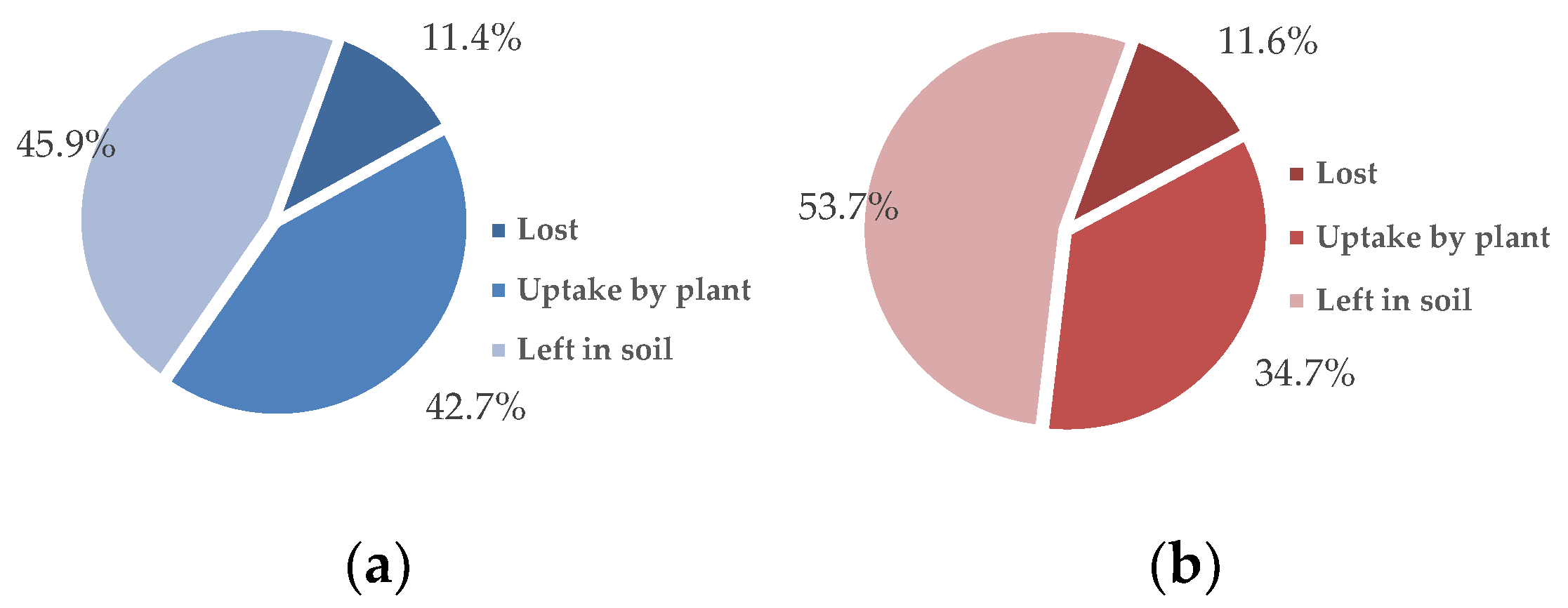Abstract
Recovering nitrogen (N) from agricultural wastewater for reuse in planting fields is a more sustainable and economical strategy to limit N pollution than using conventional treatments. Hereby, regular biochar produced by wheat straw pyrolysis and Mg-modified biochar were used as the N carriers to assess inorganic-N adsorption from simulated agricultural wastewater and the potential for reuse of the carried N in a planting system. The results showed that biochar materials have different affinities towards inorganic-N types. The amount of biochar carried-N increased with the increase in inorganic-N concentration and reached 4.44 mg/g as the maximum. The biochar carried ~4 mg/g of inorganic N substituting nearly 40% of N fertilizer following a 1% w/w addition rate for vegetable planting. After a trial season, 34.7–42.7% of the carried N from biochar was assimilated by the plant, 45.9–53.7% was retained by the soil, and only about 10% was lost. In comparison to the condition with all N inputs from chemical fertilizer, the addition of part of N by the N–biochar matrix significantly reduced the N loss by improving the plant N uptake or increasing the N content in the soil. This study demonstrates that biochar materials could be used as N carriers to recover N from wastewater for reuse in soil, carrier stability, and bioavailability preservation.
1. Introduction
While point-source pollution is currently gradually reduced, non-point-source pollution is becoming the main factor related to water pollution, a trend that unfortunately tends to worsen. Data from the second census of pollution sources showed that agricultural systems in China are responsible for the discharge of 1.4 × 109 kg nitrogen (N) and 2.1 × 108 kg phosphorus (N) in 2017, among which 50.9% of N and 35.9% of P were specifically originated from the planting industry, while 42.1% of N and 56.5% of P were generated from the livestock industry. Discharge agricultural wastewater, often in the form of runoffs driven by precipitation [1], unpolished effluents (tail water) from sewage treatment assets [2], and untreated or partially treated livestock wastewater [3], is the main cause of uncontrolled N losses in rural areas. The highest risk of N runoff loss is typically expected within short periods after fertilization, i.e., a week after N application with the N concentration in the runoffs exceeding 30 mg/L [4,5]. Additionally, based on the Chinese National Standards, tail water from domestic and livestock wastewater plants—plants that meet discharge standards—may still deliver effluent rich in nutrients with N concentrations over 10 mg/L (GB 18918 of Chinese National Standards) and near 80 mg/L (GB 18596 of Chinese National Standards), respectively. The same applies for the Western world as per UWWTD 91/271/EEC (TN ≥ 10 and TP ≥ 1.0 mg/L). Thus, agricultural—among others—wastewater discharge directly into the environment may account as a risk to surface water quality. Discharge management of the above-mentioned practices often involves individuals, such as farmers and engineers. The latter typically follow standards and specs, while the former mainly base their decision-making strategy on empirical practices. As such, the total amount of N loss from agricultural wastewater is large and is difficult to control. For the reduction in N emissions from farmlands, many strategies mainly related to fertilization dose optimization, new fertilizer production and cultivation rotation system changes have been developed [6,7,8,9] aiming to control N within the confines of farmland. However, no efficient and convenient purification technologies for the reduction in N emission from livestock wastewater produced by scattered farmers are yet known. Furthermore, little attention has been paid to the environmental pollution caused by tail water from domestic sewage works.
It should be noted that the role of N can be shifted from pollutant to a useful nutrient element if returning it back to soil to support plant growth (reuse). This principle can be more economical and convenient than treating thousands of liters per second of agricultural wastewater rich in N. Thus far, the preferred strategy for N reuse is limited to direct irrigation into the area where the runoff occurs, and only a few studies have focused on how to actually recover and reuse the N from agricultural wastewater. Therefore, constructing a closed loop to return N from wastewater to soil in a form that minimizes uncontrolled losses is critical for N reuse and N loss reduction.
The formation of a N closed loop requires the involvement of a proper carrier which must be able to yield a high N adsorption capacity, be harmless for soils that are applied, be easily accessible, and be provided at a low-cost. In recent years, biochar has received much attention due to its environmental friendliness as well as its beneficial potential for soil conditioning [10,11,12,13]. Apart from its large variety of physical–chemical properties, biochar has an excellent adsorbing capacity [14] compared to other forms of soil organic matter. It was reported that both ammonium nitrogen (ammonium-N) and nitrate nitrogen (nitrate-N) could be retained on biochar as a result of cation exchange, electrostatic interaction, and/or physical adsorption [15,16,17,18,19,20,21]. Many agricultural wastes, such as peanut hull, straw, and crop residue, can be used as raw materials for biochar production [22], ensuring easy biochar availability and low cost. Thus, it is theoretically feasible to use straw biochar as a N carrier to reduce N concentration from agricultural wastewater and realize N reuse in soil.
However, there are still two problems remaining to be solved prior to biochar being used as a carrier for N loop construction. Firstly, ammonium-N and nitrate-N coexist in agricultural wastewater. Zhang [20] and Liu [23] found the adsorptive competition among coexisting ions (including ammonium-N and nitrate-N) due to the ion exchange reaction and site specificity on the surface of biochar. As most of these studies were carried out using a single element/pollutant, no clear conclusion has yet been extracted with regards to the effect of coexisting ions on the adsorption capacity of biochar. Thus, it is still unclear how biochar would act in the presence of both ammonium-N and nitrate-N from urea hydrolysis. Adsorptive affinity towards ammonium-N [18,19,23,24,25] has been previously reported using unmodified biochar (regular biochar) application. In this case, nitrate-N adsorption becomes weak, possibly due to the presence of negatively charged functional groups on the biochar surface [14,15,26]. Biochar modifications, especially with metal ion amendment, could increase its ability in anion adsorption [16,21,27,28,29]. Masanizan [30] reported a significant improvement in dye adsorption after modification by copper. For nitrate-N adsorption, Mg modification [28,31] and iron modification [32] have been reported to greatly improve the adsorptive capacities of biochar by increasing the positive charge and the pore volume on the biochar surface. Secondly, the bioavailability of the N adsorbed by biochar is unclear. Taghizadeh-Toosi [33], Kammann [34], and Krounbi [35] used biochar as a sorbent for volatile ammonia, and proved the N adsorbed by regular biochar still maintained bioavailability. Marcińczyk [36] used biochars to recover nutrients from wastewater and found the nutrient release from biochar depends on environmental pH. However, it remains unknown whether the N adsorbed by biochar can substitute part of the fertilization N input after returning the matter to soil, and whether this could affect crop growth and N uptake efficiency compared to when active N is supplied directly via fertilization.
Therefore, a study was carried out using (i) regular and (ii) Mg-modified biochars prepared through straw pyrolysis. The adsorption capacity of the two biochar types was assayed using simulated wastewater in the presence of coexisting ions (ammonium-N and nitrate-N). After adsorption, the biochar uptaken N (the carried-N) was returned to the field to offset part of the N fertilizer input. 15N-labeled urea was employed to trace N fate from the simulated wastewater to soil and/or plant tissue [33,37]. The bioavailability of the carried N and its plant uptake efficiency were tested after one season of vegetable planting. The results of this study would help us investigate the assumption that biochar could be used as a carrier for N reuse in a closed-loop agricultural process. Nonetheless, the promising results of this study may pave new paths in the field of storm water management with many of the emerging pollutants being nutrient-related.
2. Materials and Methods
2.1. Description of Biochar Materials, Soil, and Plant
Both regular biochar and Mg-modified biochar were produced. Regular biochar was produced by gradually heating wheat straw in a vacuum tube furnace reactor (Muffle Furnace, Shanghai, China) under oxy-gen-limited conditions created by N2 gas with a flow of 400 mL/min. The temperature reached 450 °C at an increment rate of 5 °C per minute; the peak temperature was then maintained for over 5 h. For producing Mg-modified biochar, wheat straw was immersed into the MgCl2 solution with a ratio of 1 kg (straw): 5 L (MgCl2 solution) for 2 h prior to heating [28]. MgCl2 solution was prepared at a ratio of 2 g (MgCl2·6H2O): 3 mL (deionized water). After pyrolysis, the two biochar materials were crushed and sieved into 0.425 mm pieces. The properties of the two biochar types are described in Table 1.

Table 1.
Properties of two biochar materials and soil.
The soil used for the planting experiment was collected from a vegetable field (31°17′ N, 119°54′ E) in Yixing City, Jiangsu Province, China. This field is located in the Tai Lake region and is subjected to a subtropical monsoon climate, with an average annual temperature and rainfall of 15.7 °C and 1177 mm, respectively. The soil was a hydragric anthrosol evolved from lacustrine deposits with the properties shown in Table 1.
Due to its short growth period (20–35 days in a moderate climate), pak choi (Brassica chinensis L.), a type of Chinese cabbage, was selected as the trialed vegetable type for the planting experiment.
2.2. Experimental Design and Sampling
2.2.1. Batch Adsorption Experiment and Sampling
Simulated agricultural wastewater was prepared at 6 different N concentrations. Runoffs from fields, effluents/tail water from sewage plants, and livestock wastewater were the simulated agricultural wastewater of the solution here. Thus, the target inorganic N concentration of the simulated solution was in the range of 5–300 mg/L [1,4] (GB 18918 of China National Standards). Beakers of 1 L volume were prepared using 180 g of dry experiment soil (about 2cm-high in 1 L beakers) and 850 mL of solution of different N concentrations by dissolving different 15N urea (10 atom %); the soil was added, mixed, and stood for 72 h at 25 °C to simulate the surface water condition after 3 days of fertilization. The surface water in the breaker was separated as the simulated agricultural wastewater to be used for the following adsorption experiments. The inorganic-N (including ammonium-N and nitrate-N) concentrations of these simulated agricultural wastewaters is shown in Table 2.

Table 2.
Inorganic-N concentrations of the simulated agricultural wastewater used for the batch adsorption experiment.
Twelve treatments were set including the 2 biochar materials (regular biochar and Mg-modified biochar) subjected to six different N-rich solutions (Table 2) of simulated agricultural wastewater at a ratio of 1 g (biochar): 100 mL (wastewater). Considering that agricultural wastewater generally remains in a ditch or pond system for over 24 h prior to discharge, the biochar–solution mixture (12 treatments in 3 replicates) was shaken in a conical flask (250 mL) with a glass stopper at 80 rpm for 24 h at 25 °C.
Meanwhile, 2 blank treatments were prepared with 2 biochar materials, respectively, and added into deionized water to test whether any N of the biochar would release during the shaking process (controls). After that, the biochar–solution mixture was separated through filter paper. The solution passing through the filter was sampled for further determination of N concentrations and the biochar was air-dried in a shaded place and then sampled to test the measured value of inorganic-N amount in the N–biochar matrix from the solution.
2.2.2. Planting Experiment and Sampling
The planting experiment was conducted in a plastic pot filled with 2 kg of air-dried soil. Three treatments were assayed with the same N rate (100 mg/kg; 200 mg per pot), P2O5 rate (85 mg/kg; 170 mg per pot), and K2O rate (120 mg/kg; 240 mg per pot). The N–biochar matrix was added at a 1% w/w rate (equivalent of 10 g biochar per unit of dry soil and 20 g for each pot) in RBA + NC and MBA + NC treatments, and completely mixed into the soil prior to planting. However, the N input from the N–biochar matrix did not meet the N rate; some N was replenished by chemical fertilizer. All chemical fertilizers were applied as a base fertilizer. The detailed N input is shown in Table 3.

Table 3.
N input and their sources under different treatments in the planting experiment.
A week later, pak choi seeds, weighing 0.2 g (about 50–60 seeds), were directly sowed into the soil of each pot. The germination time was recorded when ≥35 seeds germinated (3–5 days after sowing). The duration of light was controlled at 12 h per day through using full-spectrum illumination lamps. The soil moisture in each pot was maintained at 60%; repetitive irrigation (2–3 times a week) was used to retain the water-holding capacity throughout the entire planting experiment. The pak choi was harvested 35 days after the germination time. The whole pak choi tissue from each plot was sampled at harvest and assessed for dry biomass and N content; the soil or biochar–soil mixtures were sampled before planting and after harvest. All treatments were designed in 3 replicate pots arranged randomly in the greenhouse at a controlled temperature of 25 ± 5 °C.
2.3. Testing
2.3.1. N Content Testing
For the water samples, the ammonium-N and nitrate-N were assessed directly using a continuous-flow analyzer (Skalar Corp., Breda, Netherlands). For the biochar or other soil samples, the inorganic N was initially extracted using 2 mol/L KCl solution with 1 g (biochar, soil, or biochar–soil mixture): 10 mL solution after 1 h shaking, and then tested using a continuous-flow analyzer. For plant matter, the entire pak choi was oven-dried at 80 °C until constant mass weighing, and then ground and filtered through a 0.149 mm sieve. The N content of the plant tissue was tested using an elemental analyzer (FlashSmart™, Thermo Scientific, Waltham, MA, USA).
2.3.2. Biochar
The basic properties of two biochar materials were also analyzed; specifically, the total carbon content was determined using an elemental analyzer, the surface area was determined using the BET method, the total pore volume using a physisorption analyzer (ASAP 2020, Micromeritics, Norcross, GA, USA) with N2 sorption isotherms at 22 °C, and the the Zeta potential via a dynamic light scatter (Zetasizer Nano ZS90, Malvern Panalytical Ltd, Malvern, UK).
2.3.3. 15N Abundance Testing
All 15N abundances in this study were measured with an isotope mass spectrometer (MAT-251, Thermo Scientific, Waltham, MA, USA). The difference between the abundance of the stable nuclide and the natural abundance determined the atom percent excess of the element due to 15N addition. The 15N abundance of plant (A(p)) was determined directly. The 15N abundance and amount of inorganic N in the soil–biochar mixture (A(s)) were determined following the method described by Zhang [38].
2.4. Calculations
2.4.1. N Removal Efficiency
The N removal efficiency of the biochar was determined with Equation (1).
where X refers to ammonium-N (AN), nitrate-N(NN), and inorganic N (IN) for calculating their removal efficiencies. RE refers to the removal efficiency in %; Cad stands for the concentration of N in different forms in the separated solution after biochar adsorption (all in mg/L); and Cin is the concentration of N in different forms in the simulated agricultural wastewater before biochar adsorption (Table 2) in mg/L.
REX = (1 − CadX/CinX) × 100%,
2.4.2. Inorganic N Adsorbed by Biochar
The theoretical value of the inorganic-N amount in the N–biochar matrix (NB(T)) was estimated as the difference in N content of the solution after adsorption minus the initial N content prior to adsorption (Equation (2)).
where NB(T) represents the theoretical value of the inorganic-N amount in the N–biochar matrix in mg/g; V is the volume of the solution, hereby 0.1 L for this study; and m is the dry weight of the biochar material added in the solution, hereby 1 g in this study.
NB(T) = (Cin(IN) − Cad(IN)) × V ÷ m,
The measured value of inorganic-N amount in the N–biochar matrix (NB(M)) was tested directly after the two biochar materials separated from the biochar–solution mixture, air-dried in the shaded place, and extracted by 2 mol/L KCl solution.
2.4.3. N Input from N–Biochar Matrix
The N input from the N–biochar matrix was calculated as per Equation (3) based on the measured value of inorganic-N amount in the N–biochar matrix.
where N(B) represents the N input from the N–biochar matrix for the planting experiment in mg; C(b) is the measured value of inorganic-N amount in the N–biochar matrix in mg/g; AR is the weight of biochar added, 10 g/kg in this study; and M refers to the weight of dry soil used for the planting experiment, herein 2 kg.
N(B) = C(b) × AR × M,
2.4.4. N Balance in Planting Experiment
After one growing season of vegetable planting, the amount of N uptake by the plant was determined from harvest data following Equation (4), and the amount of N remaining in the soil was determined from the changes in inorganic mineral content in the soil or biochar–soil mixture before fertilization and after harvest (Equation (5)). Moreover, the amount of N loss was calculated by the difference between the N input and the N uptake by the plant and residue in the soil following Equation (6).
where N(p) represents the plant N uptake (mg/pot), C(p) is the N content of the plant (mg/g), and M(p) is the plant dry biomass (in g/pot).
where N(s) represents the total soil N amount after harvest (mg/pot); C(s1) and C(s2) stand for the N content of the soil before planting and after harvest (mg/kg); and M(s) refers to the soil weight (kg/pot), herein 2 kg/pot.
where N(l) represents the N loss (mg/pot), and N(input) represents the N input (mg/pot), herein 200 mg.
N(p) = C(p) × M(p),
N(s) = (C(s2) − C(s1)) × M(s),
N(l) = N(input) − N(s) − N(p),
The fate of the carried N from the biochar was traced by 15N and its distribution was calculated with the 15N abundance of plants and the biochar–soil mixture following Equations (7)–(9), while the fate of the N from the fertilizer was also calculated with Equations (10)–(12).
where RB(p), RB(s), and RB(l) represent the ratio of N uptake by the plant, the ratio of N left in the soil system, and the ratio of N loss from the N–biochar matrix (%). A(p) and A(s) stand for the 15N abundance of the plant and soil–biochar mixture in %, and A(urea) is the 15N abundance of urea, hereby 10 atom% in this study.
where RF(p), RF(s), and RF(l) represent the ratio of N uptake by the plant, the ratio of N left in the soil system, and the ratio of N loss from the fertilizer in %, and N(F) represents the N input from the fertilizer in mg.
RB(p) = A(p) ÷ A(urea) × N(p) ÷ N(B) × 100%,
RB(s) = A(s) ÷ A(urea) × N(s) ÷ N(B) × 100%,
RB(l) = 1 − RB(s) − RB(p),
RF(p) = (N(p) − A(p) ÷ A(urea) × N(p)) ÷ N(F) × 100%,
RF(s) = (N(s) − A(s) ÷ A(urea) × N(s)) ÷ N(F) × 100%,
RF(l) = 1 − RF(s) − RF(p),
2.4.5. Statistical Analysis
The significance of the differences between the two biochar treatments during the adsorption experiment were evaluated using a statistical T-test. The significant differences in the data related to the N from the planting experiment were determined using Duncan’s multiple-range test (p < 0.05) (SPSS ver. 16.0 for Windows, SPSS Inc., Chicago, IL, USA).
3. Results
3.1. N Removal Efficiency
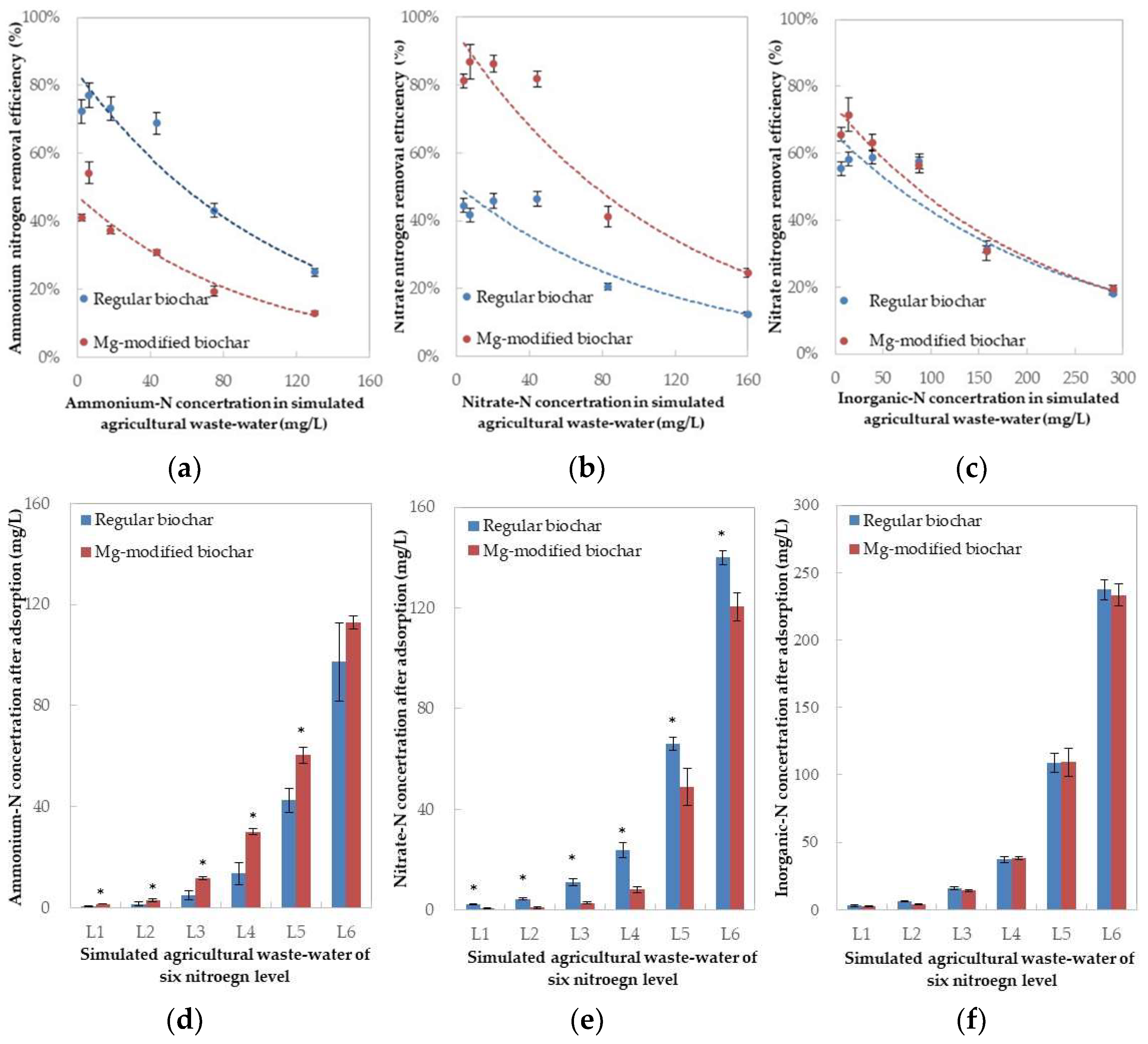
The increase in N concentration in the stimulated wastewater led to a N removal efficiency decrease for both biochar types. Adsorptive affinity on a specific N substrate (ammonium-N and nitrate-N) was apparent between the two char media. The regular biochar showed a significantly higher ammonium-N removal efficiency compared to the Mg-modified biochar at every N concentration trialed (Figure 1a), while the Mg-modified biochar achieved a better performance against nitrate-N removal (Figure 1b). The ammonium removal efficiency of regular biochar exceeded 70% for an initial ammonium-N concentration less than 40 mg/L (L3 in Table 2), and fell to less than 40% when the latter was close to 90 mg/L (L4). The ammonium-N removal efficiency of Mg-modified biochar was less than 30% at 40 mg/L, and fell to 20% at 90 mg/L. Meanwhile, the nitrate-N removal efficiency of Mg-modified biochar exceeded 80% when the nitrate-N initial concentration was 40 mg/L; this dropped to slightly over 40% at 90 mg/L of initial N. The nitrate-N removal efficiency of the regular biochar never surpassed 50%.
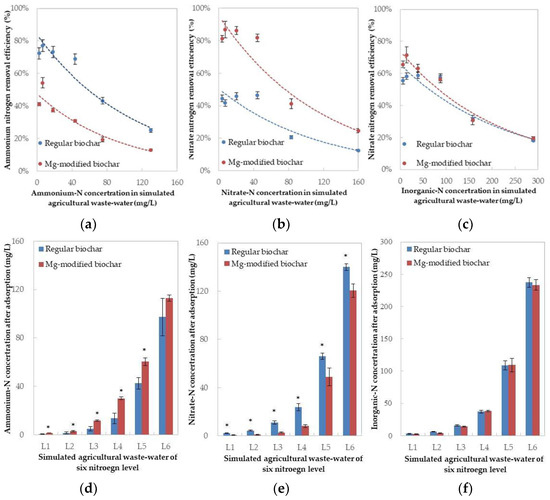
Figure 1.
N removal efficiencies of the two biochar materials and residual N concentrations in simulated agricultural wastewater after adsorption: (a) Ammonium-N removal efficiency; (b) Nitrate-N removal efficiency; (c) Inorganic-N removal efficiency; (d) Ammonium-N concentration after adsorption; (e) Nitrate-N concentration after adsorption; (f) Inorganic-N concentration after adsorption. * represents the significant differences in mean value between the treatments with regular biochar and Mg-modified biochar determined by a paired-t test (p < 0.05).
Although the ammonium and nitrate N removal efficiencies of the regular and Mg-modified biochars were different, their inorganic-N removal efficiencies were fairly similar (Figure 1c). Specifically, the inorganic-N removal efficiencies of the two biochar materials reached over 50% when the initial concentration was near 90 mg/L (L4 in Table 2), and fell to 30% at 150 mg/L (L5). After adsorption, the similar inorganic-N concentration (Figure 1f) was left in the stimulated agricultural wastewater with different ammonium and nitrate components (Figure 1d,e).
3.2. Amount of N Adsorbed by Biochars
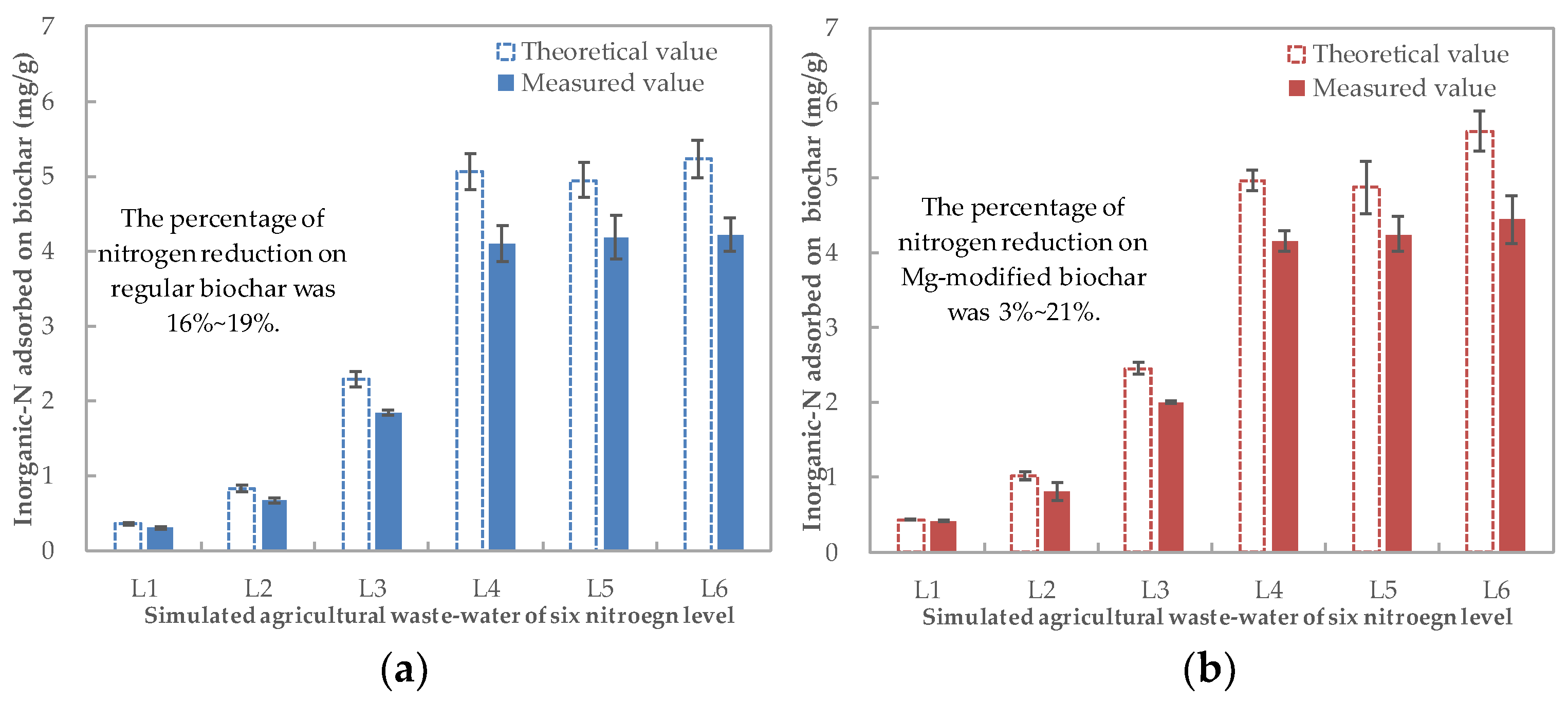
The theoretical value of inorganic N adsorbed by the biochars was calculated based on the difference in the inorganic-N concentrations between the stimulated agricultural wastewater and the measured value that was actually tested prior to planting (Figure 2). Compared to the theoretically expected value, part of the carried N from the biochar (carried N) was lost during the air-dying process: between 16 and 19% for the regular biochar, while for the Mg-modified biochar, the loss increased with the increase in wastewater N (from 3% to 21%).
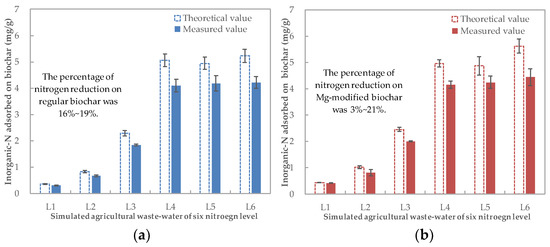
Figure 2.
Inorganic-N amount in the N–biochar mixture for (a) Regular biochar and (b) Mg-modified biochar.
Based on the measured adsorption value, one gram of regular and Mg-modified biochars could, respectively, carry 0.30–4.22 mg and 0.42–4.44 mg of inorganic N (Figure 2). For both regular and Mg-modified biochar, the carried N increased with the increase in N concentrations in the stimulated agricultural wastewater from L1 to L4 but plateaued at L5 and L6. Based on the N removal efficiency and N–biochar matrix production, the inorganic-N concentration of 100 mg/L was selected for the preparation of the biochar materials with a ratio of 1 g (biochar): 100 mL (wastewater). Hence, the N–biochar matrix produced by the regular biochar and the Mg-modified biochar adsorbing N from the stimulated agricultural wastewater at L4 (the inorganic-N concentration near to 90 mg/L) was used as carriers to recover N from wastewater and apply that onto the soil.
3.3. Bioavailability of the Carried N on Biochar
After the adsorptive and drying process, the regular biochar with 4.15 mg N/g and the Mg-modified biochar with 4.09 mg N/g were produced and employed in the planting experiment. Biochar addition changed the N distribution of the plant uptake, soil retention, and loss in the planting experiment (Table 4). The N uptake by the plant was promoted by biochar addition rising from 72.8 kg/ha (N control) to 77.4 kg/ha (MBA + NC) or 93.6 kg/ha (RBA + NC). Moreover, the soils with biochar had significantly more N than that observed in the N control soil (it rose from 57.8 kg/ha (N control) to 70.0 kg/ha (RBA + NC) or 85.9 kg/ha (MBA + NC)). Hence, the expected N loss, theoretically, was reduced from 69.2 kg/ha (N control) to 36.4 kg/ha (RBA + NC) or 36.7 kg/ha (MBA + NC). The treatments with two biochar material amendments had similar amounts of N loss but the loss pathways differed (Table 3). More N was assimilated by the plant in the case of regular biochar addition (RBA + NC), while for the Mg-modified biochar, more N was retained in the soil (MBA + NC).

Table 4.
N balance in one season of vegetable planting.
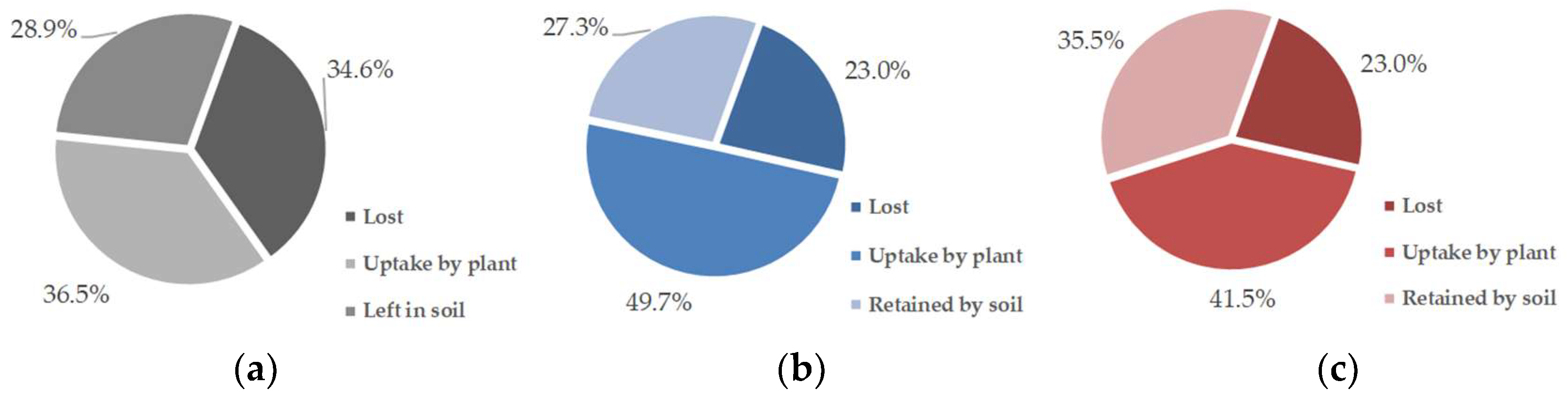
Nearly 90% of the 15N adsorbed by biochar was recovered in the plant tissue and soil after a single season of vegetable planting (Figure 3). Although the plant assimilated more N from the fertilizer than from the N–biochar matrix in the treatments with biochar amendment (Table 4), the carried N showed bioavailability at a level of 34.7–42.7% of the N assimilated by the plant. Additionally, the rate of the plant N uptake from fertilization was improved due to biochar presence from 36.4% (N control) to 41.5% (RBA + NC) and 49.7% (MBA + NC) (Figure 4). Compared to the fertilizer’s N, higher carried N from the biochar was retained by the soil, with the rate ranging from 45.9% to 53.7% (Figure 4). Only ~10% of the biochar carried-N got lost, amounting to slightly lower than the N from the fertilizer (Figure 3 and Figure 4). Biochar addition also decreased the rate of N loss from the fertilizer from 34.6% (N control) to 23% (RBA + NC and MBA + NC) (Figure 4).
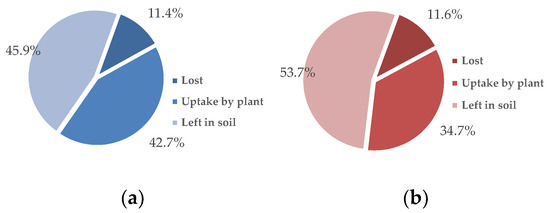
Figure 3.
Rates of the carried-N uptake by the plant, that left in soil, and that lost from the N–biochar matrix: (a) RBA + NC and (b) MBA + NC.
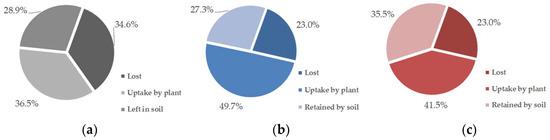
Figure 4.
Rates of the N uptake by the plant, that left in soil, and that lost from the fertilizer: (a) N control, (b) RBA + NC and (c) MBA + NC.
4. Discussion
4.1. N Adsorptive Capacity
The N adsorptive capacity of the biochar materials was an important factor assessed in this study as such media operated as N carriers to recover and reuse N from agricultural wastewater. Previous studies highlighted that the adsorbing performance of biochar mainly depended on its surface groups, porous structure, and other ions in the environment [17,28,29]. With these properties in mind, and after modification, the rough surface of Mg-modified biochar showed a metallic luster with many dense spherical particles scattered in internal and external pores, which were likely the MgO particles loaded onto the modified biochar surface (Figure S1). On the other hand, the clean and smooth surface of the regular biochar was found rich in irregular honeycomb-shaped porosity. Hydroxy, carboxyl, and aldehyde groups (all of them had negative charges) were the most abundant groups on the regular biochar, while after Mg modification, MgO- replaced part of these groups on the biochar surface. The significant differences in ion selectivity and adsorptive affinity between the regular biochar and Mg-modified biochar (Figure 1) could be explained by the charge changing on the biochar surface [25,27,28,39]. Electrostatic attraction was considered as the most likely adsorption mechanism for both ammonium-N and nitrate-N substrates [26,29]. Begum [40] believed that surface adsorption also occurred on the surface of biochar as ionic-size (radius of 0.335 nm) nitrate hydration can penetrate into the biochar. The increase in pore volume of Mg-modified biochar compared to that of regular biochar (Table 1) may support this view. However, the amount of inorganic N adsorbed by the biochar (Figure 2) remained unchanged regardless of modification, indicating that the porous structure might not be the predominant mechanism regulating the amount of N adsorption.
Although many previous studies reported the great promotion of N adsorptive capacity of biochar after modification [26,28,29], no significant difference was observed in this study focusing on the adsorptive capacity of inorganic N between regular and Mg-modified biochars. Furthermore, the amount of carried N from the biochar (Figure 2) was not as large as that used in the previously mentioned studies. Herein, the nitrate-N and ammonium-N present in the stimulated agricultural wastewater had similar N concentrations (Table 2). Based on previous findings [23,41,42,43], the coexistence of ions could increase the challenge for effective adsorption due to fewer vacant sites or blocks in the material’s pores; the phenomenon intensifies when ions’ initial concentrations become high (i.e., concentrated wastewater). This suggests that an adsorptive competition occurs due to the interferences between ammonium-N and nitrate-N in this study, and subsequently, a reduction in the N adsorptive capacity of the biochar limiting the amount of the potentially carried N from the biochar. In fact, most agricultural wastewater contains more than one form of N-based ions as well as a plethora of other ions (SO42−, PO43−, etc.) irrelevant to N. This inevitably may increase competition for active sites in the case of more complex actual wastewater. Hence, compared to the data obtained from pure ion conditions, the adsorbing data (Figure 1 and Figure 2) in this study can be more valuable in terms of practical applications.
The stability of the carried N from biochar is another key parameter and should be noted. Taghizadeh-Toosi [33] thought the N–biochar matrix was stable under ambient conditions because no significant change in total N content was observed within 12 experimental days. The different adsorptive conditions in this study may be used to explain the inconsistent results related to the stability of the carried N. In the study by Taghizadeh-Toosi [33], biochar was enriched with N by exposing the material to ammonia in a dry format, whereas in this study, ammonia from simulated agricultural wastewater was used. The dying process followed in this study provided optimal conditions for N transformations and for N loss. The loss percentage of the carried N from regular and Mg-modified biochar was estimated at around 19% and 15%, respectively (Figure 2); this suggests that the amount of actual N from the N–biochar matrix should be measured prior to biochar addition to the field. Further studies are needed to improve the stability of the carried N from biochars so bioavailability can be maintained.
4.2. Bioavailability of The Carried N from Biochar
The bioavailability of the carried N was demonstrated by the amount of N utilized by the plant during the trialed season. In this study, biochar amendment significantly increased soil inorganic-N content with more than half of it originating from the N–biochar matrix (Table. 3). Previous reviews found that soil inorganic-N content may be found to be decreased due to biochar amendment, but this can increase if the amended biochar is rich in organic matter [44,45]. The inconsistency among studies related to nutrient bioavailability from biochar implies that the changes in soil inorganic-N content can be a comprehensive result of multiple N processes that take place during the adsorption and retention of the N in the biochar [46,47]. The addition of biochar increased the soil nitrate-N content (Figure S2); nitrate-N is a major N form that can be easily lost with rain in agricultural systems and is the one that could be directly assimilated by most plants (except paddy or tea). Improving soil nitrate-N retention is essential for the optimization of N distribution in planting systems. Another study had previously reported an increase in plant N uptake for the consecutive growing seasons due to biochar amendment and a subsequent increase in soil N content [48]. Thus, it is believed that the carried N from the biochar and that present in soil today (or the current season) would still promote its bioavailability and could be assimilated by the plants the season after.
In this study, N loss between regular and Mg-modified biochar treatments was found to be similar, indicating the comparable capacity of the two biochar materials; the reduction pathway, however, was expected to differ. Compared to regular biochar, Mg-modified biochar did not directly contribute to planting growth but significantly increased the amount of N that returned in the soil (Table 3). This does not mean that the carried N from Mg-modified biochar may have had lower bioavailability than that of the regular biochar, but suggests the importance of taking into account both soil and biochar characteristics when selecting the biochar type as a N carrier. The selection of a suitable biochar based on soil characteristics would be more conducive in promoting plant growth and, subsequently, the bioavailability of the N carried by biochar. The soil collected for this study was slightly acidic (pH 5.6). Compared to the soil from the N control treatment, both regular and Mg-modified biochar amendments increased the soil pH in the beginning of the planting experiment, but only the soil subjected to regular biochar attained pH stability during vegetable planting (Figure S3). Since most biochar is alkaline, many studies applied biochar to an acidic soil environment in the expectation that biochar would neutralize part of the acid. Some of them found that biochar could significantly affect soil texture [49], N transformation [50], and microbial diversity [51,52]. However, the soil pH was not always changed significantly by biochar addition [52,53,54]. Even after a few planting seasons or many years of planting, the initial apparent increase in soil pH due to biochar addition would gradually fall back [52,53]. However, almost all studies have found that biochar could provide favorable soil conditions by increasing soil pH buffering capacity and maintaining the stability of the soil environment [49,50,51,52,53,54]. Thus, biochar would be an optimum choice as a N carrier for both plant growth and N bioavailability.
4.3. Potential of Biochar Application for N Recovery from Agricultural Wastewater
The possibility for biochar use as a N carrier was demonstrated in this study on the basis of inorganic-N adsorption from simulated agricultural wastewater and the carried-N reuse scenario in planting systems. There were still some limitations that need to be discussed related to the biochar application for N recovery from agricultural wastewater.
In terms of adsorption, the N form and concentration in wastewater should be the factors determining the choice of biochar material and its addition rate. Agricultural field runoffs, sewage tail water, and livestock wastewater are the three typical sources of N pollution. The biochar application scenarios for these agricultural wastewaters are provided as below. With regard to the runoffs from fields, the range of inorganic-N concentration is wide and often exceeds 30 mg/L [4]. There is usually more than one form of N in such runoffs, but these typically depend on the crop system type. Specifically, ammonium-N is the major form of N loss from paddy fields, while nitrate-N is the major form of N loss in upland fields [1]. It is implied that different biochar materials should be selected as the applicable N carriers to respond to different N forms. As the results from Figure 1 show, inorganic-N removal efficiencies could reach up to 60% with a ratio of 1 g (biochar): 100 mL (wastewater) when inorganic-N concentration is less than 40 mg/L (L1-L3 in Table 1). This suggested that nearly half of the inorganic N from wastewater could return to the field via reuse by biochar; this practice could effectively reduce the environmental risk from N accumulation and loss and improve N uptake efficiency in the field. With regard to effluents/tail water from sewage plants, typically, there is 15–20 mg/L of N remaining in such effluents (GB 18918 of China National Standards), and most of it is nitrate-N (partial denitrification). Therefore, Mg-modified biochar or other biochar with an affinity to nitrate-N could be selected following the ratio of 0.5–1 g (biochar): 100 mL (sewage). It is estimated that more than half of the inorganic N could be removed from sewage tail water with this process. Lastly, in the case of livestock wastewater, the ammonium-N concentration in such substrates can often be of several hundreds of mg/L. Based on the result from this study, regular biochar or other biochar types with an affinity towards ammonium-N should be selected. Its preferred addition rate could be set following the N concentration of the wastewater, such as 1 g (biochar): 100 mL (wastewater) for the wastewater with an ammonium-N concentration of 1 × 102 mg/L. Biochar application could be an effective way to realize partial (50%) ammonium-N recovery from livestock wastewater.
In terms of reuse, the amount of inorganic N adsorbed per unit of biochar weight and the addition rate are the key factors determining how much N fertilizer could be substituted by the N–biochar matrix. Additionally, the amount of carried N was determined by the inorganic-N concentration present in the wastewater. With the data from Figure 2, it was observed that when biochar was used to carry inorganic N from runoff, there was only 0.30–4.44 mg N adsorbed by one gram of biochar. With a most frequent addition rate of 0.2–1% w/w (about 4 × 103–20 × 103 kg/ha) [55], 1.6–40 kg/ha N is expected to be the N input from the biochar matrix; this is to replace 1–20% N fertilizer reducing 6–14 kg/ha N loss from runoffs (calculated with 200 kg/ha as the rate of N input for one growing season and 14% of N input getting lost into the water system). Biochar addition could improve soil properties in problem soil (such as poor aggregate stability and extreme pH) and increase N uptake, leading to an increase in plant yield and a decrease in N loss. The means of applying N–biochar matrix is not limited to recovering N from wastewater for reuse in soil but also can improve the productivity of the planting system.
5. Conclusions
Biochar materials could be used as N carriers to recover N from agricultural wastewater for reuse in soil. In this study, 0.4–4.4 mg of inorganic N from simulated agricultural wastewater was adsorbed by one gram of biochar. In this study, the N from biochar reached 40% of the total N application for a plant system with a 1% w/w addition rate to soil. The N carried by biochar retained its bioavailability with 34.7–42.7% of the N assimilated in plant tissues and 45.9–53.7% retained in soil. Only 10% of the carried N was lost, an amount lower than the N loss expected from conventional fertilization. Compared to N control treatment, the addition of biochar materials increased the amount of the N utilized by the plant and/or retained by the soil, significantly reducing nitrogen loss. Due to the loading of MgO- on the surface of Mg-modified biochar, regular biochar and Mg-modified biochar showed different affinities in the ammonia-N and nitrate-N adsorption process. This indicates that different biochar materials should be selected to meet the treatment needs of different agricultural wastewater. Furthermore, biochar material selection and optimized addition rates can assist further in removing constraints from soil cultivation, improving N uptake by plants and further reducing N loss.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/separations9110337/s1. Figure S1: SEM images for biochar materials; Figure S2: Soil inorganic nitrogen content after one season of vegetable planting; Figure S3: The change in soil pH in the planting experiment.
Author Contributions
Conceptualization, Y.Y. and L.Y.; methodology, Y.Y. and B.Y.; formal analysis, Y.Y. and J.D.; writing—original draft preparation, Y.Y.; writing—review and editing, E.P.; funding acquisition, L.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research and Development Project of China, grant number 2021YFD1700801, and the National Natural Science Foundation for Young Scholars of China, grant number 41501320.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hou, X.; Zhan, X.; Zhou, F.; Yan, X.; Gu, B.; Reis, S.; Wu, Y.; Liu, H.; Piao, S.; Tang, Y. Detection and attribution of nitrogen runoff trend in China’s croplands. Environ. Pollut. 2018, 234, 270–278. [Google Scholar] [CrossRef]
- Yin, A.J.; Duan, J.J.; Xue, L.H.; Feng, Y.F.; Petropoulos, E.; Yang, L.Z. High yield and mitigation of N-loss from paddy fields obtained by irrigation using optimized application of sewage tail water. Agr. Ecosyst. Environ. 2020, 304, 107137. [Google Scholar] [CrossRef]
- Woli, K.P.; Nagumo, T.; Hatano, R. Magnitude of nitrogen pollution in stream water due to intensive livestock farming practices. Soil Sci. Plant Nutr. 2002, 48, 883–887. [Google Scholar] [CrossRef]
- Shan, L.N.; He, Y.F.; Chen, J.; Huang, Q.; Lian, X.; Wang, H.C.; Liu, Y.L. Nitrogen surface runoff losses from a Chinese cabbage field under different nitrogen treatments in the Taihu Lake Basin, China. Agr. Water Manage. 2015, 159, 255–263. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Kong, L.L.; Zhu, L.; Xia, J.H.; Patricia, X. Fate characteristics of nitrogen in runoff from a small agricultural watershed on the south of Huaihe River in China. Environ. Earth Sci. 2012, 66, 835–848. [Google Scholar] [CrossRef]
- Xue, L.; Yu, Y.; Yang, L. Maintaining yields and reducing nitrogen loss in rice–wheat rotation system in Taihu Lake region with proper fertilizer management. Environ. Res. Lett. 2014, 9, 115010. [Google Scholar] [CrossRef]
- Tilman, D.; Cassman, K.G.; Matson, P.A.; Naylor, R.; Polasky, S. Agricultural sustainability and intensive production practices. Nature 2002, 418, 671–677. [Google Scholar] [CrossRef]
- Ju, X.T.; Xing, G.X.; Chen, X.P.; Zhang, S.L.; Zhang, L.J.; Liu, X.J.; Cui, Z.L.; Yin, B.; Christie, P.; Zhu, Z.L. Reducing environmental risk by improving N management in intensive Chinese agricultural systems. Proc. Natl. Acad. Sci. USA 2009, 106, 3041–3046. [Google Scholar] [CrossRef]
- Hou, P.F.; Jiang, Y.; Yan, L.; Petropoulos, E.; Wang, J.Y.; Xue, L.H.; Yang, L.Z.; Chen, D.L. Effect of fertilization on nitrogen losses through surface runoffs in Chinese farmlands: A meta-analysis. Sci. Total Environ. 2021, 793, 148554. [Google Scholar] [CrossRef]
- Lehmann, J. A handful of carbon. Nature 2007, 447, 143–144. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, B.; Yao, Y.; Fang, J.; Zhang, M.; Zhou, Y.; Chen, H.; Yang, L. Effects of feedstock type, production method, and pyrolysis temperature on biochar and hydrochar properties. Chem. Eng. J. 2014, 240, 574–578. [Google Scholar] [CrossRef]
- Allohverdi, T.; Mohanty, A.K.; Roy, P.; Misra, M. A review on current status of biochar uses in agriculture. Molecules 2021, 26, 5584. [Google Scholar] [CrossRef]
- Ayaz, M.; Feiziene, D.; Tilvikiene, V.; Akhtar, K.; Stulpinaite, U.; Iqbal, R. Biochar role in the sustainability of agriculture and environment. Sustainability 2021, 13, 1330. [Google Scholar] [CrossRef]
- Gai, X.; Wang, H.; Liu, J.; Zhai, L.; Liu, S.; Ren, T.; Liu, H. Effects of feedstock and pyrolysis temperature on biochar adsorption of ammonium and nitrate. PLoS ONE 2014, 9, e113888. [Google Scholar] [CrossRef]
- Cheng, C.H.; Lehmann, J.; Engelhard, M.H. Natural oxidation of black carbon in soils: Changes in molecular form and surface charge along a climosequence. Geochim. Cosmochim. Acta 2008, 72, 1598–1610. [Google Scholar] [CrossRef]
- Saleh, M.E.; Mahmoud, A.H.; El-Refaey, A.A. Removal of cadmium from aqueous solution by biochars derived from peanut hull and wheat straw. Adv. Environ. Biol. 2014, 8, 399–410. [Google Scholar]
- Vithanage, M.; Rajapaksha, A.U.; Ahmad, M.; Uchimiya, M.; Dou, X.; Alessi, D.S.; Yong, S.O. Mechanisms of antimony adsorption onto soybean stover-derived biochar in aqueous solutions. J. Environ. Manag. 2015, 151, 443–449. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, B.; Zhang, M.; Inyang, M.; Zimmerman, A.R. Effect of biochar amendment on sorption and leaching of nitrate, ammonium, and phosphate in a sandy soil. Chemosphere 2012, 89, 1467–1471. [Google Scholar] [CrossRef]
- Sayadi, M.; Farasati, M.; Mahmoodlu, M.G.; Rostamicharati, F. Removal of nitrate, ammonium, and phosphate from water using conocarpus and paulownia plant biochar. Iran. J. Chem. Chem. Eng. 2020, 39, 205–222. [Google Scholar] [CrossRef]
- Zhang, M.; Tian, Y.; Zhao, M.; Yin, B.; Zhu, Z. The assessment of nitrate leaching in a rice–wheat rotation system using an improved agronomic practice aimed to increase rice crop yields. Agr. Ecosyst. Environ. 2017, 241, 100–109. [Google Scholar] [CrossRef]
- Cengeloglu, Y.; Tor, A.; Ersoz, M.; Arslan, G. Removal of nitrate from aqueous solution by using red mud. Sep. Purif. Technol. 2006, 51, 374–378. [Google Scholar] [CrossRef]
- Shi, Y. China’s resources of biomass feedstock. Engineering Sci. 2011, 13, 16–23. [Google Scholar]
- Liu, H.W.; Dong, Y.H.; Wang, H.Y.; Liu, Y. Ammonium adsorption from aqueous solutions by strawberry leaf powder: Equilibrium, kinetics and effects of coexisting ions. Desalination 2010, 263, 70–75. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, Y.X.; Wu, W.X.; Shi, D.Z.; Yang, M.; Zhong, Z.K. Evaluation of biochar effects on nitrogen retention and leaching in multi-layered soil columns. Water Air Soil Poll. 2010, 213, 47–55. [Google Scholar] [CrossRef]
- Hollister, C.C.; Bisogni, J.J.; Lehmann, J. Ammonium, nitrate, and phosphate sorption to and solute leaching from biochars prepared from corn stover and oak wood. J. Environ. Qual. 2012, 42, 137–144. [Google Scholar] [CrossRef]
- Sanford, J.R.; Larson, R.A.; Runge, T. Nitrate sorption to biochar following chemical oxidation. Sci. Total Environ. 2019, 669, 938–947. [Google Scholar] [CrossRef]
- Hale, S.; Alling, V.; Martinsen, V.; Mulder, J.; Breedveld, G.; Cornelissen, G. The sorption and desorption of phosphate-P, ammonium-N and nitrate-N in cacao shell and corn cob biochars. Chemosphere 2013, 91, 1612–1619. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, B.; Yao, Y.; Xue, Y.; Inyang, M. Synthesis of porous MgO-biochar nanocomposites for removal of phosphate and nitrate from aqueous solutions. Chem. Eng. J. 2012, 210, 26–32. [Google Scholar] [CrossRef]
- Wang, T.T.; Zhang, D.; Fang, K.K.; Zhu, W.; Peng, Q.; Xie, Z.G. Enhanced nitrate removal by physical activation and Mg/Al layered double hydroxide modified biochar derived from wood waste: Adsorption characteristics and mechanisms. J. Environ. Chem. Eng. 2021, 9, 105184. [Google Scholar] [CrossRef]
- Masanizan, A.; Lim, C.M.; Kooh, M.R.R.; Mahadi, A.H.; Thotagamuge, R. The removal of ruthenium-based complexes N3 dye from DSSC wastewater using copper impregnated KOH-activated bamboo charcoal. Water Air Soil Pollut. 2021, 232, 388. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, P.; Li, X.; Sun, Q.; She, D. Magnesium chloride-modified potassium humate-based carbon material for efficient removal of phosphate from water. J. Taiwan Inst. Chem. E 2022, 140, 104540. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, G.; Zhang, P.; Shen, J.; Wang, S.; Li, Y. Development of iron-based biochar for enhancing nitrate adsorption: Effects of specific surface areas, electrostatic forces, and functional groups. Sci. Total Environ. 2022, 856, 159037. [Google Scholar] [CrossRef]
- Taghizadeh-Toosi, A.; Clough, T.J.; Sherlock, R.R.; Condron, L.M. Biochar adsorbed ammonia is bioavailable. Plant Soil 2012, 350, 57–69. [Google Scholar] [CrossRef]
- Kammann, C.I.; Schmidt, H.P.; Messerschmidt, N.; Linsel, S.; Steffens, D.; Müller, C.; Koyro, H.W.; Conte, P.; Joseph, S. Plant growth improvement mediated by nitrate capture in co-composted biochar. Sci. Rep. 2015, 5, 11080. [Google Scholar] [CrossRef]
- Krounbi, L.; Enders, A.; Gaunt, J.; Ball, M.; Lehmann, J. Plant uptake of nitrogen adsorbed to biochars made from dairy manure. Sci. Rep. 2021, 11, 15001. [Google Scholar] [CrossRef]
- Marcińczyk, M.; Ok, Y.S.; Oleszczuk, P. From waste to fertilizer: Nutrient recovery from wastewater by pristine and engineered biochars. Chemosphere 2022, 306, 135310. [Google Scholar] [CrossRef]
- Craswell, E.T.; Chalk, P.M.; Kaudal, B.B. Role of 15N in tracing biologically driven nitrogen dynamics in soils amended with biochar: A review. Soil Biol. Biochem. 2021, 162, 108416. [Google Scholar] [CrossRef]
- Zhang, J.; Cai, Z.; Yi, C.; Zhu, T. Denitrification and total nitrogen gas production from forest soils of Eastern China. Soil Biol. Biochem. 2009, 41, 2551–2557. [Google Scholar] [CrossRef]
- Wang, Z.H.; Guo, H.Y.; Shen, F.; Yang, G.; Zhang, Y.Z.; Zeng, Y.M.; Wang, L.L.; Xiao, H.; Deng, S.H. Biochar produced from oak sawdust by Lanthanum (La)-involved pyrolysis for adsorption of ammonium (NH4+), nitrate (NO3−), and phosphate (PO43−). Chemosphere 2015, 119, 646–653. [Google Scholar] [CrossRef]
- Begum, S.A.; Hyder, A.; Hicklen, Q.; Crocker, T.; Oni, B. Adsorption characteristics of ammonium onto biochar from an aqueous solution. J. Water Supply Res. 2021, 70, 113–122. [Google Scholar] [CrossRef]
- Yin, Q.Q.; Zhang, B.D.; Wang, R.K.; Zhao, Z.H. Biochar as an adsorbent for inorganic nitrogen and phosphorus removal from water: A review. Environ. Sci. Pollut. R. 2017, 24, 26297–26309. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Xue, Y.W.; Deng, P.Y.; Cheng, X.R.; Yang, K. Removal of aqueous ammonium by biochars derived from agricultural residuals at different pyrolysis temperatures. Chem. Spec. Bioavailab. 2015, 27, 92–97. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, B.; Chen, J.J.; Yang, L.Y. Engineered biochar reclaiming phosphate from aqueous solutions: Mechanisms and potential application as a slow-release fertilizer. Environ. Sci. Technol. 2013, 47, 8700–8708. [Google Scholar] [CrossRef]
- Nguyen, T.; Xu, C.Y.; Tahmasbian, I.; Che, R.; Xu, Z.; Zhou, X.; Wallace, H.M.; Bai, S.H. Effects of biochar on soil available inorganic nitrogen: A review and meta-analysis. Geoderma 2017, 288, 79–96. [Google Scholar] [CrossRef]
- Gao, S.; DeLuca, T.H.; Cleveland, C.C. Biochar additions alter phosphorus and nitrogen availability in agricultural ecosystems: A meta-analysis. Sci. Total Environ. 2019, 654, 463–472. [Google Scholar] [CrossRef]
- Huang, C.; Sun, X.Y.; Wang, L.J.; Storer, P.; Siddique, K.H.M.; Solaiman, Z.M. Nutrients leaching from tillage soil amended with wheat straw biochar influenced by fertiliser type. Agriculture 2021, 11, 1132. [Google Scholar] [CrossRef]
- Li, Y.; Feng, G.; Tewolde, H.; Yang, M.; Zhang, F. Soil, biochar, and nitrogen loss to runoff from loess soil amended with biochar under simulated rainfall. J. Hydrol. 2020, 591, 125318. [Google Scholar] [CrossRef]
- Zhou, Y.X.; Berruti, F.; Greenhalf, C.; Tian, X.H.; Henry, H.A.L. Increased retention of soil nitrogen over winter by biochar application: Implications of biochar pyrolysis temperature for plant nitrogen availability. Agr. Ecosyst. Environ. 2017, 236, 61–68. [Google Scholar] [CrossRef]
- Liu, X.; Wang, W.; Peñuelas, J.; Sardans, J.; Chen, X.; Fang, Y.; Tariq, A. Effects of nitrogen-enriched biochar on subtropical paddy soil organic carbon pool dynamics. Sci. Total Environ. 2022, 851, 158322. [Google Scholar] [CrossRef]
- Zheng, J.; Luan, L.; Luo, Y.; Fan, J.; Xu, Q.; Sun, B.; Jiang, Y. Biochar and lime amendments promote soil nitrification and nitrogen use efficiency by differentially mediating ammonia-oxidizer community in an acidic soil. Appl. Soil Ecol. 2022, 180, 104619. [Google Scholar] [CrossRef]
- Zhang, M.; Riaz, M.; Xia, H.; Li, Y.; Wang, X.; Jiang, C. Four-year biochar study: Positive response of acidic soil microenvironment and citrus growth to biochar under potassium deficiency conditions. Sci. Total Environ. 2022, 813, 152515. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Li, H.; Xiao, J.; Sun, J.; Liu, R.; Zhang, A. Soil multifunctionality of paddy field is explained by soil pH rather than microbial diversity after 8-years of repeated applications of biochar and nitrogen fertilizer. Sci. Total Environ. 2022, 853, 158620. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.L.; Li, K.W.; Nkoh, J.N.; He, X.; Hong, Z.N.; Xu, R.K. Effects of the increases in soil pH and pH buffering capacity induced by crop residue biochars on available Cd contents in acidic paddy soils. Chemosphere 2022, 301, 134674. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Odindo, A.; Xue, L.; Yang, L. Influences of biochar addition on vegetable soil nitrogen balance and pH buffering capacity. IOP Conf. Ser. Earth Environ. Sci. 2016, 41, 012029. [Google Scholar] [CrossRef]
- Verheijen, F.G.A.; Mankasingh, U.; Penizek, V.; Panzacchi, P.; Glaser, B.; Jeffery, S.; Bastos, A.C.; Tammeorg, P.; Kern, J.; Zavalloni, C.; et al. Representativeness of European biochar research: Part I- field experiments. J. Environ. Eng. Landsc. 2017, 25, 140–151. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).