Abstract
Secondary plant metabolites and their derivatives play a significant role in human health. Ferruginol is a diterpene phenol that has recently received attention for its pharmacological properties, including antibacterial, antitumor, antimalarial, and cardioprotective effects. Recently, we detected the ferruginol compound in the leaf and seed extracts of Juniperus procera using different analytical approaches. The present work aims at detecting phytochemical compounds in a root extract of J. procera and estimating the amount of ferruginol compound in different parts of Juniperus procera. To screen the phytochemical compounds present in the root extract of J. procera, Gas chromatography/mass spectrometry (GC/MS) was performed. For ferruginol identification and estimation, high-performance liquid chromatography (HPLC) with the ferruginol reference standard and high-resolution direct analysis in real-time (DART) time-of-flight mass spectrometry (TOFMS) (DART-TOF-MS) analysis were used. GC/MS analysis revealed more than 20 bioactive compounds related to secondary plant metabolites in the root extract of J. procera with biological activity. The DART-TOF-MS result showed the typical positive ion spectra of ferruginol, and the HPLC result confirmed that the root extract of J. procera contains the ferruginol compound. In contrast, the root extract of J. procera contained a significant amount of ferruginol compared to that in the leaf and seed extracts. All parts of the J. procera contained the ferruginol compound and proved that ferruginol might be accumulated in the roots, leaves, and seeds of J. procera.
1. Introduction
Juniperus procera Hoechst. ex Endl. is indigenous to the mountains of East Africa from East Sudan to Zimbabwe, and the southern region of the Arab Peninsula across the Red Sea; it is widely spread out throughout the southern part of Saudi Arabia [1,2]. In Saudi Arabia, J. procera is traditionally used for treating jaundice, gastrointestinal disturbances, hepatic diseases, and pharyngitis as an antirheumatic for gout and several inflammatory conditions [3]. Additionally, J. procera is a natural source of photochemical components that may have antioxidant, insecticidal, antibacterial, and anticancer properties [4,5,6,7].
Bioactive components such as phenolics, flavonoids, terpenoids, alkaloids, and their derivatives are very important compounds due to their applications in different fields such as the medical, cosmetic, pharmacological, agricultural, and food industries [8,9,10,11]. Abietane diterpenoids are a type of these bioactive compounds [12,13] that exhibit several interesting biological activities, such as antitumor, antioxidant, antimicrobial, antifungal, antiviral, cytotoxic, and anti-inflammatory activities [14,15]. In this regard, abietane-type diterpenoids are significant phytochemical compounds exhibiting a wide range of pharmacological properties [16]. The pharmacological properties of ferruginol, which include antibacterial, anticancer, antimalarial, and cardioprotective effects, have recently received attention [17,18]. Cancer cell growth is inhibited by the ferruginol compound [18]. Moreover, ferruginol showed a strong protective impact in animal gastric ulcer models [19]. In the literature, the ferruginol compound was widely investigated and showed many important biological activities [16,20,21,22]. A previous study detected ferruginol in the berries of J. procera [23], while we recently detected ferruginol in the leaves and seeds of J. procera through different analytical approaches [24,25], and it is the dominant compound there. In this context, ferruginol is widely distributed in the Juniperus genus [26,27]. Phytochemical components are ubiquitous secondary metabolites in the plant kingdom, and are not nutritional, but vital ingredients for human health maintenance [28]. For better understanding the biological activities that plant species exert, it is important to explore and to evaluate their phytochemical constituents [29]. Different plant parts dominate a pool of bioactive compounds containing potential chemical groups and may contain different amounts of these compounds [30]. New sources of bioactive compounds such as ferruginol are really needed due to their pharmacological properties. Therefore, besides the identification of phytochemical compounds in root extracts of J. procera, this research evaluates the ferruginol compound in different parts (roots, leaves, and seeds) of J. procera. Hence, GC/MS was used for bioactive compound identification from the root extracts, while DART-ToF-MS and HPLC with a ferruginol reference standard were used for the quantification of the ferruginol compound in the different parts (seeds, leaves, and roots) of J. procera.
2. Materials and Methods
2.1. Chemical Reagents
Solvents such as HPLC-grade methanol, acetonitrile, and water were purchased from Sigma-Aldrich, St. Louis, MO, USA), while the ferruginol standard (95%) was purchased from WuXi App Tec- LabNetwork (Shanghai, China).
2.2. Plant Materials and Extraction
Different parts (roots, leaves, and seeds) of J. procera were collected from the alpha region, Saudi Arabia accepting the terms and conditions of national and international standards. A voucher specimen (13,497) was deposited in the herbarium of the center. The collected plant materials were washed carefully using distilled water to remove unwanted particles and dust. Then, plant materials were dried at room temperature. One gram from each part of plant was ground using an electronic blender and placed into 20 mL of methanol. Next, the extraction was carried out using an Innova 44 Inc incubator at 120 rpm, and the temperature was maintained at 28 °C for 5 days. The organic and aqueous phases were separated via centrifugation at 5000 rpm for 15 min. The collected supernatant was filtered through a 0.45 mm nylon syringe and stored at 4 °C for further use.
2.3. GC/MS Analysis of Root Extract
The root extract of J. procera was subjected to GC/MS analysis as follows. Gas chromatography/mass spectrometry (GC/MS) was performed with an Agilent 6890 gas chromatograph (Agilent Inc., Palo Alto, CA, USA) coupled to a 5973MSD operated in electron impact mode at 70 eV ion source energy. The gas chromatograph (GC) was fitted with a 30 m × 0.25 mm fused capillary column coated with 0.25 mm film thick DB-5MS (Agilent), and helium was used as the carrier gas with flow rate 1 mL/min and a split flow of 25 mL/min. The oven temperature was adjusted from 60 °C (1 min initial hold) to 280 °C (isothermal for 10 min final time), with injector temperature of 250 °C and total run time of 50 min. The transfer line temperature was set at 250 °C. The MS was operated in electron impact mode at 70 eV ion source energy. Mass spectrometric data were acquired and processed using the GC-MS ChemStation data system (WILEY 9th edition, NIST-08 MS library, Gaithersburg, MD, USA).
2.4. High-Resolution DART-ToF-MS Analysis
Direct real-time time-of-flight mass spectrometry (DART-ToF-MS) analysis (conditions: ionization ES+ − He; temperature, 250 °C; peak voltage, 500V). AccuTOF LC-plus from JEOL (Tokyo, Japan) was used for extracted sample characterization. Volatile plant extract compounds were evaporated in a stream of helium that had been heated to 250 °C, and ionized with the excited metastable helium atoms before entering the ion source of the time-of-flight mass spectrometer. In positive ionization mode, the molecules were mainly protonated without any fragmentation.
2.5. Quantification of Ferruginol with HPLC
The ferruginol compound was identified and quantified using acetonitrile and mobile-phase methanol (40:60) (v/v). Ferruginol was quantified through HPLC analysis with an Agilent Technologies 1290 Infinity system (Agilent Inc., Palo Alto, CA, USA). The separations were conducted using ZOBRAX RX-C18 column (4.6 × 150 mm) in which the mobile phases were pumped at a flow rate of 1.000 mL/min with a 5 min run time and injection volume of 1 μL. The column temperature was maintained at 27 °C. The chromatogram was recorded at 220 nm. Ferruginol in the sample was identified through its retention time spiked with the ferruginol standard under similar conditions. Ferruginol was estimated by using linear equation y = 967.6x − 44.425 with R2 = 0.9997 on the basis of the reference standard curve prepared with different concentrations of ferruginol (0.2, 0.4, 0.6, and 0.8 µg/mL).
2.6. Statistical Analysis
SPSS one-way analysis of variance (ANOVA)–Duncan’s test was performed for evaluating statistical significance at p < 0.05. The described results represent the mean of three replicates ± standard deviation (SD).
3. Results and Discussion
3.1. GC/MS Analysis of Different Parts of Juniperus procera
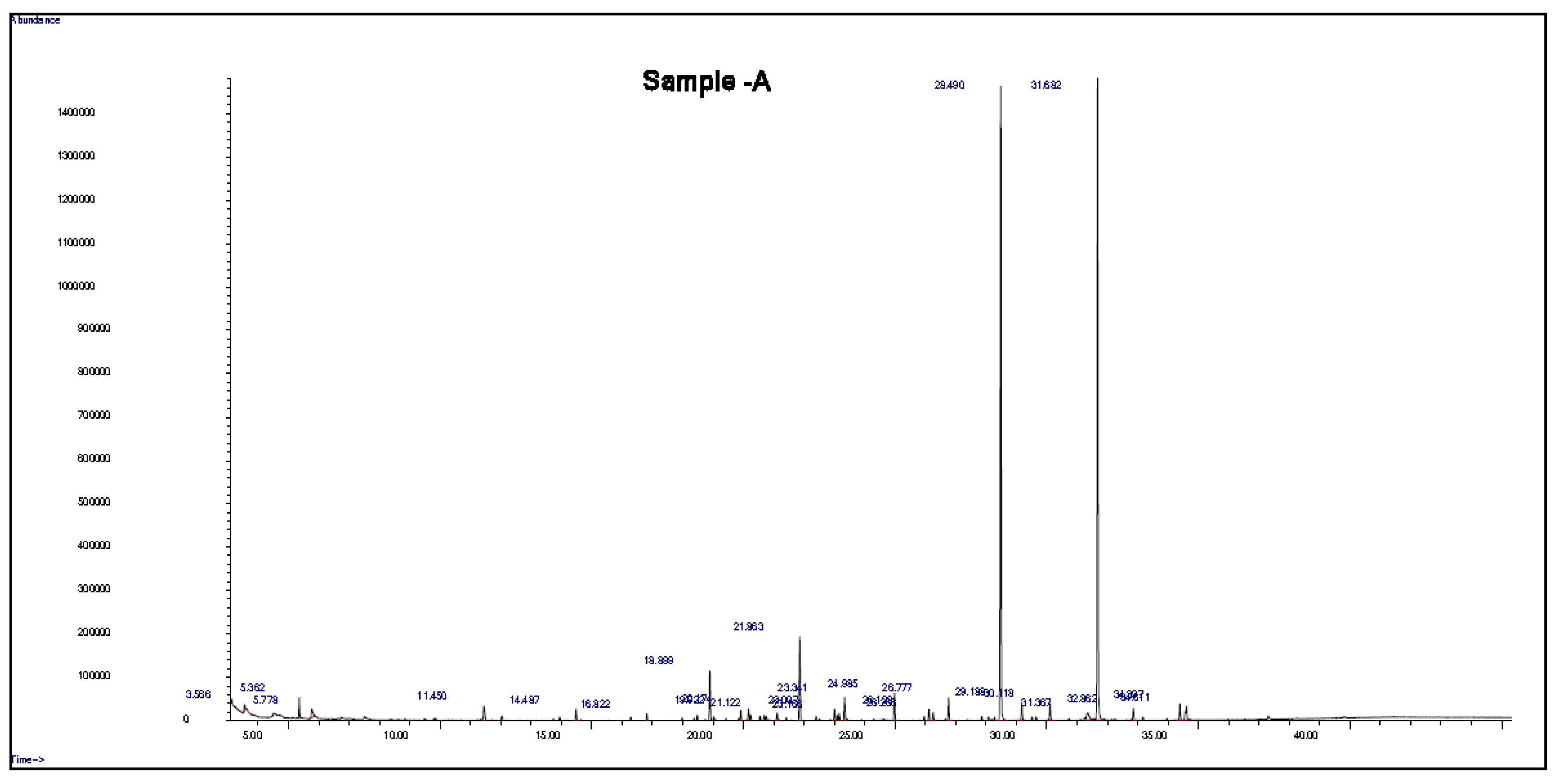
J. procera is a natural source of photochemical components that may have antioxidant, insecticidal, antibacterial, and anticancer properties. The methanolic extracts of the leaves and seeds of J. procera were subjected to GC/MS analysis, and the obtained results were published recently [24,31]. In this study, the root extract of J. procera was examined with GC-MS analysis for phytochemical component detection. The bioactive compounds in the root extract were identified using commercial libraries and a comparison of the mass spectra, match percentages, and retention times of the reference compounds. The recorded results of GC analysis reveal that the root extracts of J. procera are rich in phytochemical compounds. In this regard, J. procera is a natural source of phytochemical constituents with potential antimicrobial, insecticidal, anticancer, and antioxidant activities [4,5,6,32]. For accurate results, the methanol that was used as a solvent in the extraction process and the methanolic extract of the root of J. procera were examined with GC/MS analysis (Figure S2 and Figure 1). Then, the detected compounds in the plant sample were compared with the bank (methanol solvent) for bioactive compound identification. GC/MS analysis of the root extract showed about 20 bioactive components related to secondary plant metabolites with biological activity (Figure 1 and Table 1). These detected phytochemical compounds in the root extract of J. procera have antimicrobial, antioxidant, anticancer, and anti-inflammatory biological activities. For example, octanoic acid shows antimicrobial activity [33], while longifolene and undecanoic acid act as antifungal agents [34,35]. The extracted bioactive compounds of J. procera were effective and potent against SCC-9 cancer cells [36]. The phytochemical compounds highlighted above, other phytochemical constituents detected in the root extract of J. procera, and their biological activity are presented in Table 1. These results may support and justify the use of J. procera in traditional medicine.
Figure 1.
GC-MS chromatogram of the root extract of J. procera.

Table 1.
Secondary gas-chromatography/mass-spectrometry metabolites profile and biological activity of the root extract of J. procera.
3.2. Ferruginol Identification and Estimation
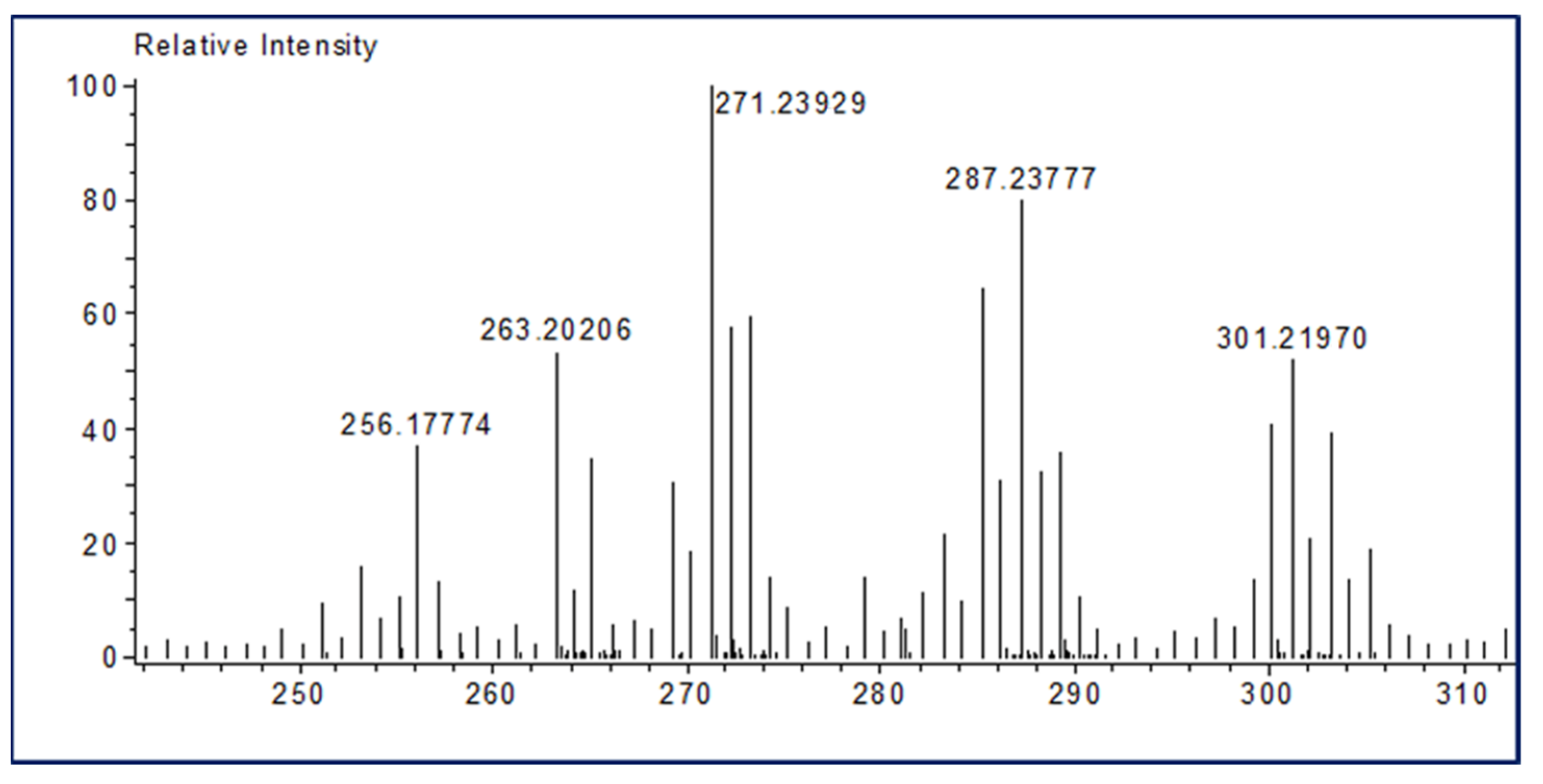
Ferruginol is a diterpene phenol that has recently received attention for its pharmacological properties, including antibacterial, antitumor, antimalarial, and cardioprotective effects [17]. In addition, ferruginol inhibits the growth rate of cancer cells [50]. We recently detected the ferruginol compound in leaf and seed extracts of African pencil cedar (J. Procera) through different analytical approaches [24,25,31]. We found that ferruginol is dominant compound in the leaves and seeds of J. procera. The DART-ToF-MS analysis of the extract showed a spectral range from 285.2 to 278.2 (Figure 2 and Table 2), indicating the presence of ferruginol in the extracts of J. procera. This result closely matched the ferruginol compound (C20H30O, mol. wt 286.5) according to the National Institute of Standards and Technology (NIST) Standard Reference Database [51]. Additionally, Kuroda K [52] reported that ferruginol normally generated peaks between 285 and 301. Thus, all these peaks (285.2, 286.2, 287.2) with chemical formulas C20H29O, C20H30O, and C20H31O, and 301. 2 are generated from J. procera tissue using DART-MS might originate from the ferruginol compound (C20H30O, mol. wt. 286.5) (Figure 2 and Table 2), which agreed with previous reports regarding the ferruginol compound [52,53,54]. DART-MS, with the characteristic of the rapid identification of bioactive compounds, is a new research tool for herbal medicine to very simply complete an experimental process [52,55,56]. As the presence of the ferruginol compound was confirmed in the different parts (roots, leaves, and seeds) of J. procera, we separated and evaluated the amount of ferruginol compound in the root, leaf and seed extracts of J. Procera. However, different factors, such as sample preparation, mobile phase, column types, and detectors should be considered for the chromatographic analysis of phenolic compounds [57]. Hence, for the separation of the ferruginol compound, HPLC with an authentic standard, and different mobile phases and conditions were tested. Among the tested mobile phases, the methanol and acetonitrile (60:40) (v/v) combination was suitable for ferruginol separation considering the conditions detailed in the methodology section (Figure 3a). In this regard, methanol and acetonitrile or their aqueous forms are the main mobile phases utilized in the HPLC quantification of phenolic compounds [58]. Ferruginol in the plant materials was identified via its retention time and by spiking with a reference standard under similar conditions, whereas ferruginol was quantified using a linear equation prepared with different concentrations of the standard (Figure S1).
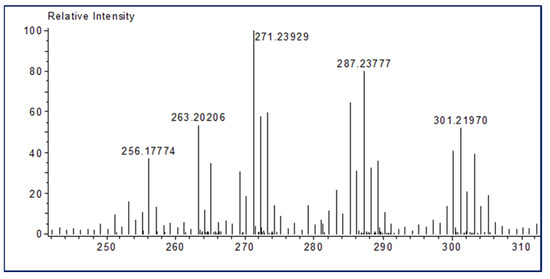
Figure 2.
DART TOF-MS chromatogram of J. procera showing the ferruginol spectrum.

Table 2.
Main phytochemical compound of the extracts of J. procera detected with DART-ToF-MS, indicating the presence of ferruginol in the extracts.
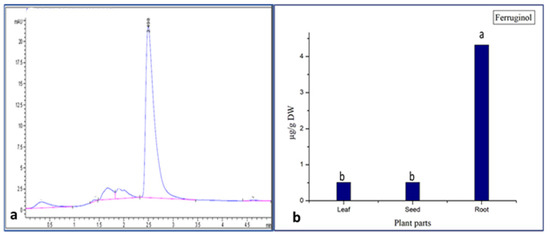
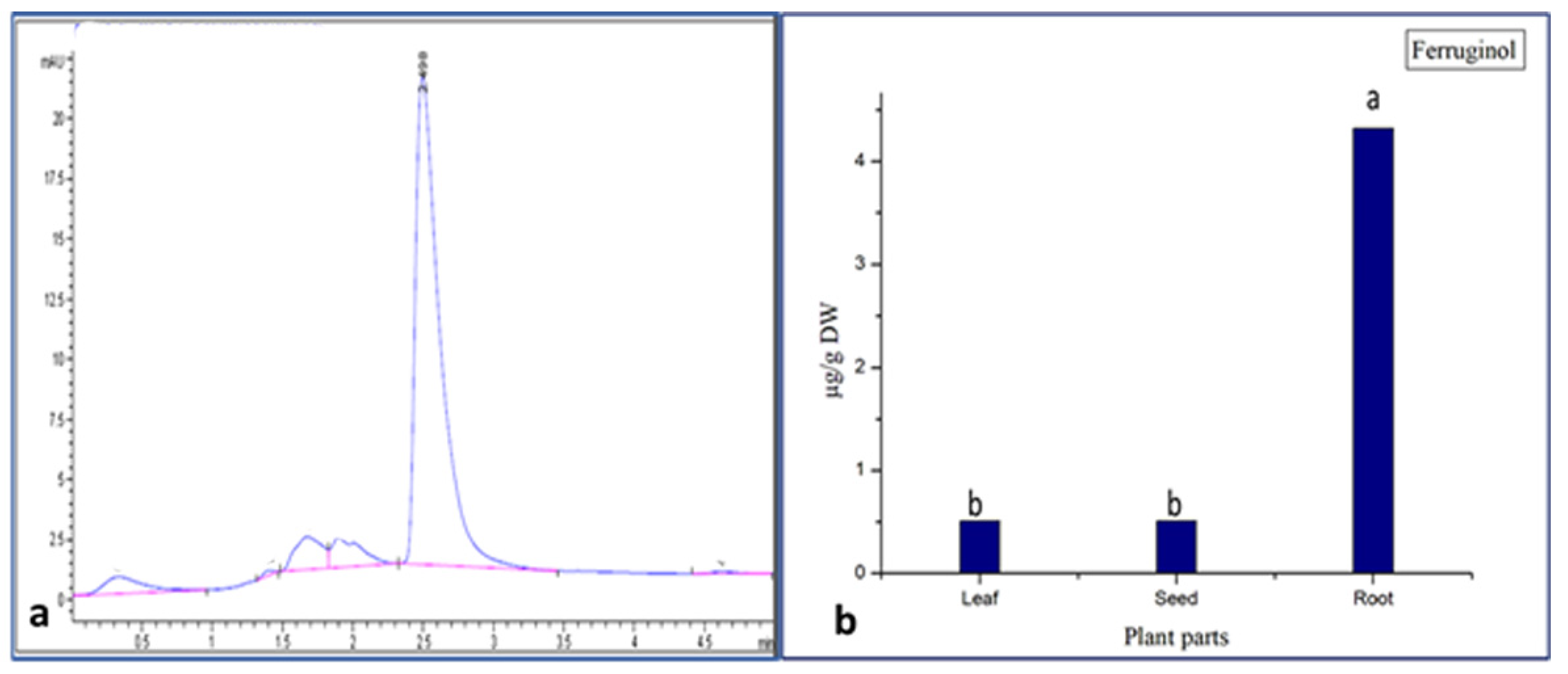
Figure 3.
(a) Ferruginol standard (RT = 2.498); (b) concentration of ferruginol in different part of Juniperus procera. a,b different superscripts within the same column differ significantly (p < 0.05). Reported results represent the mean of three replicates ± standard deviation (SD).
The obtained results confirm that all parts (roots, leaves, and seeds) of Juniperus procera contain the ferruginol compound. These results also demonstrate that, among the different parts (leaves, seeds, and roots) of J. procera, the root extract contained a higher amount of the ferruginol compound (4.4 µg), followed by the leaf (0.43 µg) and seed (0.42 µg) extracts (Figure 3b). In the literature, the ferruginol compound was detected in the berries of J. procera [23], but no previous report examined the quantification of the ferruginol compound in the different parts (leaves, seeds, and roots) of J. procera or any related plant as a comparative study. However, the authors in [53] stated that ferruginol was distributed in heartwood tissue or accumulated in intermediate wood and heartwood [52]. This study proves that the ferruginol compound might be accumulated in different plant parts. Moreover, this research reveals that the roots of J. procera are a new source of ferruginol.
4. Conclusions
In conclusion, this study confirmed that J. procera is rich in bioactive compounds, and that all parts (roots, leaves, and seeds) of J. procera contain the ferruginol compound. This research also revealed that the root extracts contained a significantly greater amount of ferruginol compared to that in other parts of the plant. Moreover, this research demonstrated that the mobile phase consisting of methanol and acetonitrile is suitable for the chromatographic separation of the ferruginol compound. Thus, these results support and justify the utilization of J. procera in traditional medicine. Further, research should be conducted to investigate the medicinal value of the root extract of J. procera.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/separations9110352/s1. Figure S1: standard ferruginol curve; Figure S2: GC-MS chromatogram of blank (methanol).
Author Contributions
A.M.S. proposed the work, and planned and performed the experiments; A.M.S., M.T., S.K., M.N., H.O.S. and S.A., methodology; F.A.-Q., lab supervisor; A.M.S. wrote the full manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available from the correspondence.
Acknowledgments
The authors extend their appreciation to researchers supporting project number RSP-2021/73 at King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors have declared no conflict of interest.
References
- Mujwah, A.A.; Mohammed, M.A.; Ahmed, M.H. First isolation of a flavonoid from Juniperus procera using ethyl acetate extract. Arab. J. Chem. 2010, 3, 85–88. [Google Scholar] [CrossRef]
- Collenette, S. Wild flowers of Saudi Arabia; East Anglian Engraving Co., Ltd.: Norwich, UK, 1999; Volume 110, pp. 274–275. [Google Scholar]
- Ghazanfar, S.A. Handbook of Arabian Medicinal Plants; CRC press: Boca Raton, FL, USA, 1994. [Google Scholar]
- Nuñez, Y.O.; Salabarria, I.S.; Collado, I.G.; Hernández-Galán, R. Screening Study of Potential Lead Compounds for Natural Product Based Fungicides from Juniperus Lucayana. Nat. Prod. Commun. 2008, 3, 132274115. [Google Scholar] [CrossRef]
- Tumen, I.; Eller, F.J.; Clausen, C.A.; Teel, J.A. Antifungal activity of heartwood extracts from three Juniperus species. BioResources 2013, 8, 12–20. [Google Scholar] [CrossRef]
- Abdel Ghany, T.; Hakamy, O.M. Juniperus procera as food safe additive, their antioxidant, anticancer and antimicrobial activity against some food-borne bacteria. J. Biol. Chem. Res. 2014, 31, 668–677. [Google Scholar]
- Hazubska-Przybył, T. Propagation of Juniper species by plant tissue culture: A mini-review. Forests 2019, 10, 1028. [Google Scholar] [CrossRef]
- Rivero-Montejo, S.d.J.; Vargas-Hernandez, M.; Torres-Pacheco, I. Nanoparticles as novel elicitors to improve bioactive compounds in plants. Agriculture 2021, 11, 134. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Na, M.; Kang, S.C. The role of bioactive substances in controlling foodborne pathogens derived from Metasequoia glyptostroboides Miki ex Hu. Food Chem. Toxicol. 2010, 48, 1945–1949. [Google Scholar] [CrossRef]
- Acquaviva, R.; Malfa, G.A.; Loizzo, M.R.; Xiao, J.; Bianchi, S.; Tundis, R. Advances on natural abietane, labdane and clerodane diterpenes as anti-cancer agents: Sources and mechanisms of action. Molecules 2022, 27, 4791. [Google Scholar] [CrossRef]
- Son, K.-H.; Oh, H.-M.; Choi, S.-K.; Han, D.C.; Kwon, B.-M. Anti-tumor abietane diterpenes from the cones of Sequoia sempervirens. Bioorganic Med. Chem. Lett. 2005, 15, 2019–2021. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, B.; Yao, S. Simultaneous determination of abietane-type diterpenes, flavonolignans and phenolic compounds in compound preparations of Silybum marianum and Salvia miltiorrhiza by HPLC-DAD-ESI MS. J. Pharm. Biomed. Anal. 2005, 38, 564–570. [Google Scholar] [CrossRef]
- Roa-Linares, V.C.; Brand, Y.M.; Agudelo-Gomez, L.S.; Tangarife-Castaño, V.; Betancur-Galvis, L.A.; Gallego-Gomez, J.C.; González, M.A. Anti-herpetic and anti-dengue activity of abietane ferruginol analogues synthesized from (+)-dehydroabietylamine. Eur. J. Med. Chem. 2016, 108, 79–88. [Google Scholar] [CrossRef]
- Bisio, A.; Pedrelli, F.; D’Ambola, M.; Labanca, F.; Schito, A.M.; Govaerts, R.; De Tommasi, N.; Milella, L. Quinone diterpenes from Salvia species: Chemistry, botany, and biological activity. Phytochem. Rev. 2019, 18, 665–842. [Google Scholar] [CrossRef]
- González, M.A. Aromatic abietane diterpenoids: Their biological activity and synthesis. Nat. Prod. Rep. 2015, 32, 684–704. [Google Scholar] [CrossRef]
- González-Cardenete, M.A.; Rivas, F.; Basset, R.; Stadler, M.; Hering, S.; Padrón, J.M.; Zaragozá, R.J.; Dea-Ayuela, M.A. Biological profiling of semisynthetic C19-functionalized ferruginol and sugiol analogues. Antibiotics 2021, 10, 184. [Google Scholar] [CrossRef]
- Wei, Y.; He, J.; Qin, H.; Wu Xa Yao, X. Determination of ferruginol in rat plasma via high-performance liquid chromatography and its application in pharmacokinetics study. Biomed. Chromatogr. 2009, 23, 1116–1120. [Google Scholar] [CrossRef]
- González, M.A.; Clark, J.; Connelly, M.; Rivas, F. Antimalarial activity of abietane ferruginol analogues possessing a phthalimide group. Bioorganic Med. Chem. Lett. 2014, 24, 5234–5237. [Google Scholar] [CrossRef]
- Espinoza, M.; Santos, L.S.; Theoduloz, C.; Schmeda-Hirschmann, G.; Rodríguez, J.A. New gastroprotective ferruginol derivatives with selective cytotoxicity against gastric cancer cells. Planta Med. 2008, 74, 802–808. [Google Scholar] [CrossRef]
- Becerra, J.; Flores, C.; Mena, J.; Aqueveque, P.; Alarcón, J.; Bittner, M.; Hernández, V.; Hoeneisen, M.; Ruiz, E.; Silva, M. Antifungal and antibacterial activity of diterpenes isolated from wood extractables of Chilean Podocarpaceae. Boletín de la Soc. Chil. de Química 2002, 47, 151–157. [Google Scholar] [CrossRef]
- Bakir, D.; Akdeniz, M.; Ertas, A.; Yilmaz, M.A.; Yener, I.; Firat, M.; Kolak, U. A GC-MS method validation for quantitative investigation of some chemical markers in Salvia hypargeia Fisch. & CA Mey. of Turkey: Enzyme inhibitory potential of ferruginol. J. Food Biochem. 2020, 44, e13350. [Google Scholar]
- Zare, S.; Hatam, G.; Firuzi, O.; Bagheri, A.; Chandran, J.N.; Schneider, B.; Paetz, C.; Pirhadi, S.; Jassbi, A.R. Antileishmanial and pharmacophore modeling of abietane-type diterpenoids extracted from the roots of Salvia hydrangea. J. Mol. Struct. 2021, 1228, 129447. [Google Scholar] [CrossRef]
- Samoylenko, V.; Dunbar, D.C.; Gafur, M.A.; Khan, S.I.; Ross, S.A.; Mossa, J.S.; El-Feraly, F.S.; Tekwani, B.L.; Bosselaers, J.; Muhammad, I. Antiparasitic, nematicidal and antifouling constituents from Juniperus berries. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2008, 22, 1570–1576. [Google Scholar]
- Salih, A.M.; Al-Qurainy, F.; Khan, S.; Tarroum, M.; Nadeem, M.; Shaikhaldein, H.O.; Alabdallah, N.M.; Alansi, S.; Alshameri, A. Mass propagation of Juniperus procera Hoechst. Ex Endl. From seedling and screening of bioactive compounds in shoot and callus extract. BMC Plant Biol. 2021, 21, 1–13. [Google Scholar] [CrossRef]
- Salih, A.M.; Al-Qurainy, F.; Khan, S.; Tarroum, M.; Nadeem, M.; Shaikhaldein, H.O.; Gaafar, A.-R.Z.; Alfarraj, N.S. Biosynthesis of zinc oxide nanoparticles using Phoenix dactylifera and their effect on biomass and phytochemical compounds in Juniperus procera. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Seca, A.; Pinto, D.; Silva, A.; Gupta, V. Bioactive Phytochemicals: Perspectives for Modern Medicine. 2015. Available online: https://www.abebooks.com/products/isbn/9789351307068/16068698672&cm_sp=snippet-_-srp1-_-PLP1 (accessed on 1 January 2020).
- Han, J.-W.; Shim, D.-W.; Shin, W.-Y.; Kim, M.-K.; Shim, E.-J.; Sun, X.; Koppula, S.; Kim, T.-J.; Kang, T.-B.; Lee, K.-H. Juniperus rigida Sieb. extract inhibits inflammatory responses via attenuation of TRIF-dependent signaling and inflammasome activation. J. Ethnopharmacol. 2016, 190, 91–99. [Google Scholar] [CrossRef]
- Patil, B.S.; Jayaprakasha, G.K.; Chidambara Murthy, K.; Vikram, A. Bioactive compounds: Historical perspectives, opportunities, and challenges. J. Agric. Food Chem. 2009, 57, 8142–8160. [Google Scholar] [CrossRef]
- Suroowan, S.; Llorent-Martínez, E.J.; Zengin, G.; Dall’Acqua, S.; Sut, S.; Buskaran, K.; Fakurazi, S.; Mahomoodally, M.F. Phytochemical Characterization, Anti-Oxidant, Anti-Enzymatic and Cytotoxic Effects of Artemisia verlotiorum Lamotte Extracts: A New Source of Bioactive Agents. Molecules 2022, 27, 5886. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef]
- Salih, A.M.; Al-Qurainy, F.; Nadeem, M.; Tarroum, M.; Khan, S.; Shaikhaldein, H.O.; Al-Hashimi, A.; Alfagham, A.; Alkahtani, J. Optimization method for phenolic compounds extraction from medicinal plant (Juniperus procera) and phytochemicals screening. Molecules 2021, 26, 7454. [Google Scholar] [CrossRef]
- Metivier, R.; Francis, F.; Clydesdale, F. Solvent extraction of anthocyanins from wine pomace. J. Food Sci. 1980, 45, 1099–1100. [Google Scholar] [CrossRef]
- Zhang, H.; Dolan, H.L.; Ding, Q.; Wang, S.; Tikekar, R.V. Antimicrobial action of octanoic acid against Escherichia coli O157: H7 during washing of baby spinach and grape tomatoes. Food Res. Int. 2019, 125, 108523. [Google Scholar] [CrossRef]
- Zhao, S.; Lin, G.; Duan, W.; Zhang, Q.; Huang, Y.; Lei, F. Design, synthesis, and antifungal activity of novel longifolene-derived diacylhydrazine compounds. ACS Omega 2021, 6, 9104–9111. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Martins, M.P.; Bitencourt, T.A.; Peres, N.T.; Rocha, C.H.; Rocha, F.M.; Neves-da-Rocha, J.; Lopes, M.E.; Sanches, P.R.; Bortolossi, J.C. Reassessing the use of undecanoic acid as a therapeutic strategy for treating fungal infections. Mycopathologia 2021, 186, 327–340. [Google Scholar] [CrossRef] [PubMed]
- AlShahrani, A.; AlShahrani, I.; Hosmani, J.; Togoo, R.A.; Sakinatulain, T.; Alam, T.; Hameed, M.S. Anticancer activity of Juniperus procera grown in southwestern region of Saudi Arabia on human oral squamous cell carcinoma cell lines. Pharmacogn. Mag. 2020, 16, 499. [Google Scholar]
- Chavan, M.J.; Wakte, P.S.; Shinde, D.B. Analgesic and anti-inflammatory activities of the sesquiterpene fraction from Annona reticulata L. bark. Nat. Prod. Res. 2012, 26, 1515–1518. [Google Scholar] [CrossRef]
- Mitić, Z.S.; Jovanović, B.; Jovanović, S.Č.; Stojanović-Radić, Z.Z.; Mihajilov-Krstev, T.; Jovanović, N.M.; Nikolić, B.M.; Marin, P.D.; Zlatković, B.K.; Stojanović, G.S. Essential oils of Pinus halepensis and P. heldreichii: Chemical composition, antimicrobial and insect larvicidal activity. Ind. Crops Prod. 2019, 140, 111702. [Google Scholar] [CrossRef]
- Fernandes, E.S.; Passos, G.F.; Medeiros, R.; da Cunha, F.M.; Ferreira, J.; Campos, M.M.; Pianowski, L.F.; Calixto, J.B. Anti-inflammatory effects of compounds alpha-humulene and (−)-trans-caryophyllene isolated from the essential oil of Cordia verbenacea. Eur. J. Pharmacol. 2007, 569, 228–236. [Google Scholar] [CrossRef]
- Ahmed, H.M.; Al-Zubaidy, A.M.A. Exploring natural essential oil components and antibacterial activity of solvent extracts from twelve Perilla frutescens L. Genotypes. Arab. J. Chem. 2020, 13, 7390–7402. [Google Scholar] [CrossRef]
- Deans, S.; Svoboda, K.P. Antibacterial activity of summer savory (Satureja hortensis L.) essential oil and its constituents. J. Hortic. Sci. 1989, 64, 205–210. [Google Scholar] [CrossRef]
- Dharni, S.; Maurya, A.; Samad, A.; Srivastava, S.K.; Sharma, A.; Patra, D.D. Purification, characterization, and in vitro activity of 2,4-di-tert-butylphenol from Pseudomonas monteilii PsF84: Conformational and molecular docking studies. J. Agric. Food Chem. 2014, 62, 6138–6146. [Google Scholar] [CrossRef]
- Simoh, S.; Zainal, A. Chemical profiling of Curcuma aeruginosa Roxb. rhizome using different techniques of solvent extraction. Asian Pac. J. Trop. Biomed. 2015, 5, 412–417. [Google Scholar] [CrossRef]
- Chowdhury, S.K.; Dutta, T.; Chattopadhyay, A.P.; Ghosh, N.N.; Chowdhury, S.; Mandal, V. Isolation of antimicrobial Tridecanoic acid from Bacillus sp. LBF-01 and its potentialization through silver nanoparticles synthesis: A combined experimental and theoretical studies. J. Nanostructure Chem. 2021, 11, 573–587. [Google Scholar] [CrossRef]
- Braga, Y.F.; Grangeiro, T.B.; Freire, E.A.; Lopes, H.L.; Bezerra, J.N.; Andrade-Neto, M.; Lima, M.A.S. Insecticidal activity of 2-tridecanone against the cowpea weevil Callosobruchus maculatus (Coleoptera: Bruchidae). An. da Acad. Bras. de Ciências 2007, 79, 35–39. [Google Scholar] [CrossRef]
- Henry, G.E.; Momin, R.A.; Nair, M.G.; Dewitt, D.L. Antioxidant and cyclooxygenase activities of fatty acids found in food. J. Agric. Food Chem. 2002, 50, 2231–2234. [Google Scholar] [CrossRef]
- Aparna, V.; Dileep, K.V.; Mandal, P.K.; Karthe, P.; Sadasivan, C.; Haridas, M. Anti-inflammatory property of n-hexadecanoic acid: Structural evidence and kinetic assessment. Chem. Biol. Drug Des. 2012, 80, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Wang, S.; Cao, M.; Xiong, W.; Wu, L. (E)-9-Octadecenoic Acid Ethyl Ester Derived from Lotus Seedpod Ameliorates Inflammatory Responses by Regulating MAPKs and NF-κB Signalling Pathways in LPS-Induced RAW264. 7 Macrophages. Evid.-Based Complement. Altern. Med. 2022, 2022. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, L.; Cui, L.; Liu, Z.; Wei, J.; Kang, W. Antioxidant and α-glucosidase inhibitiory activity of Cercis chinensis flowers. Food Sci. Hum. Wellness 2020, 9, 313–319. [Google Scholar] [CrossRef]
- Xiong, W.D.; Gong, J.; Xing, C. Ferruginol exhibits anticancer effects in OVCAR-3 human ovary cancer cells by inducing apoptosis, inhibition of cancer cell migration and G2/M phase cell cycle arrest Retraction in/10.3892/mmr. 2021.11868. Mol. Med. Rep. 2017, 16, 7013–7017. [Google Scholar] [CrossRef]
- Mikaia, A.; White, E.; Zaikin, V.; Zhu, D.; Sparkman, O.D.; Neta, P.; Zenkevich, I. NIST standard reference database 1A. In Standard Reference Data; NIST: Gaithersburg, MD, USA, 2014; Available online: https://www.nis.tgov/srd/nist-standard-reference-database-1a (accessed on 11 May 2014).
- Kuroda, K.; Fujiwara, T.; Hashida, K.; Imai, T.; Kushi, M.; Saito, K.; Fukushima, K. The accumulation pattern of ferruginol in the heartwood-forming Cryptomeria japonica xylem as determined by time-of-flight secondary ion mass spectrometry and quantity analysis. Ann. Bot. 2014, 113, 1029–1036. [Google Scholar] [CrossRef]
- Imai, T.; Tanabe, K.; Kato, T.; Fukushima, K. Localization of ferruginol, a diterpene phenol, in Cryptomeria japonica heartwood by time-of-flight secondary ion mass spectrometry. Planta 2005, 221, 549–556. [Google Scholar] [CrossRef]
- Imai, T.; Tanabe, K.; Kato, T.; Fukushima, K. Determination of Ferruginol, a Heartwood Diterpene Phenol, in Cryptomeria japonica by Time-of-flight Secondary Ion Mass Spectrometry (TOF-SIMS). In 59th Appita Annual Conference and Exhibition: Incorporating, Proceedings of the 13th ISWFPC (International Symposium on Wood, Fibre and Pulping Chemistry), Auckland, New Zealand, 16–19 May 2005; Appita Inc.: Victoria, Australia, 2005; p. 153. [Google Scholar]
- Cody, R.B.; Fouquet, T.N.; Takei, C. Thermal desorption and pyrolysis direct analysis in real time mass spectrometry for qualitative characterization of polymers and polymer additives. Rapid Commun. Mass Spectrom. 2020, 34, e8687. [Google Scholar] [CrossRef]
- Saito, K.; Watanabe, Y.; Shirakawa, M.; Matsushita, Y.; Imai, T.; Koike, T.; Sano, Y.; Funada, R.; Fukazawa, K.; Fukushima, K. Direct mapping of morphological distribution of syringyl and guaiacyl lignin in the xylem of maple by time-of-flight secondary ion mass spectrometry. Plant J. 2012, 69, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Stalikas, C.D. Extraction, separation, and detection methods for phenolic acids and flavonoids. J. Sep. Sci. 2007, 30, 3268–3295. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A. Phenolic Antioxidants in Foods: Chemistry, Biochemistry and Analysis; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).