Abstract
This study’s goal is to use a Box–Behnken design [BBD] methodology to create a new reverse-phase high-performance liquid chromatography diode-array detection [RP-HPLC-DAD] method for the simultaneous quantification of Amitriptyline and Propranolol in tablet dosages. The amitriptyline and propranolol standard drug peaks were obtained using a C-18 column with a dimension of 4.6 × 100 mm and a particle size packing of 2.5 µm at the retention time of 5.328 and 7.48 min, respectively. The mobile phase composition was a 75:25 mixture of methanol and 0.1 percent orthophosphoric acid, flowing at 1.0 mL/min at 26 °C. The peaks were identified at 257 nm after injecting 20 µL of the sample. An assay of the marketed tablets was performed, and the result was 101.33 and 99.4% for amitriptyline and propranolol, respectively, when compared to the standard calibration curve. Forced degradation investigations, such as acid, base, H2O2, and neutral condition, were performed. The results for both medications in term of % degradation were as follows: amitriptyline (16.07, 91.92, 26.98, and 0.64) and propranolol (15.84, 11.52, 9.09, and 3.62). According to the ICH criteria, the findings of the validation parameters were within an acceptable range. The new RP-HPLC-DAD method with BBD application is easy, accurate, and time-saving.
1. Introduction
Amitriptyline’s IUPAC name is 3-(5, 6-dihydrodibenzo [2,1-b:2′,1′-f][7]annulen-11-ylidene)-N,N dimethylpropan-1-amine (Figure 1). It is a dibenzocycloheptene-derivative tricyclic antidepressant [TCA] and analgesic. It has a tricyclic ring structure with an alkyl amine substitution on the central ring. Amitriptyline [AMPL] has been shown to improve mood in patients who are depressed. TCAs have a strong inhibitory effect on serotonin and norepinephrine reuptake [].
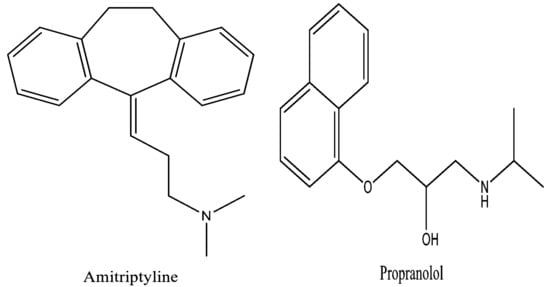
Figure 1.
Chemical structure of Amitriptyline and Propranolol.
Propanolol’s IUPAC name is 1-(isopropylamino)-3-(naphthalen-1-yloxy) propan-2-ol in Figure 1, Propranolol [PROL]. It is a beta-adrenoceptor blocking medication (beta-blocker) that is primarily used to treat angina and hypertension. It is the only medication that has proven to be effective in the prevention of migraine headaches in kids. PROL hydrochloride is a generic medication that is accessible in tablet, oral solution, and syrup forms. PROL is also used to treat hypertension, irregular heartbeats, cardiovascular disease, and even certain types of tremor [].
Both drugs in single-dose form have pharmacological action. AMPL blocks the membrane pump mechanism that is liable for serotonin uptake in serotonergic neurons. Serotonin levels rise, which reduces the brain’s electrical activity. This is the pathway understood to play a role in migraine prevention. PROL tries to compete with sympathomimetic neurotransmitters, such as catecholamines, for binding at beta (1)-adrenergic receptors, resulting in decreased vasodilator responses to beta-adrenergic stimulation. Migraine treatment is aided by membrane stabilising action on cranial blood vessels. Both mechanisms work simultaneously, which are advantageous to the treatment of migraine [].
A literature review of the analysis of AMPL and PROL has been conducted, single and in combination with different analytical techniques. A quantitative HPLC analysis of psychotherapeutic medications had been reported as the simultaneous determination of AMPL hydrochloride and perphenazine [], and the quantities of AMPL and its principal metabolite, nortriptyline, in human plasma were determined using a HPLC in combination with UV and particle beam mass spectrometry []. To evaluate AMPL and nortriptyline in serum samples for clinical monitoring, a micellar liquid chromatographic technique was devised []. AMPL residues in waste were determined using ionic liquid-based immersed droplet micro-extraction and HPLC []. HPLC-DAD measurement of AMPL and its principal metabolites in human plasma had also been conducted []. The simultaneous determination of AMPL hydrochloride and chlordiazepoxide had been performed in tablet dose forms using spectrophotometry and chromatography []. Tricyclic antidepressant urine sample was analysed using a supported liquid membrane approach for HPLC []. PROL determination in human plasma had been analysed using a HPLC with UV detector []. A HPLC technique for the measurement of PROL and its primary metabolite, 4-hydroxypropranolol, was devised, which was simple, sensitive, and selective []. PROL enantiomers were quantified in tiny blood samples from rats using a RP-HPLC after chiral derivatization []. With the use of a HPLC-fluorescence analytical technique, an immobilised polysaccharide-based chiral stationary phase was used to measure PROL enantioselectively in rat serum []. In ex-vivo rat intestinal permeability studies, RP-HPLC with UV detection was employed to simultaneously quantify indinavir and PROL []. Using RP-HPLC, the enantiomers PROL and 4-hydroxypropranolol were determined simultaneously []. Using HPTLC, the simultaneous estimation of AMPL, atenolol, and PROL had been reported for almost 98% of organic mobile phases of different combinations of benzene, methanol, and acetone [].
LC analytical procedures have several drawbacks, such as being dull; being costly; causing column blockage because of the high strength buffer; requiring an infrequent solvent, such as tetra butyl; and providing less precise results due to the high flow rate [].
The above weaknesses can be counterbalanced in analysis by employing QbD strategy. Researchers have developed simple and reliable methods for estimating multiple medications in a single-dose form. For qualitative and quantitative analyses, optimising chromatographic conditions with the QbD technique can take less time, be more accurate, and be less expensive. Previously, QbD approaches to analysis were documented in API and herbal formulations [,].
The multiple factorial analysis coupled in blocks was first proposed by Box and Behnken in 1960. The reduction in the number of experiments is this method’s main benefit. The configurations where all of the variables are either at their highest or lowest levels are not included in the BBD, and it can be used to analyse chromatographic data and improve chromatographic workflow. In a previous study, an ANOVA analysis identified many interdependent relationships as well as the most important factors in the drug separation. They were watched for the factors that did not matter when being analysed separately [].
With the help of the available literature, the method for this study was designed. The selection of factors which could influence the response, such as retention time and column performance, took priority. In simultaneous estimation, peak resolution and column performance are very important. The BBD applications for the optimisation of chromatographic conditions were given a best result using the selected factors.
This study was conducted to estimate AMPL and PROL in their marketed formulations using a BBD methodology for optimising chromatographic conditions in a RP-HPLC-DAD analytical technique method development, and validation was performed in accordance with the ICH recommendations. It is a novel, easy, accurate, and precise method. To the best of our knowledge, no research has been published on the simultaneous estimation of AMPL and PROL in tablet dosage form using a BBD approach. This method will be useful for routine analysis in quality control. Because the analytical time is reduced, it is more cost-effective.
2. Materials and Methods
2.1. Chemicals
From Sigma Aldrich, we purchased standard AMPL and PROL, and, from S.D., we purchased fine chemical, Mumbai HPLC grade methanol. Other chemicals, such as KH2PO4 and ortho-phosphoric acid [OPA] (85–88% purity), were purchased from Loba Chemie Pvt. Ltd. Mumbai, Maharashtra 400005, India.
2.2. Instrumentation
The details of the HPLC AGILENT (1100), gradient system, DAD detector, and software (Chemstation 1290 infinity II) were provided. The C-18 column had a dimension of 4.6 × 100 mm × 2.5 µm.
2.3. QbD Concept Use to Optimize the Chromatographic Conditions
Analytical target profile [ATP]: The goal is to improve the peak precision in simultaneous estimation by optimising the separation settings. The peaks must have a low tailing factor and be well-resolved. ATP should meet the quantitative method’s quality standard [].
2.4. Risk Assessment Studies
The aim of risk assessment studies is to investigate the effects of numerous elements that influence the target method’s quality profile [TMQP] []. Critical analytical attributes [CAAs] enable researchers to appraise the ties between the critical method parameters of the TMQP prior to risk assessment investigations. The data from risk assessment studies aid in determining the root causes of the problems and the sources of faults, variances, defects, or failures. Another function of risk assessment studies is to collect information on an individual’s risk factors, which can then be classified into three levels and designated as low, medium, and high risk. A total of seven factors were examined for screening in this investigation. Three parameters were chosen for the systemic optimization based on their high-, medium-, and low-risk scores [].
2.5. Optimization
The chromatographic conditions were optimised using BBD (Design Expert 13.0.3.0 software Stat-Ease Inc., Minneapolis, MN, USA). In the given sets of factors and responses, seventeen trials were conducted, from which five trials were in optimised conditions. The effects of the interactions between the factors were observed in the remaining twelve trials. In the simultaneous estimation of AMPL and PROL, three factors were considered, which affected the retention time and tailing factor responses in the chromatogram. The trials are presented in Table 1 [,].

Table 1.
Box-Behnken Design Summary.
2.6. RP-HPLC-DAD Method Development
The mobile phase composition and ratio were finalized as methanol (10 mM of KH2PO4 at pH 4.0) with OPA at a ratio of 75:25, respectively, using a flow rate of 1.0 mL/min at 26 °C. Absorbance was detected at 257 nm and the volume of the sample for the experiment was used at 20 µL. The sample run time in the system was 10 min; the retention times of AMPL and PROL were 5.324 and 7.480 min, respectively; and the theoretical plate values were 9535 and 10,938. The height of the peak expressed in mAU in the chromatogram and the peak value of AMPL and PROL were 5.07 and 18.84, respectively.
(a) Stock solutions: The standard solution was prepared in methanol, with accurately weighted 50 mg of AMPL and 100 mg of PROL, and then dissolved in 100 mL of methanol in a volumetric flask; this is the initial stock solution, with concentrations of 500 µg/mL AMPL and 1000 µg/mL PROL.
(b) Determination of ʎ max: The sample was scanned at 200–400 nm with a drug concentration of 0.5 µg/mL AMPL and 1 µg/mL PROL, and the ʎ max was set at 257 nm.
(c) Preparation of calibration curve: Five dilutions of each drug were prepared for the calibration curve. The AMPL range was 5–25 µg/mL and the PROL range was 20–100 µg/mL. The dilutions were prepared from the respective stock solutions. The limit of detection (LOD) and the limit of quantification (LOQ) were determined. The numerical value of each is presented in Table 2.

Table 2.
Calibration curve details.
2.7. RP-HPLC-DAD Method Validation
In accordance with the ICH recommendations, the RP-HPLC-DAD method was validated. Experiments covering various validation factors, such as system suitability, accuracy, precision, linearity, LOD, LOQ, robustness, and solution stability, were performed [,,].
(a) System suitability test: The performance of the HPLC equipment was tested by executing a system suitability test in which the experiment results had to be reproducible, and it was performed in accordance with USP 24/NF 19. Six batches of the sample were examined before the analysis to ensure the repeatability of the chromatographic apparatus. Two factors were considered, the retention time and the tailing factor, and were calculated to obtain the percentage relative standard deviation (% RSD) values [].
(b) Linearity: This test was performed by injecting standard solutions of each medication across the range (n = 3). A calibration curve was drawn, with the Y-axis representing average peak areas and the X-axis representing concentrations. The calibration curve was used to calculate the coefficient of correlation, slope, and intercept to determine linearity [].
(c) Precision and accuracy: In terms of inter-day and intra-day precision, the experiments were performed by taking into account three quality control samples LQC, MQC, and HQC with the concentrations of AMPL at 05 μg/mL, 15 μg/mL, and 25 μg/mL, respectively, whereas the PROL concentrations were 20 μg/mL, 60 μg/mL, and 100 μg/mL. In each experiment, the % RSD of each concentration level was measured. To determine accuracy, the traditional approach of percentage recovery was used; a pre-measured concentration of 05 μg/mL of the AMPL sample and 20 μg/mL of PROL were obtained, and, thereafter, 0, 50, 100, and 150% drug content solution were introduced [].
(d) Solution stability: The quality control samples were used for the stability studies. Both drug samples (15 μg/mL AMPL) and (60 μg/mL PROL) were kept for fourteen days at 25 °C and for thirty days at 2–8 °C. After performing the analysis, the results were reported in terms of % recovery and % RSD [].
(e) Robustness study: The robustness of the newly developed RP-HPLC-DAD method was tested. Practicality was performed with intentional changes in the chromatographic conditions, and the results were examined. The established method was deemed robust if the variability in the results stayed within the allowable threshold specified in the guidelines. Flow rate, mobile phase composition, and wavelength all could have an effect on the symmetry of the peak and the retention time [].
2.8. Quantitative Analysis of Tablets
Twenty tablets were weighed and crushed (each containing both AMPL and PROL); the overall weight of the powder was 1.6 gm, and the mean weight of the tablet was 0.08 gm (80 mg). The concentration of this stock solution was 100 μg/mL of AMPL and 400 μg/mL of PROL after transferring 80 mg into a volumetric flask and adding 100 mL of methanol. The solution was sonicated for a duration of 30 min, and, after that, it was filtered to 0.45 μm. Across the calibration range, a series of dilutions were prepared. The sample was injected six times at 15 μg/mL and 60 μg/mL concentrations, and its percentage assay was determined from the calibration curve.
2.9. Forced Degradation Study
The standard mixes of both medications were subjected to various circumstances, and the degraded products were analysed under specific chromatographic conditions [,].
(a) Acid hydrolysis: The stock solutions of AMPL (500 μg/mL) and PROL (1000 μg/mL) were made into a solution with the concentrations of AMPL at 25 μg/mL and PROL at 100 μg/mL and 5 mL of 0.1 N HCl to make a total volume of 20 mL; three sets were prepared in round bottom flasks. The solution was refluxed for 45 min at 80 °C on a heated mantle. For the test, 20 µL of the sample was injected three times.
(b) Alkali hydrolysis: AMPL (500 μg/mL) and PROL (1000 μg/mL) stock solutions were prepared into a solution with the concentrations of AMPL at 25μg/mL and PROL at 100 μg/mL and 5 mL of 0.1 N NaOH to make a total volume of 20 mL; three sets were prepared in round bottom flasks. The solution was refluxed for 60 min on a heated mantle at 80 °C. For the test, 20 µL of the sample was injected three times.
(c) Oxidative degradation: A solution with AMPL (500 μg/mL) and PROL (1000 μg/mL) was transferred into three 250 flasks. In each flask, 20 mL of 3 percent H2O2 was added and then refluxed on a heated mantle at 80 °C for two hours. For the test, 20 µL of the sample was injected three times.
(d) Neutral hydrolysis: AMPL (500 μg/mL) and PROL (1000 μg/mL) stock solutions were prepared into a solution with the concentrations of AMPL at 25μg/mL and PROL at 100 μg/mL. A volume of 20 µL was injected three times into the HPLC.
3. Results and Discussion
3.1. Optimization
Following the risk assessment, the [BBD] application selected the following factors: mobile phase composition (% methanol) in the mobile phase, flow rate of the mobile phase, and detecting wavelength. The medication AMPL’s response was recorded as retention time and theoretical plate, as well as for PROL.
(a) The retention time of AMPL: Table 1 depicts AMPL in its optimal state. Five of the seventeen experiments had been optimised (13–17). The quadratic model’s inbuilt ANOVA is noteworthy. The model’s F-value is 491.54, as implied. The majority of the model’s term values are less than 0.0500, indicating that the model as a whole is significant. The model summary statistics are shown in Table 3. The signal-to-noise ratio was measured based on the acceptable precision value. The value in this case is 79.762, suggesting a sufficient signal. The signal-to-noise ratio was also measured using adequate precision. A ratio greater than 4 is preferred. A ratio of 79.762 suggests that the signal is satisfactory. The inbuilt model graphs are shown in Figure 2 and Figure 3. The final equation is presented in terms of coded factors A (−0.0061), B (+0.0246), C (−0.0128), AB (−0.0288), AC (+0.1065), BC (−0.0310), A2 (−0.0871), B2 (+0.0134) and C2 (−0.0214).

Table 3.
Model Summary Statistics.

Figure 2.
Contour plots AMPL retention time in terms of AB, AC and BC.
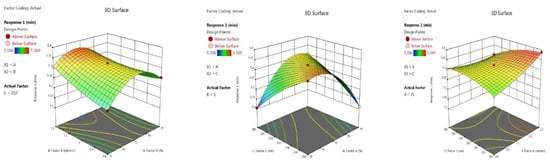
Figure 3.
3D responses AMPL retention time in terms of AB, AC and BC.
(b) Theoretical plate value of AMPL: The column efficiency was proven by the theoretical plate value, which was concluded by the BBD application with inbuilt AVOVA for the quadratic model. The optimised trial is shown in Table 2, and the model summary statistics are shown in Table 3. An F-value of 139.39 indicates that it is important, and the p-values are less than 0.0500. The model graphs of this response in terms of contour plots (AB, AC, and BC) are shown in Figure 4 and the 3D responses (AB, AC, and BC) in Figure 5. The inbuilt model graphs are presented in Figure 4 and Figure 5. The coded factors A (+28.00), B (+92.00), C (−42.50), AB (−233.50), AC (+47.50), BC (−17.50), A2 (+26.75), B2 (+6.75) and C2 (+25.75) are presented in the final equation.

Figure 4.
Contour plots AMPL theoretical plate value in terms of AB, AC and BC.

Figure 5.
3D responses AMPL theoretical plate number in terms of AB, AC and BC.
(c) The retention time of PROL: Table 1 shows PROL in the optimal condition. The ANOVA included into the quadratic model is significant. The model’s f-value is 146.92. The model term’s value is less than 0.0500, indicating that the model is significant across the board. Table 3 displays the model summary statistics. The appropriate precision number evaluates the signal-to-noise ratio; in this case, the value is 33.745, indicating an adequate signal. A ratio greater than 4 is preferred. The inbuilt model graphs are presented in Figure 6 and Figure 7. The coded factors A (+0.0300), B (+0.1450), C (−0.0075), AB (+0.0150), AC (+0.1500), BC (−0.0250), A2 (−0.0025), B2 (−0.0325) and C2 (+0.0075) values are presented in the final equation.
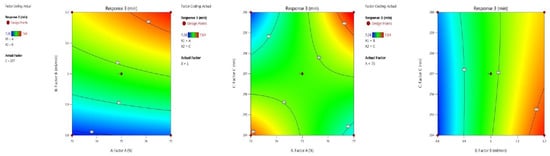
Figure 6.
Contour plots PROL retention time in terms of AB, AC and BC.

Figure 7.
3D responses PROL retention time in terms of AB, AC and BC.
(d) Theoretical plate value of PROL: The column efficiency, as confirmed by the value of the theoretical plate, in the inbuilt ANOVA for the quadratic model was determined with the application of BBD. The optimal trials are presented in Table 2, and Table 3 presents the model summary statistics. The F-value is 170.47, which indicates that it is significant with a p-value less than 0.0500.
Figure 8 and Figure 9 are the model graphs. The coded factors A (+13.63), B (+439.50), C (+33.13), AB (+1.50), AC (+522.25), BC (−125.00), A2 (+21.37), B2 (−8.38) and C2 (+83.38) values are presented in the final equation.
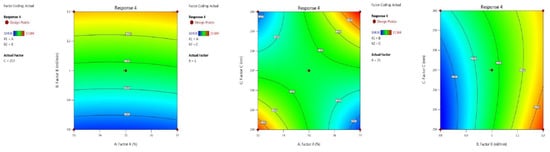
Figure 8.
Contour plots PROL theoretical plate value in terms of AB, AC and BC.

Figure 9.
3D responses PROL theoretical plate number in terms of AB, AC and BC.
3.2. Calibration Curve
The concentration and area of AMPL (Y = 2.1861x − 0.4417 and R2 = 0.9997) and PROL (Y = 2.6451x − 0.0854 and R2 = 0.9999) were found to have linear relationship (Figure 10). The mean standard deviation and percent RSD for AMPL are 0.34 and 1.46, respectively, and 0.97 and 0.93 for PROL. Figure 11 shows a chromatogram of the standard drug at a given retention time.
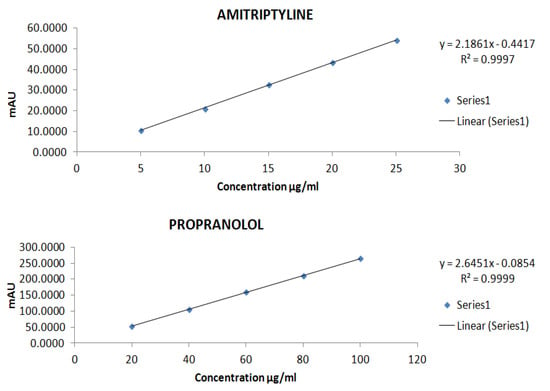
Figure 10.
Calibration curve of both drug AMPL and PROL over optimised range.
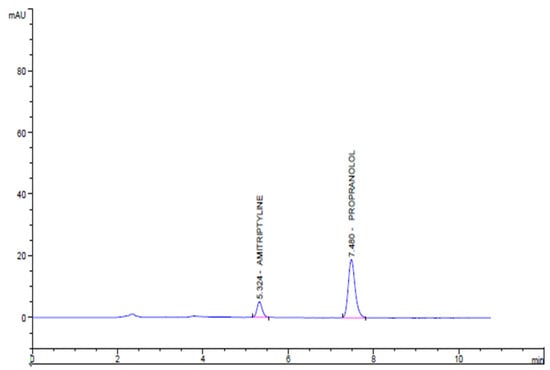
Figure 11.
Chromatogram of the standard drugs AMPL (20 µg) and PROL (80 µg) on optimised chromatographic conditions.
3.3. Method Validation
(a) System suitability test: An experiment was performed to conduct the system suitability test (n = 6). The retention time and tailing factor were considered for the calculation. The results of the experiment were founded as follows: for AMPL, the retention time was calculated for all six samples and the numerical values of average retention time, SD, and % RSD were 5.31 min, 0.05 and 1.04, respectively. The values of the tailing factor of the drug were as follows: the average tailing factor, SD, and % RSD values were 0.83, 0.07, and 9.58, respectively. The results of PROL retention time were calculated in the same way: average retention time (7.46 min), SD (0.11), and % RSD (1.40). The values of the tailing factor were as follows: average tailing factor (0.78), SD (0.06), and % RSD (7.93). The results of the experiment indicate that the device has performed admirably.
(b) Linearity: The experiments were conducted in triplicate manner (n = 3). The results were analysed based on the peak area and concentration. AMPL in the concentration range (5–25 µg/mL) had an average SD peak area of 0.34 and an average % RSD of 1.46. PROL in the concentration range (20–100 µg/mL) had an average SD peak area of 0.97 and an average % RSD of 0.92.
(c) Precision and accuracy: Table 4 shows the precision experiment results in terms of intra-day and inter-day of AMPL and PROL. The accuracy was determined in terms of percentage recovery. For this, a predetermined concentration of 10 µg/mL was considered and an amount of 80 %, 100 %, and 120 % of the drug was added. The results are presented in Table 4.

Table 4.
Precision and Accuracy.
(d) Solution stability: The outcome of the experiment in terms of % recovery and % RSD of AMPL (15 μg/mL) and PROL (60 μg/mL) were 98.15% and 0.88 and 99.05% and 0.98, respectively, at a temperature of 25 °C after 14 days. It was conducted in a triplicate manner. After 30 days at 2–8 °C, the experiments were conducted with same concentration. The results in terms of % recovery and % RSD were determined for AMPL (99.15% and 1.23) and PROL (101.45% and 0.98).
(e) Robustness study: The experiments were conducted in a triplicate manner. Both drugs at a concentration 15 μg/mL (AMPL) and a concentration of 60 μg/mL (PROL) were used. The results of the intended changes in parameter flow rate, mobile phase composition, and detection wavelength are presented in Table 5 in terms of the mean area, SD, and % RSD. A RSD value of less than 2 % suggests that the developed approach is resilient.

Table 5.
Robustness Study.
3.4. Quantitative Analysis of Tablet
With reference to the benchmark of both drugs, the assay results of the tablet (Brand name: TRIPTOLOL) were determined in terms of % of drug content, SD, and % RSD for AMPL (101.33, 0.04, and 0.16) and PROL (99.4%, 0.94, and 0.94).
3.5. Forced Degradation Study
The results of the forced degradation studies are presented in the Table 6 in different conditions, including (a) acidic degradation, (b) basic degradation, (c) H2O2 degradation, and (d) neutral condition, and its chromatogram is presented in Figure 12. The observation of the stability studies indicate that the medications should be protected from stressed conditions. The results in the different conditions show that AMPL in a neutral condition degrades very little compared to other conditions. The presence of tertiary amine in the side chain may cause the most degradation in the basic condition. AMPL has greater degradation compared to PROL in the acidic, basic, and H2O2 conditions. In this study, there is no interference on the degradation chromatogram.

Table 6.
Forced degradation studies.
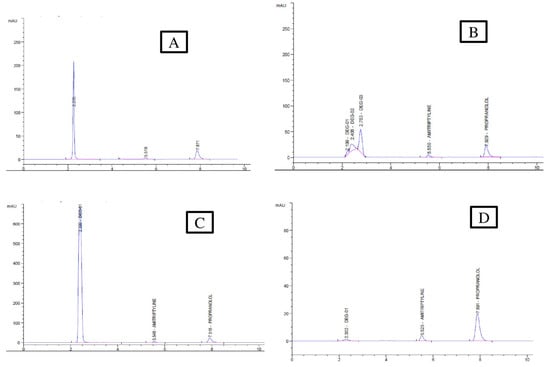
Figure 12.
Chromatogram of the degradation studies (A) 0.1 N HCl (B) 0.1N NaOH (C) 3% H2O2 (D) Neutral.
Studies of forced degradation are conducted to learn a variety of facts, including the pathways by which drug substances and drug products degrade, the distinction between the degradation products of drugs and non-drugs in a formulation, and the inherent stability of drugs in formulation. In addition to these forced degradation studies, they support formulation development, clarify the chemical characteristics of drug molecules, and address stability-related issues.
4. Conclusions
The purpose of this work was to develop a simple, accurate, and precise RP-HPLC-DAD method for estimating both AMPL and PROL in their marketed formulations. The use of BBD in chromatographic optimization for drug estimation is a novel strategy with the benefits of reducing time and money, while improving analytical quality by focusing on quality in the process steps. The QbD inbuilt ANOVA result indicates that it is significant. The chromatogram clearly shows both peaks and the theoretical plate value indicates that the column is efficient. The validation parameter results are within an acceptable range. The experiment’s results are within 90–110 percent of the designated formulation’s assay.
The results of the degradation studies indicate that the tablet dosage form is stable under various stressed conditions. AMPL degradation is greater in basic conditions than in other stressed conditions. Previously, several stability studies in single and combined AMPL were reported [,,,]. To the best of our knowledge, no RP-HPLC-DAD method for simultaneous estimation of AMPL and PROL has been reported. This report’s novel method is the first of its kind. The developed method had a short analytical time and, thus, can be easily performed.
Author Contributions
Conceptualization, M.K.; methodology, M.K. and S.A.; software, S.A.; validation, M.K. and S.A.; formal analysis, M.K.; investigation, M.K.; resources, S.A.; data curation, S.A.; writing, M.K.; writing—review and editing, M.K. and S.A.; visualization, M.K.; supervision, M.K.; project administration, M.K.; funding acquisition, M.K. All authors have read and agreed to the published version of the manuscript.
Funding
The authors extend their appreciation to the Deputyship for research & Innovation, Ministry of Education in Saudi Arabia, for funding this research work through the project number (IF-PSAU-2021/03/18910).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not Applicable.
Data Availability Statement
Not Applicable.
Acknowledgments
The authors acknowledge there sincere thanks to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for providing scientific environment and ambience for writing this research article (IF-PSAU-2021/03/18910).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shaikh, S.; Jadhav, P. RP-HPLC Method Development and Validation for the Simultaneous Estimation of Amitriptyline Hydrochloride and Pantoprazole Sodiumin Bulk and Capsule Dosage Form. J. Drug Deliv. Ther. 2019, 9, 37–42. [Google Scholar]
- Shabir, G.A. Development and Validation of RP-HPLC Method for the Determination of Methamphetamine and Propranolol in Tablet Dosage Form. Indian J. Pharm. Sci. 2011, 73, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Sprenger, T.; Viana, M.; Tassorelli, C. Current Prophylactic Medications for Migraine and Their Potential Mechanisms of Action. Neurotherapeutics 2018, 15, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Ferguson Glenda, K. Quantitative HPLC Analysis of a Psychotherapeutic Medication: Simultaneous Determination of Amitriptyline Hydrochloride and Perphenazine. J. Chem. Educ. 1998, 75, 1615–1618. [Google Scholar] [CrossRef]
- Kudo, K.; Jitsufuchi, N.; Imamura, T. Selective determination of amitriptyline and nortriptyline in human plasma by HPLC with ultraviolet and particle beam mass spectrometry. J. Anal. Toxicol. 1997, 21, 185–189. [Google Scholar] [CrossRef][Green Version]
- Bose, D.; Durgbanshi, A.; Martinavarro-Domínguez, A.; Capella-Peiró, M.E.; Carda-Broch, S.; Esteve-Romero, J.; Gil-Agustí, M. Amitriptyline and nortriptyline serum determination by micellar liquid chromatography. J. Pharmacol. Toxicol. Methods 2005, 52, 323–329. [Google Scholar] [CrossRef]
- Hamed Mosavian, M.T.; Es’haghi, Z.; Razavi, N.; Banihashemi, S. Pre-concentration and determination of amitriptyline residues in waste water by ionic liquid based immersed droplet microextraction and HPLC. J. Pharm. Anal. 2012, 2, 361–365. [Google Scholar] [CrossRef]
- Linden, R.; Antunes, M.V.; Ziulkoski, A.L.; Wingert, M.; Tonello, P.; Tzvetkov, M.; Souto, A.A. Determination of amitriptyline and its main metabolites in human plasma samples using HPLC-DAD: Application to the determination of metabolic ratios after single oral dose of amitriptyline. J. Braz. Chem. Soc. 2008, 19, 35–41. [Google Scholar] [CrossRef]
- Patel, S.; Patel, N.J. Spectrophotometric and chromatographic simultaneous estimation of amitriptyline hydrochloride and chlordiazepoxide in tablet dosage forms. Indian J. Pharm. Sci. 2009, 71, 472–476. [Google Scholar] [CrossRef]
- Salman, S.A.; Sulaiman, S.A.; Ismail, Z.; Gan, S.H. Quantitative determination of propranolol by ultraviolet HPLC in human plasma. Toxicol. Mech. Methods 2010, 20, 137–142. [Google Scholar] [CrossRef]
- Abdel-Hamid, M.E. High-performance liquid chromatographic determination of propranolol and 4-hydroxypropranolol in serum. J. Clin. Pharm. Ther. 1988, 13, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Guttendorf, R.J.; Kostenbauder, H.B.; Wedlund, P.J. Quantification of propranolol enantiomers in small blood samples from rats by reversed-phase high-performance liquid chromatography after chiral derivatization. J. Chromatogr. 1989, 489, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Alanazi, A.M.; Hefnawy, M.M.; AL-Majed, A.A.A.; AL-Suwailem, A.K.; Kassem, M.G.; Mostafa, G.A.; Attia, S.M.; Khedr, M.M. HPLC-fluorescence method for the enantioselective analysis of propranolol in rat serum using immobilized polysaccharide-based chiral stationary phase. Chirality 2014, 26, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Panchagnula, R.; Bansal, T.; Varma, M.V.; Kaul, C.L. Reversed-phase liquid chromatography with ultraviolet detection for simultaneous quantitation of indinavir and propranolol from ex-vivo rat intestinal permeability studies. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2004, 806, 277–282. [Google Scholar] [CrossRef]
- John, C.; Chainulu, C.; Ghosh, P.; Srivastava, S.; Shukla, S.; Satyanarayana, S. Validated High Performance Thin Layer Chromatography Method for the Simultaneous Determination of Amitriptyline, Atenolol and Propranolol. Asian J. Res. Chem. 2011, 4, 1059–1063. [Google Scholar]
- Schaefer, H.G.; Spahn, H.L.M.; Derendorf, H. Simultaneous determination of propranolol and 4-hydroxypropranolol enantiomers after chiral derivatization using reversed-phase high-performance liquid chromatography. J. Chromatogr. 1990, 527, 351–359. [Google Scholar] [CrossRef]
- Jain, P.; Taleuzzaman, M.; Kala, C.; Kumar, G.D.; Ali, A.; Aslam, M. Quality by design (Qbd) assisted development of phytosomal gel of aloe vera extract for topical delivery. J. Liposome Res. 2021, 31, 381–388. [Google Scholar] [CrossRef]
- Czyrski, A.; Sznura, J. The application of Box-Behnken-Design in the optimization of HPLC separation of fluoroquinolones. Sci. Rep. 2019, 19, 19458. [Google Scholar] [CrossRef]
- Kaur, J.; Anwer, M.K.; Sartaj, A.; Panda, B.P.; Ali, A.; Zafar, A.; Kumar, V.; Gilani, S.J.; Kala, C.; Taleuzzaman, M. ZnO Nanoparticles of Rubia cordifolia Extract Formulation Developed and Optimized with QbD Application, Considering Ex Vivo Skin Permeation, Antimicrobial and Antioxidant Properties. Molecules 2022, 27, 1450. [Google Scholar] [CrossRef]
- ICH Harmonization for Better Health, Final Concept Paper, ICH Q14: Analytical Procedure Development and Revision of Q2 (R1) Analytical Validation. (November 2018). Endorsed by the Management Committee on 15 November 2018. Available online: https://database.ich.org/sites/default/files/Q2R2-Q14_EWG_Concept_Paper.pdf (accessed on 19 October 2022).
- Parab, G.V.; Mannur, V.K.; Hullatti, K. Quality assessment and Analytical Quality by Design-based RP-HPLC method development for quantification of Piperine in Piper nigrum L. Futur. J. Pharm. Sci. 2022, 8, 16. [Google Scholar] [CrossRef]
- Ameeduzzafar; El-Bagory, I.K.; Alruwaili, N.; Imam, S.S.; Alomar, F.A.; Elkomy, M.H.; Ahmad, N.; Elmowafy, M. Quality by design (QbD) based development and validation of bioanalytical RP-HPLC method for dapagliflozin: Forced degradation and preclinical pharmacokinetic study. J. Liq. Chromatogr. Relat. Technol. 2020, 43, 53–65. [Google Scholar] [CrossRef]
- Prajapati, P.B.; Patel, A.S.; Shah, S.A. DoE-Based Analytical-FMCEA for Enhanced AQbD Approach to MEER-RP-HPLC Method for Synchronous Estimation of 15 Anti-Hypertensive Pharmaceutical Dosage Forms. J. AOAC Int. 2022, 105, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Haque, S.M. Optimized Box-Behnken experimental design based response surface methodology and Youden’s robustness test to develop and validate methods to determine nateglinide using kinetic spectrophotometry. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 268, 120712. [Google Scholar] [CrossRef]
- ICH Guideline. Validation of Analytical Procedures: Text and Methodology. In Proceedings of the International Conference on Harmonization, Topic Q2 (R1), Geneva, Switzerland, Complementary Guideline on Methodology dated 6 November 1996 incorporated in November 2005. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/q2r1-validation-analytical-procedures-text-and-methodology-guidance-industry (accessed on 19 October 2022).
- FDA. FDA. Analytical Procedures and Methods Validation for Drugs and Biologics Guidance for Industry; FDA: Silver Spring, MD, USA, 2015. [Google Scholar]
- Yadav, P.; Taleuzzaman, M.; Kumar, P. Simultaneous Estimation of Aspirin, Atorvastatin Calcium and Clopidogrel Bisulphate in a Combined Dosage form by RP-HPLC. Asian J. Chem. Sci. 2019, 6, 1–10. [Google Scholar] [CrossRef]
- Alquadeib, B.T. Development and validation of a new HPLC analytical method for the determination of diclofenac in tablets. Saudi Pharm. J. 2019, 27, 66–70. [Google Scholar] [CrossRef]
- Thangabalan, B.; Kahsay, G.; Eticha, T. Development and Validation of a High-Performance Liquid Chromatographic Method for the Determination of Cinitapride in Human Plasma. J. Anal. Methods Chem. 2018, 28, 8280762. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, A.; Pandey, A.; Gupta, V.; Malasoni, R.; Srivastava, A.; Pandey, R.R.; Satyanarayana, M.; Pratap, R.; Dwivedi, A.K. Assay method for quality control and stability studies of a new anti-diabetic and anti-dyslipidemic flavone (S002-853). Pharmacogn. Mag. 2015, 11, S53–S59. [Google Scholar] [CrossRef][Green Version]
- Bhimanadhuni, C.N.; Garikapati, D.R.; Usha, P. Development and validation of an RP-HPLC method for the simultaneous determination of Escitalopram Oxalate and Clonazepam in bulk and its pharmaceutical formulations. ICPJ 2012, 1, 193–198. [Google Scholar] [CrossRef]
- Verma, D.; Mirza, M.A.; Taleuzzaman, M.; Khuroo, T.; Talegaonkar, S.; Kumar, R.; Sahu, P.L.; Iqbal, Z. Development and validation of RP-HPLC method to simultaneously detect lactone and carboxylate form of Topotecan along with Thymoquinone: Application to nanoparticulate anticancer formulation system. J. Anal. Chem. 2020, 4, 503–509. [Google Scholar] [CrossRef]
- Tome, T.; Žigart, N.; Časar, Z.; Obreza, A. Development and Optimization of Liquid Chromatography Analytical Methods by Using AQbD Principles: Overview and Recent. Adv. Org. Process Res. Dev. 2019, 23, 1784–1802. [Google Scholar] [CrossRef]
- Al-Rimawi, F. Development and validation of a simple reversed-phase HPLC-UV method for determination of oleuropein in olive leaves. J. Food Drug Anal. 2014, 22, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Rabie, S.; Ashraf, M.A. RP-HPLC determination of amitriptyline hydrochloride in tablet formulations and urine. Asian J. Res. Chem. 2011, 4, 24–27. [Google Scholar]
- Faraat, A.; Singha, G.N.; Sahua, P.; Nagarab, R.; Nagara, M.; Tyagi, A. Application of an LC/HPLC method development and validation for the simultaneous estimation of amitriptyline hydrochloride and chlordiazepoxide in tablet dosage form by using a reverse phase technique. Pharm Lett. 2015, 7, 172–177. [Google Scholar]
- Srikantha, D.; Raju, R. Method development and validation of chlordiazepoxide and amitriptyline hydrochloride inpharmaceutical formulations by RP-HPLC. Asian J. Biomed. Pharm. Sci. 2014, 4, 8–14. [Google Scholar]
- Karchaliya, C.V.; Patel, P.B. Development and validation of analytical methods for simultaneous estimation of amitriptyline hydrochloride and methylcobalamin in their tablet dosage form by UV spectrophotometric method. Pharma Tutor Mag. 2015, 3, 46–50. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).