Abstract
Recently, neoadjuvant treatment has turned out to be a feasible alternative for individuals suffering from locally advanced breast cancer. The neoadjuvant therapy is a type of chemotherapy that is given either before or after surgeries to diminish a tumor and minimize the likelihood of recurrence. This article demonstrates the development of a unique bioanalytical validated sensitive method by means of an ultra high performance liquid chromatography–tandem mass spectrometry (UPLC–MS/MS) approach for the concurrent estimation of neoadjuvant treatments including 5-Fluorouracil, Doxorubicin, and Capecitabine in rat plasma. Samples were prepared using the fine minor QuEChERS process and analyzed using a Shimadzu-C18 column via an isocratic separation. Acetonitrile:water in the ratio of (30:70) (both containing 0.1 percent formic acid v/v) was the mobile phase employed at a flow rate of 0.20 mL/min. At concentrations of 50.00–500.00 ng/mL for 5-Fluorouracil, 25.00–500.00 ng/mL for Doxorubicin, and 5.00–100.00 ng/mL for Capecitabine, the procedure was shown to be linear. The limit of detection (LOD) was assessed in ng/mL and varied from 1.33 to 13.50. Relative standard deviations for precision were below 2.47 percent over the whole concentration range. For all analytes, the average recovery rate varied from 73.79 to 116.98 percent. A preliminary pharmacokinetic study was successfully performed in real rats to evaluate the procedure efficiency.
1. Introduction
The most prevalent form of breast cancer found in females in their forties is locally progressed breast cancer (LABC). LABC applies to broad breast tumors (diameter > 5 cm) with chest wall or skin invasion or disease progression to the axillary, supraclavicular, or ipsilateral internal mammary lymph nodes [1]. Although (LABC) is a rare occurrence, its control remains a challenge. With just a five-year survival rate of 30–40%, LABC has a poor prognosis. Despite the fact that it is incurable, the majority of people require therapy to alleviate symptoms and extend their lives [2].
Neoadjuvant chemotherapy (NAC) is a term that relates to chemotherapy provided before surgical treatment. NAC’s purpose was to hasten tumor shrinkage so that a major mastectomy or radiation could be performed later [3]. In the last 60 years, the management of (LABC) has changed significantly. Radical mastectomy was initially the first line of treatment for females with LABC. Patients with LABC were eventually managed with systemic neoadjuvant chemotherapy in addition to surgery and radiation, based on the poor outcomes of surgery and radiation, as well as the early encouraging findings of adjuvant chemotherapy in females with axillary node-positive illness [4].
Interest in neoadjuvant treatment has grown in recent years [5]. When compared to full breast excision, breast conservation can be achieved by neoadjuvant chemotherapy, which is linked to lower morbidity and a better body image [6]. One of the prospective benefits of this approach is that it can downstage large tumors, increasing the number of patients who can get breast-conserving surgery [7].
Capecitabine is an orally administered fluoropyrimidine carbamate utilized to cure metastatic breast cancer that has returned after earlier therapy where tissues and lymph nodes in the neck, chest, and under the breastbone are affected (locally advanced breast cancer). Other parts in the body may be impacted as well (secondary breast cancer). It is a prodrug that is transformed into the active metabolite 5-Fluorouracil via an enzymatic mechanism at the tumor site. It is presently approved for use as a third-line therapy for metastatic illness in a number of countries [8]. Capecitabine is frequently utilized in conjunction with other anticancer drugs. It was discovered to be beneficial in individuals with HER2-negative breast cancer who developed invasive disease following conventional neoadjuvant treatment [9]. Patients with a poor prognosis, including those with triple-negative disease and suffering the expected adverse effects, benefitted from adjuvant Capecitabine therapy, which increased disease-free and overall survival.
Doxorubicin is an anthracycline chemotherapeutic drug utilized to cure different types of cancer such as lymphoma, Kaposi’s sarcoma, bladder cancer, breast cancer, and acute lymphocytic leukemia. Doxorubicin is a DNA intercalator that stops cancer cells from multiplying by blocking topoisomerase II. It is an effective therapy for breast cancer when used alone or in combination with other drugs [10,11]. It has also been used as a neoadjuvant chemotherapy for LABC [12]. In the treatment of metastatic triple-negative and locally advanced breast cancer, Doxorubicin and Capecitabine have shown encouraging results [13,14], with 77% of the patients achieving a clinical response. This strategy was feasible with adequate tolerability [15].
5-Fluorouracil is the active cancer-fighting form of Capecitabine. 5-Fluorouracil inhibits the nucleotide synthetic enzyme (thymidylate synthase) and its metabolites from integrating into RNA and DNA, preventing them from performing their normal function. It is commonly used to treat a range of cancers, particularly colorectal, breast, and digestive tract tumors, and it may also be used with other chemotherapeutic medications to enhance response and survival rates [16]. In individuals with advanced breast cancer, 5-Fluorouracil plus Doxorubicin is typically a well-tolerated first-line treatment [17].
Current problems in antineoplastic treatment include sub-therapeutic concentrations and serious toxic effects, which can both be addressed by using therapeutic drug monitoring (TDM)-guided dosage to keep concentrations within the therapeutic window and improve treatment outcomes [18].
Pharmacokinetic (PK) investigations of chemotherapeutics in cancer victims are crucial for dosage selection and dosing intervals in clinical applications. As soon as the chemotherapy is administered, it passes through a number of metabolic pathways; in order to figure out which ones, researchers must monitor the drugs in biological samples using various analytical methodologies. Furthermore, in patients undergoing multi-drug cancer therapy regimens, multi-drug quantitation approaches can provide a variety of benefits, including reduced sampling time and processing costs [19].
Sample preparation is a crucial feature of bioanalytical estimates since biological samples are exceedingly complex matrices with numerous components that might interfere with excellent separations and/or good mass spectrometer signals [20]. In this study, the QuEChERS technique was customized for extraction of the target analytes. It was commonly used for extraction of pesticide residues in food [21,22,23]. Recently, its use has been tailored to include drugs [24] and other poisons [25] in biological fluids.
Quick, Easy, Cheap, Effective, Rugged, and Safe are all parts of the term “QuEChERS”. It pertains to a testing process based on dispersive solid phase extraction that was developed in 2003 to measure pesticide residue on fruits and vegetables. In addition to being practical, quick, and affordable, QuEChERS can be used for analytes other than pesticides. Its acceptance in the food testing industry and other sectors consequently skyrocketed [26]. QuEChERS has typically been paired with either (GC-MS) or (LC-MS) analysis. Through the selection of various extraction solvents, salt formulations, and buffers for salting-out partitioning, as well as various d-SPE and SPE sorbents for the clean-up procedure, it is possible to modify the extraction procedures for target analytes for better selectivity, sensitivity, and specificity [27].
Several methods, including liquid–liquid extraction, protein precipitation, solid–liquid phase microextraction, and solid phase extraction, have been developed for the estimation of Capecitabine and its metabolites (including 5-Fluorouracil in human plasma by HPLC/UV [28], LC-MS/MS [29,30,31,32,33,34,35,36], and in mouse plasma [37]). However, no approaches utilizing the QuEChERS technique for plasma extraction and quantifying Capecitabine, Doxorubicin, and 5-Fluorouracil have been reported.
The purpose of this research is to investigate a new bioanalytical eco-friendly strategy using (UPLC-MS/MS) for concurrent determination of Capecitabine, Doxorubicin, and 5-Fluorouracil in rat plasma with a low limit of detection, small sample volume, minimal solvents for the extraction of chemotherapeutics from plasma samples through the use of an unprecedented mini-QuEChERS approach. A brief preliminary pharmacokinetic investigation on actual rats was correspondingly conducted using these findings.
2. Experimental
2.1. Materials
2.1.1. Pure Standards
Capecitabine, Doxorubicin, and 5-Fluorouracil were kindly supplied from Hikma Pharmaceuticals (Cairo, Egypt). Caffeine (used as an internal standard) was purchased from Fluka (Chemie GmbH, Buchs, Switzerland). All standards were labeled to be more than 98% purity. Figure 1 depicts the chemical structures of the target compounds and the internal standard.
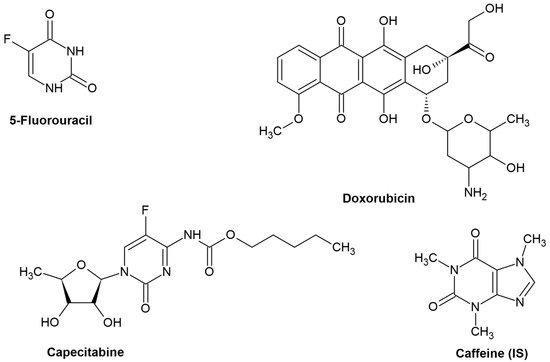
Figure 1.
Chemical structure of the studied compounds and the utilized IS.
2.1.2. Chemical Reagents
All of the solvents employed in this study were LC-MS grade. Solvents such as acetonitrile, methanol, Dimethyl sulfoxide (DMSO), and water were obtained from Supelco (Darmstadt, Germany). Formic acid (purity > 99%) was purchased from (Carlo Erba, Val de Reuil, France). Anhydrous magnesium sulphate and sodium chloride were supplied from (Fisher Chemical, Analytical reagent AR, Loughborough, UK). Primary secondary amine (PSA) bulk packing (50 µm, 70 Å) was obtained from Phenomenex (Torrance, CA, USA). Rat plasma (adult male Sprague Dawley rats) was purchased from El-Nile Company (Cairo, Egypt). Vacutainer EDTA tubes (Vacuette K3E) for plasma were supplied from Greiner-Bio-One Gmb (Frickenhausen, Germany).
2.2. Methods
2.2.1. Instrumentation and HPLC Conditions
Isocratic chromatography was performed on Nexera 2040 C Liquid chromatograph (Shimadzu, Japan) using a C18 column (150.0 × 2.1 mm, 1.9 µm) secured by a similar C18 guard column (10.0 × 2.1 mm, 1.9 µm) (Shimadzu, Kyoto, Japan) using Caffeine as the IS, with the mobile phase comprising acetonitrile:water in the ratio of (30:70, v/v) and 0.1 percent formic acid (v/v) at 0.2 mL/min as the flow rate. Centrifuge Z 36 HK, Super High Speed Refrigerated (Hermle Labortechnik, Wehingen, Germany), Mechanical Shaker (Heidolph, Schwabach, Germany), and Vortex Mixer (ZX3, Alfa medical Westbury, Shanghai, China) were also employed during the analysis.
2.2.2. Mass Spectrometric Conditions
In the multiple reaction monitoring (MRM) mode, Triple Quadrupole Mass Spectrometer (Shimadzu MS-8045) with an electrospray ion (ESI) source was employed to gather both the parent and fragment ion spectra for analyte recognition and verification. Except for 5-Fluorouracil, which was polarized in the negative ESI mode, all of the compounds were polarized in the positive ESI mode. Lab Solutions LCMS software (Version 5.97 SP1, Shimadzu Corporation, Kyoto, Japan) was utilized to process data from the Shimadzu equipment. The interface temperature was adjusted at 300 °C, while the heat block temperature at 400 °C, the desolvation temperature was set to 250 °C, 10.0 L/min, 3.0 L/min, and 10.0 L/min were the flow of drying gas, flow of the nebulizing gas, and the flow of heating gas, respectively. Table 1 displays the mass parameters of the target analytes and the utilized IS employed in this investigation.

Table 1.
Mass detection parameters and retention times of the target analytes and the utilized IS.
2.3. Solutions and Standards
Standard stock solutions of Caffeine (IS) and Capecitabine (1.00 mg/mL) were diluted in acetonitrile to obtain working solutions of (1.00 µg/mL). While, Doxorubicin and 5-Fluorouracil stock solutions (1.00 mg/mL) were diluted in water and DMSO, respectively, to produce (10.00 µg/mL) working solutions. Working solutions were then serially diluted to produce concentrations of (50.00, 100.00, 150.00, 200.00, 250.00, 300.00, 500.00 ng/mL) for 5-Fluorouracil, (25.00, 50.00, 100.00, 250.00, 450.00, 500.00 ng/mL) for Doxorubicin, and (5.00, 10.00, 20.00, 30.00, 70.00, 90.00, 100.00 ng/mL) for Capecitabine. Dilutions were carried out in the mobile phase. Stock and working solutions were stored at −20 °C while not in use and were freshly prepared to undergo analysis and validation.
Spiked and Real Plasma Samples
Working solutions (1.00 µg/mL) for Capecitabine or (10.00 µg/mL) for Doxorubicin and 5-Fluorouracil were loaded into 50.0 µL of blank rat plasma in specific proportions (5.0–100.0 µL). 5-Fluorouracil concentrations were (50.00, 100.00, 150.00, 200.00, 250.00, 300.00, 500.00 ng/mL), Doxorubicin concentrations were (25.00, 50.00, 100.00, 250.00, 450.00, 500.00 ng/mL) and concentrations of Capecitabine were (5.00, 10.00, 20.00, 30.00, 70.00, 90.00, 100.00 ng/mL). Caffeine was utilized in all assays at 50.00 ng/mL. QC levels of 5-Fluorouracil were (50.00, 250.00, and 500.00 ng/mL), whereas those for Doxorubicin were (25.00, 250.00, 500.00 ng/mL) and for Capecitabine were (5.00, 50.00, and 100.00 ng/mL).
Following intraperitoneal administration of the examined combination into rats, samples were taken. While the rat was immobilized, blood was extracted from its venous sinus. The eye was allowed to protrude and the neck was lightly scuffed. Laterally, medially, or dorsally, a capillary tube was introduced. Capillarity allowed blood to flow into the capillary tube. Samples were maintained at −20 °C in vacutainers containing EDTA until analysis. The calibration standard samples and QC samples were both freshly made.
2.4. Sample Preparation
First, 50.0 µL of rat plasma (blank or spiked) was vortexed with 700.0 µL of acetonitrile in Eppendorf tubes using a minor QuEChERS technique. Sodium chloride (0.05 g) and anhydrous magnesium sulphate (0.10 g) were added and mixed for another five minutes. At a speed of 6000 rpm, tubes were centrifuged for twenty minutes. The top layer comprising acetonitrile was then transported to new Eppendorfs containing 7.00 mg PSA and 40.00 mg anhydrous magnesium sulphate, which were mixed for one minute, then eventually centrifuged at 6000 rpm for another twenty minutes. To perform UPLC-MS/MS analysis, the purified extract was evaporated to dryness before being reconstituted in the 1.0 mL mobile phase.
2.5. Method Validation
Linearity, intra-day and interday precision, accuracy, selectivity, analyte recovery, lower limit of quantification (LLOQ), and stability following sample preparation were all subjected to a thorough validation protocol in compliance with US FDA guidelines [38].
2.5.1. Linearity
As previously stated, six different standard concentrations were prepared in blank rat plasma and each was evaluated five times. Calibration curves for the analytes were created using ratios of peak areas of the target analytes to the used IS versus the nominal concentrations of the calibration standards.
Except for LLOQ, which was set at 20%, the acceptance criteria for each concentration level was 15% departure from the nominal value [38].
2.5.2. Precision and Accuracy
Five replicates were analyzed at three different QC levels (n = 15) in addition to the other three calibration standards as mentioned above to assess inter-, intra-assay precision, and accuracy. The suggested method’s accuracy was stated as a percentage recovery. With the exception of LLOQ, which was set at 20% of the supposed values, it should not exceed 15% for all QC levels. Additionally, precision (expressed as RSD) should be 15% for QC levels, but 20% RSD for LLOQ [38].
2.5.3. Specificity and Selectivity
The method’s specificity was established by evaluating six distinct batches of blank rat plasma samples to show that endogenous plasma components did not cause chromatographic interference. Selectivity was measured at the LLOQ level and chromatograms were compared to those of blank plasma.
Peak areas of co-eluting substances should be no more than 20% of that of the analyte at the LLOQ or 5% of that of the internal standard area. In LLOQ samples, there should be less than a 20% variation from real concentrations [36].
2.5.4. Matrix Effect and Absolute Recovery
The influence of plasma components on 5-Fluorouracil, Doxorubicin, and Capecitabine ionization was investigated. This was accomplished by comparing the spiked plasma samples’ responses (post extraction) to the analyte’s reaction from standard sample solutions at equivalent concentrations. They were computed and reported as a percentage of recovery. Recoveries were calculated by comparing responses of the examined pharmaceuticals in spiked samples to their responses in pure solvents at equal concentrations.
2.5.5. Stability
Stability of the analytes 5-Fluorouracil, Doxorubicin, and Capecitabine was assessed at successive stages at LQC (50.00, 25.00, 5.00 ng/mL) and HQC (500.00, 500.00, 100.00 ng/mL), respectively, using freshly produced spiked samples as a reference. Stability of the compounds in plasma after twelve hours at ambient temperature and in the autosampler for 48.0 h at 4 °C was tested in five replicates. Additionally, stability in plasma after three freeze-thaw rounds (−20 °C) was assessed. Samples were tested for long-term stability after freezing for two weeks (−20 °C). Analytes are stable when they recover 90–110% of the original concentration in stock and working solutions. However, in biological extracts when 85–115% of the original concentration is restored, they are called stable [38].
2.6. Method Application in Real Animals
All laboratory animal care and use protocols were carried out in compliance with existing laws and institutional guidelines [39]. A brief pharmacokinetic study in healthy adult male rats (n = 7, 8 weeks, 150 g) was performed to evaluate the analytical method described. Rats were maintained in polystyrene cages at the animal facility, Faculty of Pharmacy, Ain Shams University, under consistent settings of temperature and humidity. Clean drinking water was offered to the rats throughout the study, but they were deprived of food only one day before the procedure and were acclimatized to this habitat for several days before the trial.
The drugs were all administered intraperitoneally. The dose of 5-Fluorouracil was modified to 100.00 mg/kg according to [40], the dose of Doxorubicin was adjusted to 5.00 mg/kg according to [41], and the dose of Capecitabine was adjusted to 540.00 mg/kg [42]. At various time intervals, blood samples were collected (0.5, 1.0, 2.0, 4.0, 6.0, and 8.0 h) then moved to EDTA tubes for centrifugation at 6000 rpm for ten minutes (4 °C) to separate the plasma, which then was withdrawn and kept at −20 °C.
2.7. Ethical Statement
The ARRIVE (Animal Research: Reporting of In Vivo Research) guidelines for reporting experiments involving animals were followed for all animal investigations. Animal care and testing protocols were authorized by the Research Ethics Committee, Faculty of Pharmacy, Ain Shams University (Approval no. ACUC-FP-ASU RHDIRB20201110301 REC#79, 13 April 2022)
3. Results and Discussion
Several LC/MS/MS methods for analyzing Capecitabine and its metabolites in biological matrices have been published [43]. Chromatographic methods for Capecitabine and other cytostatic drugs are indicated in Table S1. However, no methodologies for simultaneous detection of Capecitabine and Doxorubicin have been reported except for a method which used a magnetic solid phase extraction technique for the pretreatment of human plasma samples.
In this study, we used a new technology called “miniaturized QuEChERS” to establish a novel UPLC method for detecting Capecitabine, Doxorubicin, and 5-Fluorouracil in rat plasma with minimum quantities of sample and chemical reagents. The proposed method proved to be more simple and sensitive than the reported method [13] as shown in Table S2.
3.1. Selection of the Internal Standard
A multi-step sample method of preparation, for example, where volumetric loss of the sample is likely, demands the use of an internal standard to aid with analyte quantification. For each analyte, the internal standard should ideally be a deuterated analogue or a molecule with a similar structure. However, when multiple analytes are involved, commercial availability and high cost may be a constraint. Confirming that, the matrix affects the internal standard and analyte signal intensities proportionately is the major goal. Additionally, the internal standard needs to be compatible with the matrix and with no spectral interferences with the other analytes. Hence, in our investigation, a variety of drugs, including rosuvastatin, Caffeine, and midazolam, were initially evaluated. Caffeine was selected because it met the criteria listed above, in addition to its structural similarity to 5-Fluorouracil, while interferences occurred with the other inspected chemicals (midazolam & rosuvastatin).
3.2. Optimization of Chromatographic Conditions
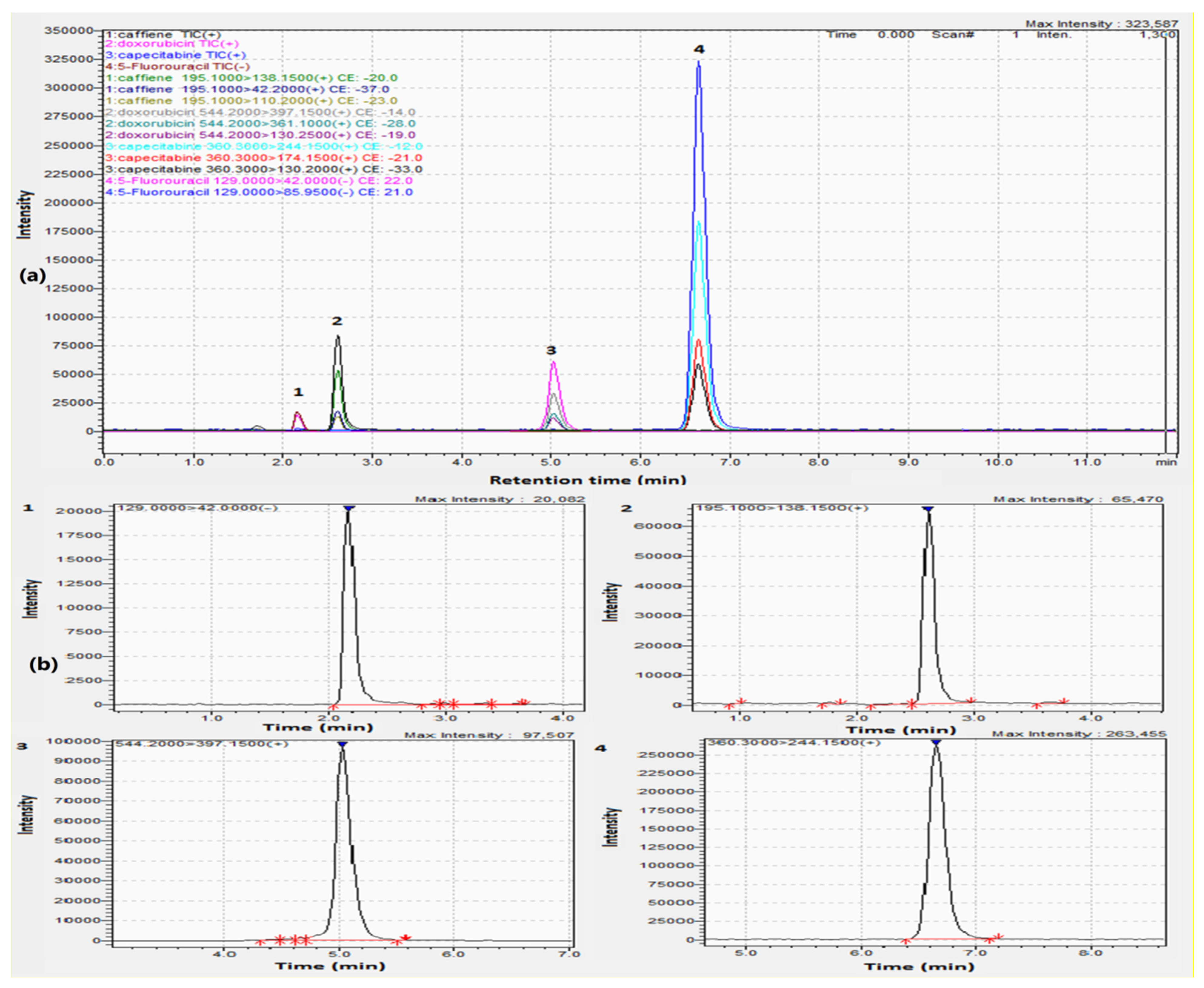
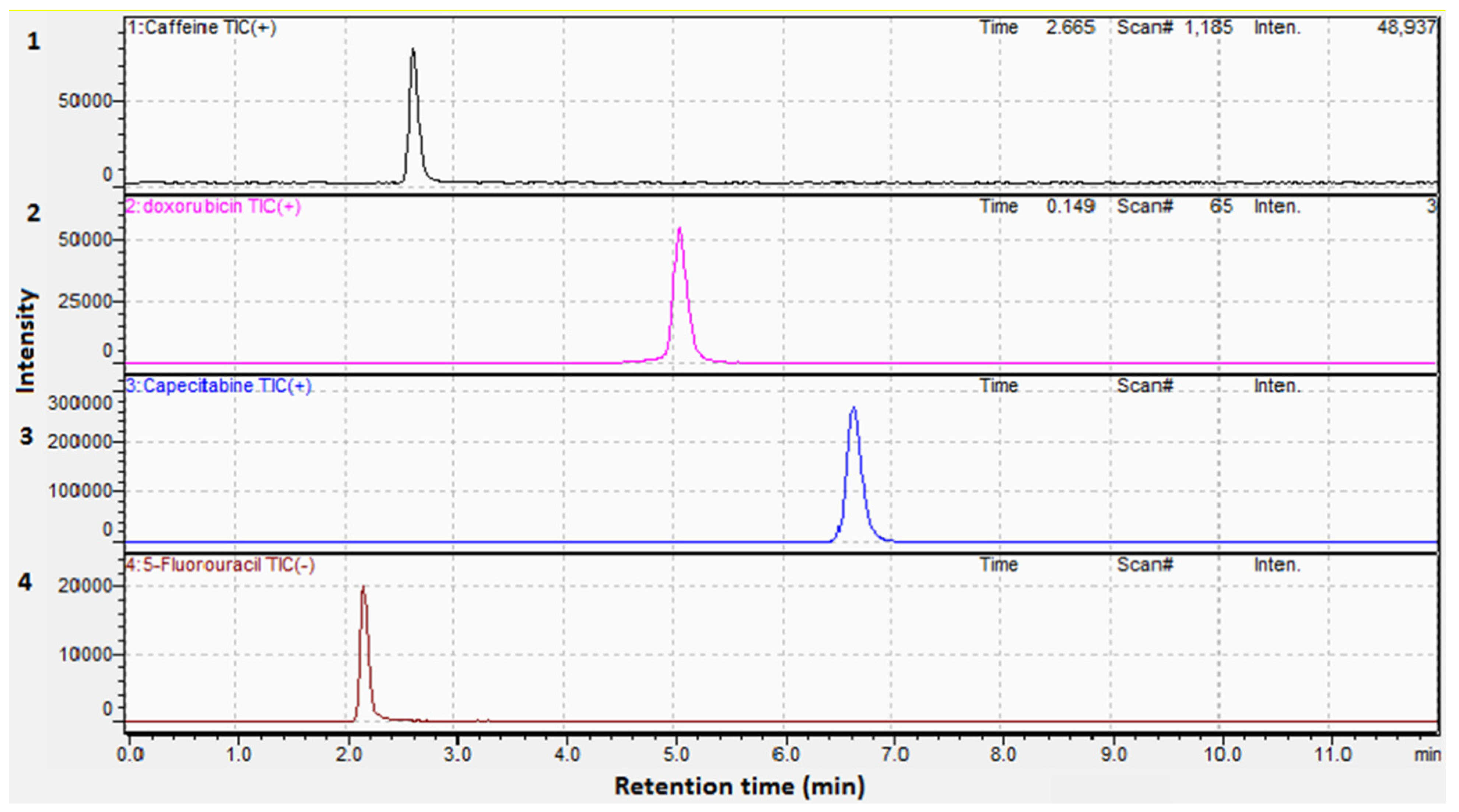
Establishing a sensitive and selective strategy for the concurrent estimation of drugs, especially those with different polarities, proved to be challenging. In this approach, we utilized a C18 column (150.0 × 2.1 mm, 1.9 µm) to improve the efficiency of separation. Mobile phases as acetonitrile:water and methanol:water (each containing 0.1% formic acid) were tested and the optimal resolution was obtained using acetonitrile. Isocratic elution was also attempted using different ratios of both solvents, the best chromatographic separation was conducted using acetonitrile:water (30:70, v/v). Different flow rates were also tried and 0.20 mL/min was chosen, yielding a run time of eleven minutes as illustrated in Figure 2a,b and Figure 3 for the total ion chromatogram (TIC), extracted ion chromatograms (XIC), and MRMs of the examined drugs, respectively.
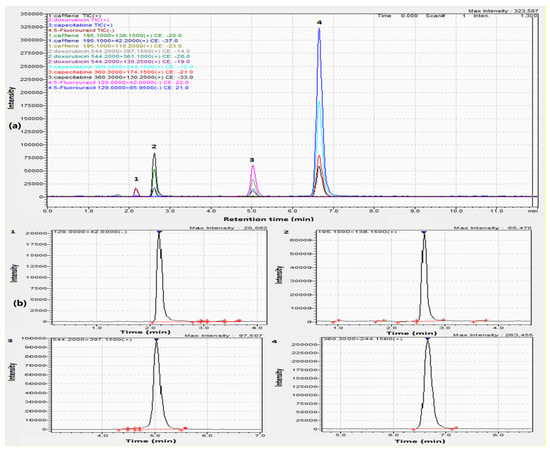
Figure 2.
(a) TIC and (b) extracted ion chromatograms of 1. (200.00 ng/mL) 5-Fluorouracil, 2. (50.00 ng/mL) Caffeine, 3. (100.00 ng/mL) Doxorubicin, and 4. (20.00 ng/mL) Capecitabine.
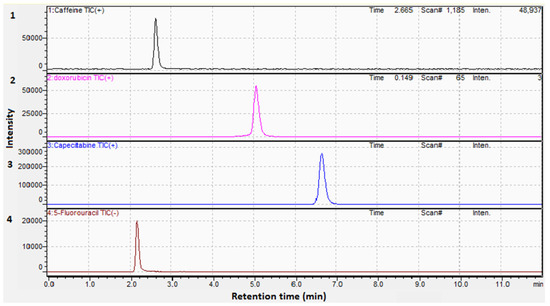
Figure 3.
MRM chromatograms of (1). (50.00 ng/mL) Caffeine, (2). (100.00 ng/mL) Doxorubicin, (3). (20.00 ng/mL) Capecitabine, and (4). (200.00 ng/mL) 5-Fluorouracil.
3.3. Optimization of MS Parameters
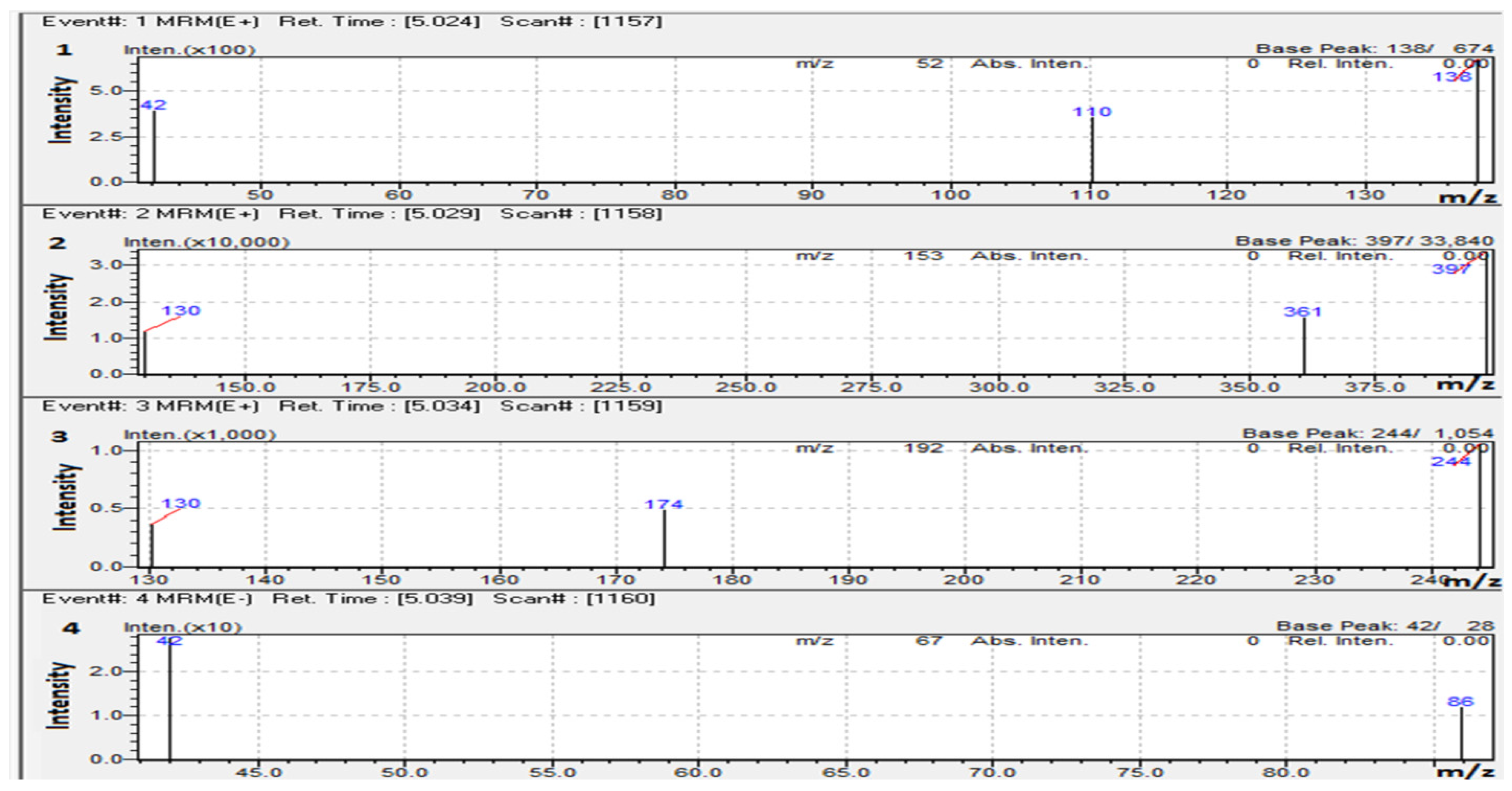
Except for 5-Fluorouracil, which was investigated in the negative ion mode, the responses for most of the compounds, such as Doxorubicin, Capecitabine, and Caffeine (IS), performed substantially better when ESI was in the positive ion mode. Detection technique of MRM was employed where the most dominant fragment ions for each molecule was used to obtain good selectivity and sensitivity. MRM transitions for the analytes of interest and IS, and collision energies (CE), are shown in Table 1, and the product ion mass spectra are shown in Figure 4.
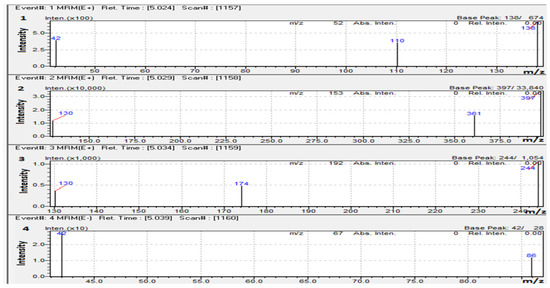
Figure 4.
Product ion mass spectra of (1). (50 ng/mL) Caffeine, (2). (100 ng/mL) Doxorubicin, (3). (20 ng/mL) Capecitabine, and (4). (200 ng/mL) 5-Fluorouracil.
3.4. Sample Preparation Development
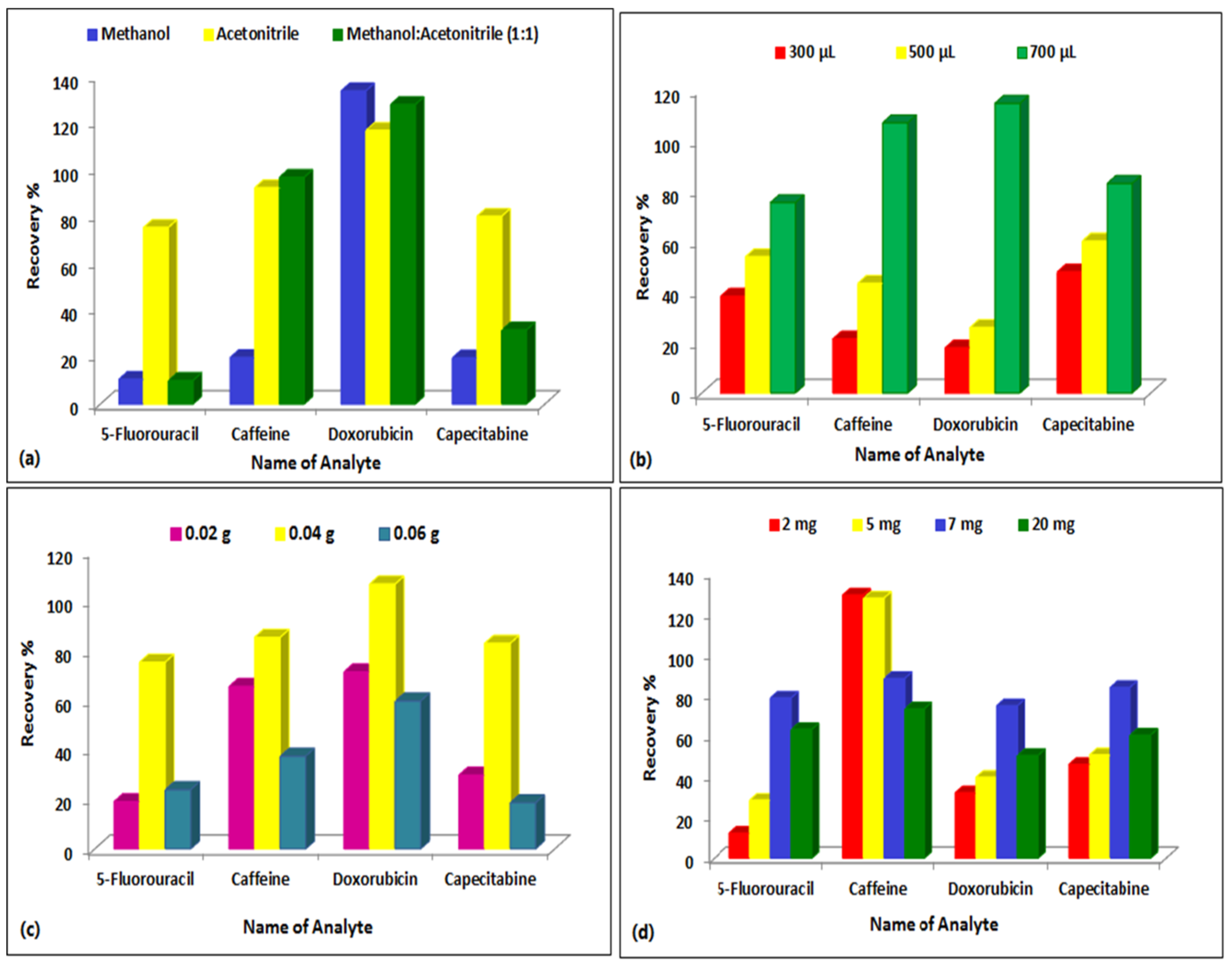
The extraction technique was investigated once the chromatographic parameters were appropriately adjusted. Owing to the complicated structure of plasma, sample preprocessing is frequently required prior to LC–MS/MS analysis to eliminate protein and other undesirable interferences. Miniaturization of the apparatus and the sample preparation procedure is amongst the most advantageous approaches for high-efficiency and high-speed analysis. Biological matrices were extracted and purified using the miniaturized QuEChERS technique with only 50.0 µL sample volume and 700.0 µL extraction solvent. Several solvents were investigated to extract the analytes such as acetonitrile, methanol, and a combination of both in equal proportions. Acetonitrile proved to be the optimum extraction solvent for all the compounds where the overall recovery was greater and more reliable as presented in Figure 5. In the clean-up procedure, C18 and PSA were investigated as adsorbents. PSA yielded better recoveries and thus was the adsorbent of choice. The quantity of magnesium sulphate was also adjusted, with 0.04 g proving to be the most effective as presented in Figure 5.
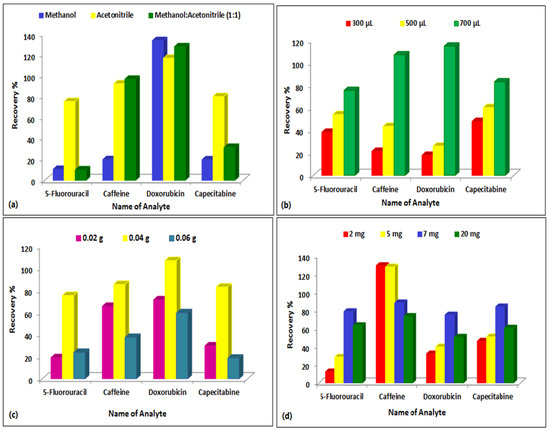
Figure 5.
Effect of clean-up step on the extraction efficiency of the compounds from spiked rat plasma using different solvents, amounts of acetonitrile, PSA, and MgSO4. (a) Effect of type of solvent, (b) Effect of volume of acetonitrile, (c) Effect of amount of MgSO4, (d) Effect of amount of PSA.
3.5. Method Validation
3.5.1. Selectivity
The absence of peaks co-eluting with the analytes indicate that all drugs were effectively separated. This can be demonstrated by comparing blank and spiked rat plasma samples at (LLOQ). The chromatogram of rat plasma is shown in (Supplementary Figure S1).
3.5.2. Linearity and Sensitivity
5-Fluorouracil, Doxorubicin, and Capecitabine exhibited linear calibration curves with regression coefficients (r) > 0.999 for ranges of concentration of 50.00–500.00 ng/mL, 25.00–500.00 ng/mL, and 5.00–100.00 ng/mL, respectively (Supplementary Figure S2). Table 2 shows the regression parameters. LODs were considered to be 13.50, 4.02, and 1.33 ng/mL for 5-Fluorouracil, Doxorubicin, and Capecitabine, respectively, corresponding to the S/N ratio of 3.3:1, whereas LOQs were set as 44.91 for 5-Fluorouracil, 12.18 for Doxorubicin, and 4.03 for Capecitabine (S/N ratio of 10). Sensitivity is indicated by the LLOQ’s accuracy and precision within 20%.

Table 2.
Validation parameters of the proposed method for the investigated analytes in rat plasma.
3.5.3. Accuracy and Precision
For all the studied pharmaceuticals, the proposed method demonstrated good accuracy with satisfactory interday and intraday precision in plasma. Percent RSD were all less than 2.47 at low, middle, and high QC samples (50.00, 250.00, and 500.00 ng/mL) for 5-Fluorouracil, (25.00, 250.00, 500.00 ng/mL) for Doxorubicin, and (5.00, 50.00, and 100.00 ng/mL) for Capecitabine. The concentrations were computed by dividing the average peak areas by the Caffeine’s area, then substituting the results into the regression equations. The ratio of the estimated to the actual concentration multiplied by 100 was used to determine recoveries. Table S3 summarizes these findings.
3.5.4. Recovery
Table 3 shows that for all analytes, the standard deviations were smaller than 10.38 and recoveries varied from 73.79 to 116.98%.

Table 3.
Absolute recovery and matrix effect at five different concentration levels for the studied analytes in rat plasma using UPLC-MS/MS, (n = 15).
3.5.5. Matrix Effect
The matrix effect is caused by the existence of co-eluting compounds in the sample, which causes the analytical assay to vary. The matrix effect is investigated to see if ionization changes due to matrix different components. It was determined using the peak area ratios of spiked samples after preparation (compared with those of the compounds in their pure forms at similar concentrations). Capecitabine and Caffeine (IS) exhibited ion enhancement, while ion suppression was observed at all levels of Doxorubicin and 5-Fluorouracil. The permissible limits for % recoveries and their RSD are summarized in Table 3.
3.5.6. Stability
Table 4 highlights the stability findings, indicating that all chemicals were stable in rat plasma for up to 12 h at 20–25 °C, except for 5-Fluorouracil, which was stable for up to 6 hr. After storage in the autosampler for 48 h, all samples remained steady, indicating reproducibility. Freeze–thaw cycles for three times (at −20 °C) were likewise successful in rat plasma samples. Finally, the final extract was examined for long-term stability, with all compounds remaining stable at −4 °C for up to 2 weeks. 5-Fluorouracil and Doxorubicin were no longer stable after two weeks in working and stock solutions, the other analytes studied were rather stable in working and stock solutions for up to one month.

Table 4.
Stability data for 5-Fluorouracil, Doxorubicin, and Capecitabine by the proposed method.
3.6. Method Application for Determination of Real Plasma Samples
Figure S3 and Table 5 illustrate the pharmacokinetic parameters and concentrations of the studied compounds following intraperitoneal administration in rats over a six-hour period, demonstrating the efficiency of the suggested procedure to be used for quantifying the investigated drugs in rat plasma for a short pharmacokinetic study. All drugs showed a sharp rise in plasma concentration with Doxorubicin being the fastest, reaching its maximum concentration in half an hour, while the other drugs reached maximum plasma concentration in one hour. 5-Fluorouracil had the greatest peak concentration, which can be explained by the fact that it is an active metabolite of Capecitabine. Doxorubicin had the lowest peak concentration.

Table 5.
Concentrations of the investigated drugs in plasma samples collected from male rats following intraperitoneal injection of a single dose of anticancer combination (n = 7).
3.7. Analytical Eco-Scale Tool for Evaluation of the Proposed Method’s Greenness (ESA)
The ESA tool was proposed in 2012 [44] and it was based on the production of a numerical total score that classifies the level of greenness of the analytical procedure. The ideal green procedure receives a score of 100 overall with no deducted points. The method’s negative ecological impacts are represented by penalty points, which are deducted from the final score of 100 points. Hazardous solvent use, high energy usage, and waste disposal have a negative impact on the environment. The green technique with a total score greater than 75 points, the reasonably green method with a total score between 50 and 75 points, and the inadequate green analysis with a total score of less than 50 points are the three classifications made by this assessment tool [44,45]. The reported chromatographic approach [33], as well as the proposed methodology, exhibit an outstanding Eco-Scale score. The penalty points for the proposed method are discriminated from those of the reported method, as shown in Table 6.

Table 6.
Penalty points for the greenness assessment of the proposed chromatographic method as compared with the reported method.
4. Conclusions
We present a new, rapid, and sensitive eco-friendly UPLC-MS/MS technique for the concurrent determination of some drugs used for locally advanced breast cancer named 5-Fluorouracil, Doxorubicin, and Capecitabine in rat plasma using a simple QuEChERS extraction strategy. The established approach displayed great accuracy and reproducibility. This assay is believed to be appropriate for TDM through a brief preliminary pharmacokinetic study in actual rats.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/separations9120403/s1, Figure S1: TIC and MRM Chromatograms of blank plasma; Figure S2: Linearity of the peak area ratios versus the corresponding concentrations of each analyte using the proposed UPLC-MS/MS method; Figure S3: Concentration vs time curves of (A) 5-Fluorouracil, (B) Doxorubicin and (C) Capecitabine in plasma samples collected from male rats following intraperitoneal injection of a single dose of anticancer combination (n = 7); Table S1: Summary of the different LC/MS methods in the literature used for the determination of the studied compounds in biological samples; Table S2: Comparison between the proposed method and the other reported method [11]; Table S3: Accuracy, Intra and inter-day precision data in rat plasma at three concentration levels (n = 15). References 46–55 are cited in the supplementary material [46,47,48,49,50,51,52,53,54,55].
Author Contributions
Conceptualization, N.F.E.A. and N.M.E.Z.; Data curation, N.F.E.A.; Formal analysis, N.F.E.A., F.A.B. and S.T.A.-R.; Funding acquisition, F.A.B. and S.T.A.-R.; Investigation, N.F.E.A. and S.O.; Methodology, N.F.E.A. and N.M.E.Z.; Resources, N.F.E.A., F.A.B., S.T.A.-R., S.O. and N.M.E.Z.; Software, N.F.E.A. and S.O.; Supervision, S.O. and N.M.E.Z.; Validation, N.F.E.A.; Writing—original draft, N.F.E.A.; Writing—review and editing, N.F.E.A., F.A.B., S.T.A.-R., S.O. and N.M.E.Z. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge financial support from the Researchers Supporting Project number (RSP-2021/103), King Saud University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
The animal study protocol was approved by Research Ethics Committee of Faculty of Pharmacy, Ain Shams University (Protocol code: ACUC-FP-ASU RHDIRB20201110301 REC#79, date of approval: 13 April 2022).
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this article.
Conflicts of Interest
The authors state that there were no financial or personal relations that may be seen as a possible conflict of interest during the study.
References
- Singletary, S.E.; Allred, C.; Ashley, P.; Bassett, L.W.; Berry, D.; Bland, K.I.; Borgen, P.I.; Clark, G.; Edge, S.B.; Hayes, D.F. Revision of the American Joint Committee on Cancer staging system for breast cancer. J. Clin. Oncol. 2002, 20, 3628–3636. [Google Scholar] [CrossRef] [PubMed]
- Terjung, A.; Kummer, S.; Friedrich, M. Simultaneous 24 h-infusion of high-dose 5-fluorouracil and sodium-folinate as alternative to capecitabine in advanced breast cancer. Anticancer. Res. 2014, 34, 7233–7238. [Google Scholar] [PubMed]
- Charfare, H.; Limongelli, S.; Purushotham, A. Neoadjuvant chemotherapy in breast cancer. J. Br. Surg. 2005, 92, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, B.L.; Demetriou, G.S.; Moodley, S.D.; Benn, C.A. When and how do I use neoadjuvant chemotherapy for breast cancer? Curr. Treat. Options Oncol. 2014, 15, 86–98. [Google Scholar] [CrossRef]
- Sachelarie, I.; Grossbard, M.L.; Chadha, M.; Feldman, S.; Ghesani, M.; Blum, R.H. Primary systemic therapy of breast cancer. Oncologist 2006, 11, 574–589. [Google Scholar] [CrossRef] [PubMed]
- Mieog, J.; Van der Hage, J.; Van De Velde, C. Neoadjuvant chemotherapy for operable breast cancer. J. Br. Surg. 2007, 94, 1189–1200. [Google Scholar] [CrossRef]
- Graham, P.J.; Brar, M.S.; Foster, T.; McCall, M.; Bouchard-Fortier, A.; Temple, W.; Quan, M.L. Neoadjuvant Chemotherapy for Breast Cancer, Is Practice Changing? A Population-Based Review of Current Surgical Trends. Ann. Surg. Oncol. 2015, 22, 3376–3382. [Google Scholar] [CrossRef]
- Smorenburg, C.H.; Bontenbal, M.; Verweij, J. Capecitabine in breast cancer: Current status. Clin. Breast Cancer 2001, 1, 288–293. [Google Scholar] [CrossRef]
- Masuda, N.; Lee, S.-J.; Ohtani, S.; Im, Y.-H.; Lee, E.-S.; Yokota, I.; Kuroi, K.; Im, S.-A.; Park, B.-W.; Kim, S.-B. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. New Engl. J. Med. 2017, 376, 2147–2159. [Google Scholar] [CrossRef]
- Bergh, J.; Jönsson, P.-E.; Glimelius, B.; Nygren, P. A systematic overview of chemotherapy effects in breast cancer. Acta Oncol. 2001, 40, 253–281. [Google Scholar] [CrossRef]
- Fossati, R.; Confalonieri, C.; Torri, V.; Ghislandi, E.; Penna, A.; Pistotti, V.; Tinazzi, A.; Liberati, A. Cytotoxic and hormonal treatment for metastatic breast cancer: A systematic review of published randomized trials involving 31,510 women. J. Clin. Oncol. 1998, 16, 3439–3460. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Pan, S.; Fan, X.; Jiang, X.; Yang, Y.; Jin, J.; Liu, Y. Pegylated liposomal doxorubicin as neoadjuvant therapy for stage II–III locally advanced breast cancer. J. Chemother. 2020, 32, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, P.; Buyse, M.E.; Swain, S.M.; Jacobs, S.A.; Robidoux, A.; Liepman, M.K.; Pajon, E.R.; Dy, P.A.; Posada, J.G., Jr.; Melnik, M.K. Concurrent Bevacizumab with a Sequential Regimen of Doxorubicin and Cyclophosphamide Followed by Docetaxel and Capecitabine as Neoadjuvant Therapy for HER2− Locally Advanced Breast Cancer: A Phase II Trial of the NSABP Foundation Research Group. Clin. Breast Cancer 2011, 11, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Skrypnikova, M.; Frolova, M.; Ignatova, E.; Pokataev, I.; Stenina, M.; Tjulandin, S. Primary systemic therapy with metronomic doxorubicin, cyclophosphamide, and capecitabine in locally advanced and metastatic triple-negative breast cancer. J. Clin. Oncol. 2011, 29, 1110. [Google Scholar] [CrossRef]
- Manga, G.P.; Shahi, P.K.; Ureña, M.M.; Pereira, R.Q.; Plaza, M.I.P.; Peron, Y.I.; Val, R.G.D.; Carrión, J.B.; Cañón, E.P.; Alfonso, P.G. Phase II study of neoadjuvant treatment with doxorubicin, docetaxel, and capecitabine (ATX) in locally advanced or inflammatory breast cancer. Breast Cancer 2010, 17, 205–211. [Google Scholar] [CrossRef]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef]
- Bontenbal, M.; Creemers, G.-J.; Braun, H.J.; de Boer, A.C.; Janssen, J.T.; Leys, R.B.; Ruit, J.B.; Goey, S.H.; van der Velden, P.C.; Kerkhofs, L.G. Phase II to III study comparing doxorubicin and docetaxel with fluorouracil, doxorubicin, and cyclophosphamide as first-line chemotherapy in patients with metastatic breast cancer: Results of a Dutch Community Setting Trial for the Clinical Trial Group of the Comprehensive Cancer Centre. J. Clin. Oncol. 2005, 23, 7081–7088. [Google Scholar]
- Mueller-Schoell, A.; Groenland, S.L.; Scherf-Clavel, O.; van Dyk, M.; Huisinga, W.; Michelet, R.; Jaehde, U.; Steeghs, N.; Huitema, A.D.; Kloft, C. Therapeutic drug monitoring of oral targeted antineoplastic drugs. Eur. J. Clin. Pharmacol. 2021, 77, 441–464. [Google Scholar] [CrossRef]
- Sabourian, R.; Mirjalili, S.Z.; Namini, N.; Chavoshy, F.; Hajimahmoodi, M.; Safavi, M. HPLC methods for quantifying anticancer drugs in human samples: A systematic review. Anal. Biochem. 2020, 610, 113891. [Google Scholar] [CrossRef]
- Prabu, S.L.; Suriyaprakash, T. Extraction of Drug from the Biological Matrix: A Review; IntechOpen: London, UK, 2012. [Google Scholar]
- Abdel-Ghany, M.F.; Hussein, L.A.; El Azab, N.F.; El-Khatib, A.H.; Linscheid, M.W. Simultaneous determination of eight neonicotinoid insecticide residues and two primary metabolites in cucumbers and soil by liquid chromatography–tandem mass spectrometry coupled with QuEChERS. J. Chromatogr. B 2016, 1031, 15–28. [Google Scholar] [CrossRef]
- Abdel-Ghany, M.F.; Hussein, L.A.; El Azab, N.F. Multiresidue analysis of five neonicotinoid insecticides and their primary metabolite in cucumbers and soil using high-performance liquid chromatography with diode-array detection. J. AOAC Int. 2017, 100, 176–188. [Google Scholar] [CrossRef] [PubMed]
- Farouk, M.; Hussein, L.A.E.A.; El Azab, N.F. Simultaneous determination of three neonicotinoid insecticide residues and their metabolite in cucumbers and soil by QuEChERS clean up and liquid chromatography with diode-array detection. Anal. Methods 2016, 8, 4563–4575. [Google Scholar] [CrossRef]
- El Azab, N.F. A validated UHPLC-MS/MS method for simultaneous quantification of some repurposed COVID-19 drugs in rat plasma: Application to a pharmacokinetic study. Microchem. J. 2022, 178, 107321. [Google Scholar] [CrossRef]
- El Azab, N.F.; Hotar, S.F.; Trabik, Y.A. Investigation of a QuEChERS-Based Method for Determination of Polycyclic Aromatic Hydrocarbons in Rat Plasma by GC–MS. J. Anal. Toxicol. 2021, 46, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Perestrelo, R.; Silva, P.; Porto-Figueira, P.; Pereira, J.A.; Silva, C.; Medina, S.; Câmara, J.S. QuEChERS-Fundamentals, relevant improvements, applications and future trends. Anal. Chim. Acta 2019, 1070, 1–28. [Google Scholar] [CrossRef]
- Kim, L.; Lee, D.; Cho, H.-K.; Choi, S.-D. Review of the QuEChERS method for the analysis of organic pollutants: Persistent organic pollutants, polycyclic aromatic hydrocarbons, and pharmaceuticals. Trends Environ. Anal. Chem. 2019, 22, e00063. [Google Scholar] [CrossRef]
- Zufıa, L.; Aldaz, A.; Giráldez, J. Simple determination of capecitabine and its metabolites by liquid chromatography with ultraviolet detection in a single injection. J. Chromatogr. B 2004, 809, 51–58. [Google Scholar] [CrossRef]
- Siethoff, C.; Orth, M.; Ortling, A.; Brendel, E.; Wagner-Redeker, W. Simultaneous determination of capecitabine and its metabolite 5-fluorouracil by column switching and liquid chromatographic/tandem mass spectrometry. J. Mass Spectrom. 2004, 39, 884–889. [Google Scholar] [CrossRef]
- Deng, P.; Ji, C.; Dai, X.; Zhong, D.; Ding, L.; Chen, X. Simultaneous determination of capecitabine and its three nucleoside metabolites in human plasma by high performance liquid chromatography–tandem mass spectrometry. J. Chromatogr. B 2015, 989, 71–79. [Google Scholar] [CrossRef]
- Forough, M.; Farhadi, K.; Molaei, R.; Khalili, H.; Shakeri, R.; Zamani, A.; Matin, A.A. Capillary electrophoresis with online stacking in combination with AgNPs@ MCM-41 reinforced hollow fiber solid-liquid phase microextraction for quantitative analysis of Capecitabine and its main metabolite 5-Fluorouracil in plasma samples isolated from cancer patients. J. Chromatogr. B 2017, 1040, 22–37. [Google Scholar]
- Salvador, A.; Millerioux, L.; Renou, A. Simultaneous LC-MS-MS analysis of capecitabine and its metabolites (5′-deoxy-5-fluorocytidine, 5′-deoxy-5-fluorouridine, 5-fluorouracil) after off-line SPE from human plasma. Chromatographia 2006, 63, 609–615. [Google Scholar] [CrossRef]
- Deenen, M.J.; Rosing, H.; Hillebrand, M.J.; Schellens, J.H.; Beijnen, J.H. Quantitative determination of capecitabine and its six metabolites in human plasma using liquid chromatography coupled to electrospray tandem mass spectrometry. J. Chromatogr. B 2013, 913, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Švobaitė, R.; Solassol, I.; Pinguet, F.; Mazard, T.; Ivanauskas, L.; Ychou, M.; Bressolle, F.M. A liquid chromatography-mass spectrometry method for the simultaneous determination of capecitabine, 5′-deoxy-5-fluorocytidine, 5′-deoxy-5-fluorouridine, 5-fluorouracil, and 5-fluorodihydrouracil in human plasma. J. Liq. Chromatogr. Relat. Technol. 2010, 33, 1705–1719. [Google Scholar] [CrossRef]
- Li, G.; Zhao, M.; Zhao, L. Ultra-performance liquid chromatography-tandem mass spectrometry for simultaneous determination of 12 anti-tumor drugs in human plasma and its application in therapeutic drug monitoring. J. Pharm. Biomed. Anal. 2021, 206, 114380. [Google Scholar] [CrossRef] [PubMed]
- Negreira, N.; Mastroianni, N.; de Alda, M.L.; Barceló, D. Multianalyte determination of 24 cytostatics and metabolites by liquid chromatography–electrospray–tandem mass spectrometry and study of their stability and optimum storage conditions in aqueous solution. Talanta 2013, 116, 290–299. [Google Scholar] [CrossRef]
- Dhananjeyan, M.R.; Liu, J.; Bykowski, C.; Trendel, J.A.; Sarver, J.G.; Ando, H.; Erhardt, P.W. Rapid and simultaneous determination of capecitabine and its metabolites in mouse plasma, mouse serum, and in rabbit bile by high-performance liquid chromatography. J. Chromatogr. A 2007, 1138, 101–108. [Google Scholar] [CrossRef]
- Food and Drug Administration, USA. Bioanalytical Method Validation Guidance for Industry; US Department of Health and Human Services: Washington, DC, USA, 2018; pp. 1–41.
- Council, N.; Studies, D. Research ILA, Animals CUGCUL. In Guide for the Care and Use of Laboratory Animals; The National Academic Press: Washington, DC, USA, 2010; Volume 8. [Google Scholar]
- Jarugula, V.R.; Lam, S.S.; Boudinot, F.D. Nonlinear pharmacokinetics of 5-fluorouracil in rats. J. Pharm. Sci. 1997, 86, 756–758. [Google Scholar] [CrossRef]
- Colombo, T.; Donelli, M.; Urso, R.; Dallarda, S.; Bartosek, I.; Guaitani, A. Doxorubicin toxicity and pharmacokinetics in old and young rats. Exp. Gerontol. 1989, 24, 159–171. [Google Scholar] [CrossRef]
- Onodera, H.; Kuruma, I.; Ishitsuka, H.; Horii, I. Pharmacokinetic study of capecitabine in monkeys and mice; species differences in distribution of the enzymes responsible for its activation to 5-FU. Drug Metab. Pharmacokinet. 2000, 15, 439–451. [Google Scholar] [CrossRef][Green Version]
- Knikman, J.E.; Rosing, H.; Guchelaar, H.J.; Cats, A.; Beijnen, J.H. A review of the bioanalytical methods for the quantitative determination of capecitabine and its metabolites in biological matrices. Biomed. Chromatogr. 2020, 34, e4732. [Google Scholar] [CrossRef]
- Gałuszka, A.; Migaszewski, Z.M.; Konieczka, P.; Namieśnik, J. Analytical Eco-Scale for assessing the greenness of analytical procedures. TrAC Trends Anal. Chem. 2012, 37, 61–72. [Google Scholar] [CrossRef]
- Gałuszka, A.; Migaszewski, Z.; Namieśnik, J. The 12 principles of green analytical chemistry and the SIGNIFICANCE mnemonic of green analytical practices. TrAC Trends Anal. Chem. 2013, 50, 78–84. [Google Scholar] [CrossRef]
- Sambasivam, G.; Shewade, D.G.; Dubashi, B.; Sundaram, R. A simple and rapid method for simultaneous quantification of doxorubicin, epirubicin, cyclophosphamide and 5-fluorouracil in human plasma by LCMS/MS. World J. Pharm. Res. 2016, 5, 747–757. [Google Scholar]
- da Silva, C.B.P.; Julio, I.P.; Donadel, G.E.; Martins, I. UPLC-MS/MS method for simultaneous determination of cyclophosphamide, docetaxel, doxorubicin and 5-fluorouracil in surface samples. J. Pharmacol. Toxicol. Methods 2016, 82, 68–73. [Google Scholar] [CrossRef]
- Guichard, S.M.; Mayer, I.; Jodrell, D.I. Simultaneous determination of capecitabine and its metabolites by HPLC and mass spectrometry for preclinical and clinical studies. J. Chromatogr. B 2005, 826, 232–237. [Google Scholar] [CrossRef]
- Radovanovic, M.; Schneider, J.J.; Shafiei, M.; Martin, J.H.; Galettis, P. Measurement of 5-fluorouracil, capecitabine and its metabolite concentrations in blood using volumetric absorptive microsampling technology and LC-MS/MS. J. Chromatogr. B 2022, 1188, 123075. [Google Scholar] [CrossRef]
- Alrobaian, M.; Panda, S.S.; Almalki, W.H.; Afzal, O.; Kazmi, I.; Alossaimi, M.A.; Al-Abbasi, F.A.; Katouah, H.A.; Rub, R.A.; Kumar, B. Development and Validation of Chemometrics-Assisted Green UPLC-MS/MS Bioanalytical Method for Simultaneous Estimation of Capecitabine and Lapatinib in Rat Plasma. J. Chromatogr. Sci. 2021, 60, 559–570. [Google Scholar] [CrossRef]
- Vaudreuil, M.-A.; Duy, S.V.; Munoz, G.; Furtos, A.; Sauvé, S. A framework for the analysis of polar anticancer drugs in wastewater: On-line extraction coupled to HILIC or reverse phase LC-MS/MS. Talanta 2020, 220, 121407. [Google Scholar] [CrossRef]
- Montange, D.; Bérard, M.; Demarchi, M.; Muret, P.; Piédoux, S.; Kantelip, J.P.; Royer, B. An APCI LC-MS/MS method for routine determination of capecitabine and its metabolites in human plasma. J. Mass Spectrom. 2010, 45, 670–677. [Google Scholar] [CrossRef]
- Vainchtein, L.D.; Rosing, H.; Schellens, J.H.; Beijnen, J.H. A new, validated HPLC-MS/MS method for the simultaneous determination of the anti-cancer agent capecitabine and its metabolites: 5′-deoxy-5-fluorocytidine, 5′-deoxy-5-fluorouridine, 5-fluorouracil and 5-fluorodihydrouracil, in human plasma. Biomed. Chromatogr. 2010, 24, 374–386. [Google Scholar] [CrossRef]
- Licea-Perez, H.; Wang, S.; Bowen, C. Development of a sensitive and selective LC-MS/MS method for the determination of α-fluoro-β-alanine, 5-fluorouracil and capecitabine in human plasma. J. Chromatogr. B 2009, 877, 1040–1046. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Grem, J.L. Liquid chromatography–mass spectrometry method for the analysis of the anti-cancer agent capecitabine and its nucleoside metabolites in human plasma. J. Chromatogr. B 2003, 783, 273–285. [Google Scholar] [CrossRef] [PubMed][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).