.5diCQA | 3.4diCQA | 5-FQA | 4-CQA | 5-CQA | 3-CQA | TOTAL CGA | Caffeine | Trigonelline |
| Water | 25 | MS-AE | 1.12 ± 0.01 jd | 4.59 ± 0.01 id | 1.01 ± 0.02 ie | 31.1 ± 0.2 fc | 36.5 ± 0.4 ie | 173.3 ± 0.4 jd | 18.9 ± 1.5 hd | 277.37 ± 0.08 h | 123.77 ± 0.04 ba | 110.41± 0.09 aa |
| ChCl 50 wt.% | 20.9 ± 0.1 fa | 24.68 ± 0.08 eb | 16.91 ± 0.04 cb | 44.16 ± 0.07 db | 81.4 ± 0.1 ba | 306.8 ± 0.2 ba | 47.2 ± 0.1 ba | 541.34 ± 0.02 ba | 113.4± 0.2 cb | 66.7 ± 2.8 bb | ||
| CCLA | 18.24 ± 0.06 ic | 25.41 ± 0.03 da | 12.6 ± 0.1 hd | 27.56 ± 0.05 hd | 33.7 ± 0.7 jd | 212.4 ± 0.7 gb | 20.57 ± 0.01 gc | 350.3 ± 0.9 fb | 61.8 ± 0.2 hc | 28.3 ± 1.7 cc | ||
| CCP | 19.48 ± 0.02 fb | 25.14 ± 0.01 da | 13.04 ± 0.02 gc | 23.51 ± 0.06 je | 40.1 ± 0.9 hc | 203.3 ± 1.1 hc | 20.01 ± 0.05 gc | 345.6 ± 0.2 fc | 55.73 ± 0.09 id | 14.4 ± 0.2 dd | ||
| CCGY | 18.9 ± 0.1 hc | 22.1 ± 0.2 gc | 45.1 ± 0.1 aa | 65.2 ± 0.2 aa | 47.1 ± 0.8 fb | 207.7 ± 0.5 hb | 29.01 ± 0.03 db | 338.87 ± 0.02 gd | 37.49 ± 0.02 je | 31.45 ± 0.08 cc | ||
| CCXY | - | - | - | - | - | - | - | - | - | - | ||
| Water | UT-AE | 6.98 ± 0.02 if | 15.44 ± 0.03 he | 7.52 ± 0.01 he | 45.45 ± 0.04 cb | 67.79 ± 0.04 cb | 300.1 ± 1.1 cb | 36.93 ± 0.03 cb | 413.21 ± 0.03 dc | 127.4 ± 0.4 aa | 112.43 ± 0.01 aa | |
| ChCl 50 wt.% | 31.15 ± 0.01 aa | 41.89 ± 0.01 aa | 23.91 ± 0.02 ba | 54.18 ± 0.01 ba | 90.4 ± 0.3 aa | 384.5 ± 0.8 aa | 49.94 ± 0.08 aa | 675.92 ± 0.01 aa | 113.4 ± 0.5 bb | 71.6 ± 2.9 bb | ||
| CCLA | 23.75 ± 0.07 db | 33.1 ± 0.1 bb | 16.76 ± 0.03 cb | 36.63 ± 0.03 ec | 49.1 ± 1.1 dc | 268.1 ± 0.2 ec | 27.27 ± 0.06 ec | 454.17 ± 0.01 cb | 94.2 ± 0.4 dc | 27.9 ± 0.6 cc | ||
| CCP | 18.58 ± 0.01 id | 23.41 ± 0.04 fd | 13.24 ± 0.02 gd | 23.52 ± 0.02 jf | 39.1 ± 0.3 if | 200.62 ± 0.08 if | 22.19 ± 0.08 ie | 339.91 ± 0.01 gd | 83.9 ± 0.1 ed | 25.9 ± 0.5 cd | ||
| CCGY | 18.81 ± 0.03 id | 23.73 ± 0.06 fd | 14.42 ± 0.01 fd | 25.58± 0.05 ie | 43.9 ± 0.4 ge | 210.7 ± 0.3 ge | 20.6 ± 3.2 gf | 357.17 ± 0.03 ee | 72.3 ± 0.3 fe | 28.17 ± 0.09 cc | ||
| CCXY | 22.43 ± 0.04 ec | 29.1 ± 0.1 cc | 15.61 ± 0.08 dc | 29.1 ± 0.1 gd | 48.9 ± 0.7 ed | 249.7 ± 0.9 fd | 26.5 ± 0.1 fd | 419.7 ± 1.7 dc | 64.1 ± 0.3 gf | 28.8 ± 1.5 cc | ||
| Water | 45 | MS-AE | 7.91 ± 0.02 if | 18.09 ± 0.03 ie | 8.79 ± 0.04 if | 48.96 ± 0.04 cb | 75.1 ± 0.2 cb | 287.6 ± 1.6 db | 39.4 ± 0.3 cb | 503.8 ± 0.2 cb | 142.06 ± 0.09 aa | 131.3 ± 0.9 aa |
| ChCl 50 wt.% | 27.14 ± 0.09 ba | 34.6 ± 0.2 ba | 21.96 ± 0.06 ba | 54.1 ± 0.1 ba | 95.3 ± 0.3 aa | 370.7 ± 1.6 ba | 54.1 ± 0.1 aa | 657.78 ± 0.02 ba | 135.8 ± 0.1 cb | 80.4 ± 0.2 cb | ||
| CCLA | 17.76 ± 0.01 gd | 24.92 ± 0.01 gc | 12.55 ± 0.05 gd | 28.54 ± 0.02 ic | 39.3 ± 0.1 jd | 210.7 ± 0.2 je | 21.82 ± 0.03 kd | 355.58 ± 0.01 je | 70.4 ± 0.2 ed | 45.1± 0.5 ed | ||
| CCP | 21.86 ± 0.07 eb | 26.71 ± 0.07 fb | 15.64 ± 0.06 eb | 28.01 ± 0.05 jd | 52.9 ± 0.2 fc | 229.1 ± 0.4 hc | 25.84 ± 0.06 ic | 400.05 ± 0.05 hc | 105.2 ± 0.1 dc | 57.1 ± 0.3 dc | ||
| CCGY | 20.57 ± 0.02 fc | 26.85 ± 0.01 fb | 14.42 ± 0.01 fc | 27.17 ± 0.02 jd | 44.35 ± 0.09 ie | 225.6 ± 0.2 id | 24.83 ± 0.01 jc | 383.9 ± 0.6 id | 66.7 ± 0.4 je | 25.1 ± 0.5 je | ||
| CCXY | 16.32 ± 0.01 he | 21.72 ± 0.02 hd | 11.52 ± 0.01 he | 21.26 ± 0.01 ke | 33.5 ± 2.6 kf | 186.7 ± 0.3 kf | 20.57 ± 0.01 le | 311.2 ± 0.3 kf | 46.81 ±0.02 kf | 12.2 ± 1.1 kf | ||
| Water | UT-AE | 3.29 ± 0.01 je | 10.95 ± 0.03 je | 3.74 ± 0.01 je | 42.51 ± 0.05 db | 58.2 ± 0.1 ec | 259.2 ± 0.4 fd | 31.01 ± 0.03 ev | 477.31 ± 0.01 db | 132.14 ± 0.09 bb | 118.4 ± 0.5 ba | |
| ChCl 50 wt% | 36.61 ± 0.03 aa | 49.97 ± 0.07 aa | 26.14 ± 0.08 aa | 56.5 ± 0.8 aa | 91.77 ± 0.05 ba | 415.3 ± 1.2 aa | 51.8 ± 0.1 ba | 727.8 ± 0.2 aa | 147.7 ± 0.7 ba | 117.6 ± 1.3 ba | ||
| CCLA | 20.23 ± 0.01 fd | 28.42 ± 0.09 ed | 14.45 ± 0.02 fd | 34.29 ± 0.04 fd | 45.3 ± 1.4 hf | 250.4 ± 0.4 ge | 26.55 ± 0.05 he | 419.7 ± 0.01 ge | 97.3 ± 0.5 gc | 27.8 ± 0.7 ie | ||
| CCP | 23.14 ± 0.06 dc | 31.5 ± 0.1 dd | 16.42 ± 0.01 dc | 33.66 ± 0.06 ge | 51.5 ± 0.5 gd | 273.8 ± 0.4 ec | 29.21 ± 0.03 fd | 459.1 ± 0.1 ec | 98.13 ± 0.06 fc | 34.4 ± 0.2 gc | ||
| CCGY | 23.80 ± 0.03 dc | 30.04 ± 0.07 dc | 16.49 ± 0.04 dc | 31.48 ± 0.07 hf | 49.59 ± 0.06 fe | 261.1 ± 0.6 lf | 27.93 ± 0.02 ge | 440.9 ± 0.8 fd | 81.1 ± 0.2 ie | 30.2 ± 0.9 hd | ||
| CCXY | 26.07 ± 0.03 cb | 33.1 ± 0.1 cb | 18.58 ± 0.05 cb | 36.1 ± 0.3 ec | 61.6 ± 0.3 db | 300.6 ± 0.2 cb | 34.26 ± 0.09 db | 511.3 ± 3.3 cf | 87.21 ± 0.07 ed | 38.1± 0.8 fb | ||
| Water | 65 | MS-AE | 25.57 ± 0.04 dc | 40.4 ± 0.1 dc | 21.44 ± 0.04 dc | 78.76 ± 0.03 aa | 123.94 ± 0.06 aa | 382.1 ± 1.6 cb | 36.93 ± 0.03 db | 811.9 ± 0.2 ba | 202.1± 0.1 aa | 201.1 ± 0.3 aa |
| ChCl 50 wt.% | 34.01 ± 0.05 ba | 43.4 ± 0.2 ba | 27.19 ± 0.06 ba | 62.1 ± 0.1 cb | 109.7 ± 0.3 bb | 422.4 ± 0.7 ba | 62.05 ± 0.04 aa | 760.76 ± 0.01 cb | 145.9 ± 0.6 bb | 70.9 ± 3.4 db | ||
| CCLA | 18.2 ± 0.1 he | 25.14 ± 0.01 ie | 12.76 ± 0.01 ie | 30.01 ± 0.02 jd | 39.3 ± 0.4 je | 219.4 ± 0.4 je | 22.7 ± 0.1 je | 367.87 ± 0.01 je | 73.5 ± 0.4 gc | 31.6 ± 0.3 hc | ||
| CCP | 34.59 ± 0.06 bb | 43.7 ± 0.1 cb | 24.21 ± 0.04 cb | 42.05 ± 0.06 ec | 61.1 ± 0.3 fc | 365.8 ± 1.7 dc | 35.05 ± 0.06 cc | 606.43 ± 0.01 dc | 68.51 ± 0.06 hd | 22.5 ± 0.9 je | ||
| CCGY | 19.79 ± 0.05 gd | 26.85 ± 0.04 gd | 13.04 ± 0.04 hd | 27.22 ± 0.04 ke | 42.8 ± 0.1 jd | 224.3 ± 0.5 id | 23.76 ± 0.02 id | 378.5 ± 0.6 ie | 66.1 ± 0.1 ie | 24.1 ± 0.6 jd | ||
| CCXY | 16.57 ± 0.07 if | 22.06 ± 0.07 jf | 11.62 ± 0.05 jf | 21.56 ± 0.04 lf | 34.4 ± 0.1 kf | 185.7 ± 0.4 kf | 19.37 ± 0.01 kf | 311.2 ± 0.6 kf | 49.19 ± 0.08 jf | 18.1 ± 0.9 jf | ||
| Water | UT-AE | 10.98 ± 0.01 jf | 19.07 ± 0.01 ke | 3.74 ± 0.01 je | 46.1 ± 0.1 db | 74.1 ± 0.2 db | 293.4 ± 0.5 ec | 39.39 ± 0.05 cb | 486.13 ± 0.06 fc | 128.6 ± 0.3 cb | 114.1 ± 0.8 ba | |
| ChCl 50 wt.% | 41.78 ± 0.08 aa | 57.7 ± 0.1 aa | 30.55 ± 0.06 aa | 65.1 ± 0.1 ba | 106.6 ± 0.3 ca | 460.1 ± 1.7 aa | 60.01 ± 0.03 ba | 821.14 ± 0.01 aa | 132.1 ± 0.7 ba | 84.1 ± 3.6 cb | ||
| CCLA | 23.14 ± 0.01 fe | 32.62 ± 0.02 gc | 16.55 ± 0.01 gd | 36.81 ± 0.01 gd | 49.1 ± 0.1 if | 275.8 ± 0.3 he | 28.6 ± 0.1 gd | 462.41 ± 0.01 he | 97.9 ± 0.5 dc | 40.5 ± 0.3 fc | ||
| CCP | 25.23 ± 0.01 dc | 33.91 ± 0.02 fc | 17.51 ± 0.03 fc | 33.48 ± 0.01 ie | 50.9 ± 0.6 he | 285.56 ± 0.09 fd | 29.2 ± 0.2 fd | 475.8± 1.1 gd | 85.23 ± 0.01 dc | 33.4 ± 0.7 gd | ||
| CCGY | 24.91 ± 0.02 ed | 31.41 ± 0.04 hd | 17.58 ± 0.04 fc | 34.29 ± 0.01 he | 57.3 ± 0.5 gd | 278.21 ± 0.04 gf | 27.9 ± 3.3 he | 472.44 ± 0.04 gc | 60.9± 0.5 fe | 26.1 ± 0.1 ie | ||
| CCXY | 29.4 ± 0.5 cb | 38.78 ± 0.01 eb | 20.43 ± 0.01 eb | 39.01 ± 0.03 fc | 63.2 ± 0.5 ec | 319.9 ± 0.3 eb | 34.3 ± 0.1 ec | 544.9 ± 3.8 eb | 86.8 ± 0.2 ed | 42.4± 0.3 ec | ||
| Explanatory Variables | Chlorogenic Acids (mg 100g−1) | Alkaloids | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Solvent | T (°C) | Technique | 4.5diCQA | 3.5diCQA | 3.4diCQA | 5-FQA | 4-CQA | 5-CQA | 3-CQA | TOTAL CGAs | Caffeine | Trigonelline |
| Water | 45 | MS-AE | 36.8 ± 0.8 cb | 42.2 ± 0.9 cb | 24.2 ± 0.2 ec | 9.4 ± 0.6 db | 128.09 ± 0.42 dc | 268.1 ± 2.9 db | 117.7 ± 0.6 aa | 710.8 ± 0.3 db | 214.2 ± 0.6 bs | 41.3 ± 0.2 cb |
| ChCl 50 wt.% | 48.29 ± 0.06 ba | 46.26 ± 0.06 ba | 48.1± 5.2 ba | 129.8 ± 0.2 ba | 79.7 ± 0.4 ed | 422.9 ± 0.8 aa | 107.8 ± 0.5 bb | 882.73 ± 0.01 ba | 199.1 ± 0.3 cb | 54.8 ± 0.3 aa | ||
| CCLA | 31.02 ± 0.08 dc | 24.97 ± 0.02 ec | 28.2 ± 0.1 db | 63.46 ± 0.01 fc | 147.5 ± 0.2 ba | 212.2 ± 0.8 ec | 51.2 ± 0.8 ec | 558.48 ± 0.09 fc | 114.6 ± 0.5 ec | 23.51 ± 0.09 ec | ||
| CCP | 22.1 ± 0.3 fd | 17.2 ± 0.1 gd | 18.9 ± 0.1 fd | 39.2 ± 0.1 hd | 137.8 ± 0.2 cb | 108.2 ± 0.7 gd | 26.62± 0.08 gd | 370.02 ± 0.09 ed | 64.1 ± 0.6 gd | 15.54 ± 0.04 fd | ||
| Water | UT-AE | 30.2 ± 0.2 dc | 28.7 ± 0.4 ec | 35.1 ± 1.3 cb | 123.1± 0.8 cb | 50.6 ± 0.1 fb | 359.4 ± 0.6 cb | 103.1 ± 0.6 cb | 729.57 ± 0.09 cb | 117.1 ± 0.4 ec | 34.04 ± 0.06 db | |
| ChCl 50 wt.% | 56.3 ± 0.6 aa | 49.7 ± 0.3 aa | 53.9 ± 0.4 aa | 136.5 ± 1.4 aa | 157.1 ± 0.3 aa | 405.4 ± 1.1 ba | 112.2 ± 0.8 ba | 971.23 ± 0.03 aa | 221.9 ± 0.4 aa | 47.4 ± 0.6 ba | ||
| CCLA | 35.9 ± 0.1 cb | 31.63 ± 0.07 db | 35.2 ± 0.1 cb | 80.7 ± 0.3 ec | 31.28 ± 0.03 gc | 298.07 ± 0.05 dc | 74.7 ± 0.7 dc | 586.7 ± 0.2 ec | 162.1 ± 0.4 db | 31.6 ± 0.1 db | ||
| CCP | 25.56 ± 0.02 ed | 22.51 ± 0.04 fd | 24.1 ± 2.1 ec | 51.59 ± 0.01 gd | 21.61 ± 0.02 hd | 172.2 ± 0.5 fd | 41.1 ± 0.1 fd | 358.72 ± 0.06 gd | 112.2 ± 0.9 fd | 24.02 ± 0.07 ec | ||
| Water | 65 | MS-AE | 47.01 ± 0.03 cb | 46.01 ± 0.09 ba | 49.17 ± 0.03 ba | 160.1 ± 0.1 aa | 86.4 ± 0.2 db | 410.1 ± 0.7 cb | 129.4± 0.2 bb | 923.5 ± 0.2 cb | 239.4 ± 0.5 aa | 52.2 ± 0.1 ba |
| ChCl 50 wt.% | 78.7 ± 0.6 aa | 44.55 ± 0.07 cb | 48.9 ± 0.2 ba | 122.1 ± 1.5 cb | 40.9 ± 0.4 gd | 466.3 ± 1.1 aa | 133.1 ± 0.3 aa | 934.6 ± 0.3 ba | 214.1 ± 0.5 bb | 54.35 ± 0.03 as | ||
| CCLA | 36.76 ± 0.03 dc | 30.2 ± 0.6 fc | 34.7 ± 0.7 eb | 79 ± 1 fc | 108.73 ± 0.04 ba | 279.3 ± 0.7 fc | 69.6 ± 1.6 ec | 638.36 ± 0.01 fd | 149.7 ± 0.6 ec | 32.73 ± 0.06 db | ||
| CCP | 23.8 ± 0.4 gd | 18.5 ± 0.3 hd | 21.2 ± 0.1 gc | 44.4 ± 0.1 hd | 99.1 ± 0.2 cc | 134.9 ± 0.3 hd | 34.1 ± 0.8 gd | 375.89 ± 0.06 hd | 71.9 ± 0.2 gd | 16.97 ± 0.03 ec | ||
| Water | UT-AE | 34.9± 0.3 ec | 33.4 ± 1.6 eb | 38.7 ± 0.3 dc | 115.4 ± 0.4 db | 11.9 ± 0.3 hd | 393.1 ± 0.2 db | 104.7 ± 1.6 cb | 733.1 ± 0.2 db | 194.5 ± 0.1 cb | 34.1 ± 0.2 db | |
| ChCl 50 wt.% | 62.1 ± 0.7 ba | 56.8 ± 0.1 aa | 60.9 ± 0.2 aa | 148.1 ± 0.9 ba | 118.4 ± 0.4 aa | 430.9 ± 0.7 ba | 119.3 ± 0.3 ba | 996.5 ± 0.3 aa | 217.1 ± 0.2 ba | 43.9 ± 0.5 ca | ||
| CCLA | 45.3 ± 0.8 cb | 38.67 ± 0.06 db | 42.2 ± 0.2 cb | 100.3 ± 0.9 ec | 70.1 ± 0.2 eb | 326.3 ± 0.6 ec | 80.1 ± 0.5 dc | 702.9 ± 0.5 eb | 168.6 ± 0.9 dc | 35.2 ± 0.2 db | ||
| CCP | 32.8 ± 0.9 fd | 27.48 ± 0.02 gc | 30.17 ± 0.05 fd | 68.78 ± 0.09 gd | 60.32 ± 0.04 fc | 228.4 ± 0.9 gd | 52.2 ± 0.7 fd | 500.2 ± 0.5 gd | 97.6 ± 0.4 fd | 33.4 ± 0.1 db | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ronko, L.Z.; Antoniassi, M.A.; Ueda, K.M.; Leal, F.C.; Toci, A.T.; Igarashi-Mafra, L.; Mafra, M.R.; Farias, F.O. Valorization of Raw Coffee Beans (Coffea arabica and Coffea canephora) through Solvent Development and Extraction of Bioactive Compounds. Separations 2022, 9, 423. https://doi.org/10.3390/separations9120423
Ronko LZ, Antoniassi MA, Ueda KM, Leal FC, Toci AT, Igarashi-Mafra L, Mafra MR, Farias FO. Valorization of Raw Coffee Beans (Coffea arabica and Coffea canephora) through Solvent Development and Extraction of Bioactive Compounds. Separations. 2022; 9(12):423. https://doi.org/10.3390/separations9120423
Chicago/Turabian StyleRonko, Letícia Zander, Maria Alice Antoniassi, Karina Mayumi Ueda, Fernando Castro Leal, Aline Theodoro Toci, Luciana Igarashi-Mafra, Marcos R. Mafra, and Fabiane Oliveira Farias. 2022. "Valorization of Raw Coffee Beans (Coffea arabica and Coffea canephora) through Solvent Development and Extraction of Bioactive Compounds" Separations 9, no. 12: 423. https://doi.org/10.3390/separations9120423
APA StyleRonko, L. Z., Antoniassi, M. A., Ueda, K. M., Leal, F. C., Toci, A. T., Igarashi-Mafra, L., Mafra, M. R., & Farias, F. O. (2022). Valorization of Raw Coffee Beans (Coffea arabica and Coffea canephora) through Solvent Development and Extraction of Bioactive Compounds. Separations, 9(12), 423. https://doi.org/10.3390/separations9120423