Achillea fragrantissima Essential Oil: Composition and Detailed Pharmacodynamics Study of the Bronchodilator Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemicals
2.3. Preparation of AFO
2.4. GC-MS Analysis
2.5. GC Analysis
2.6. Bronchoduilator Study
2.6.1. Animals
2.6.2. Isolated Tissue Preparation
2.6.3. Determination of the Possible Mechanisms of Action
2.7. Statistical Analysis
3. Results
3.1. Preparation of AFO and GC-MS Study
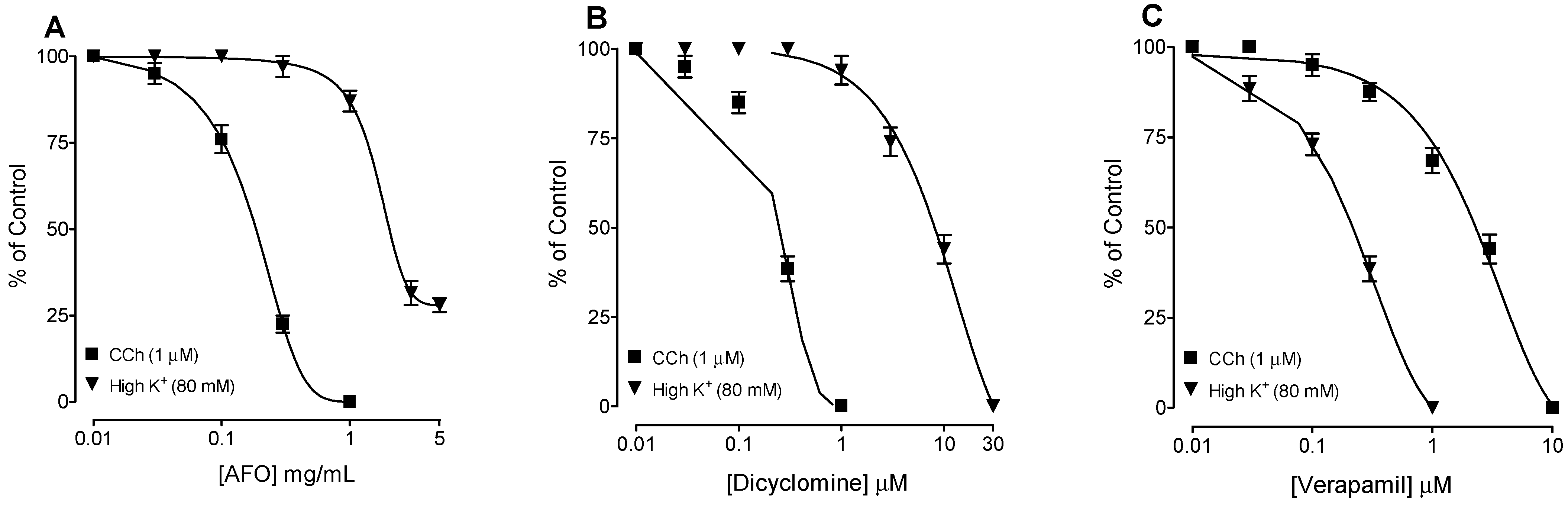
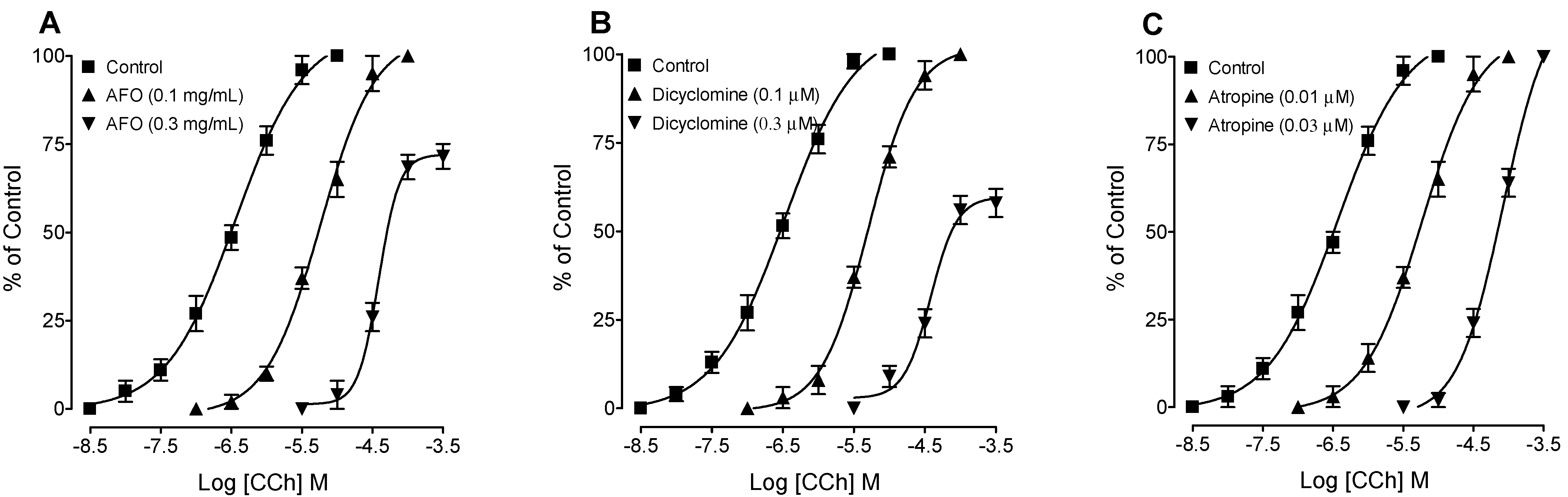
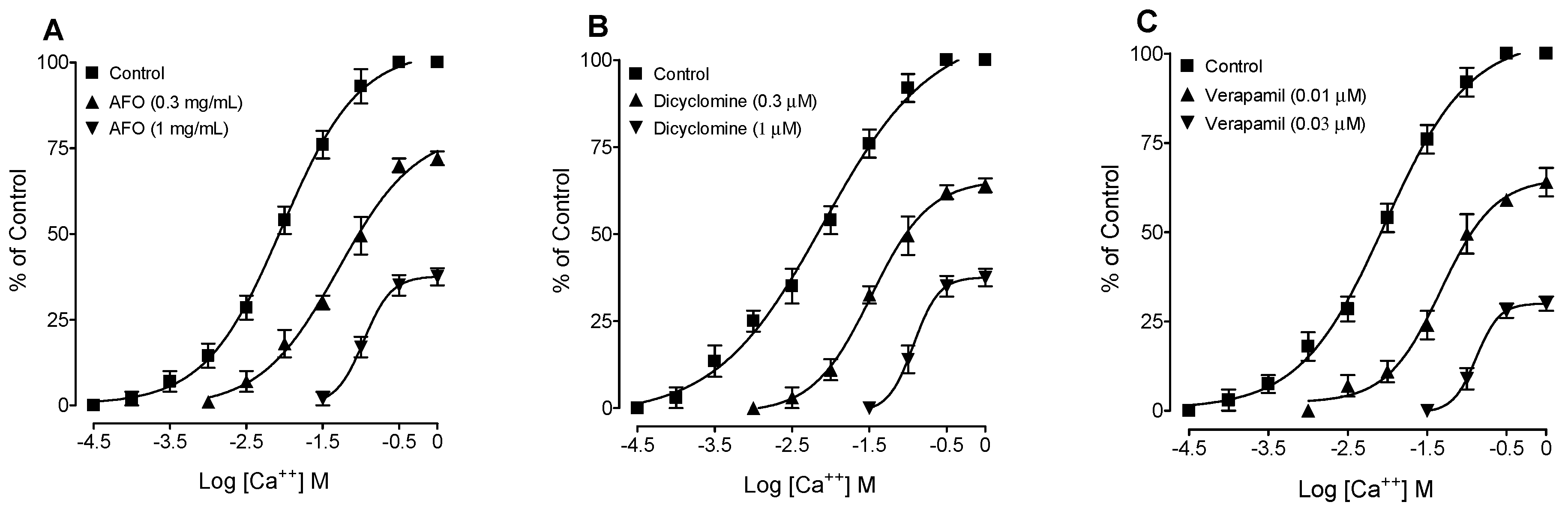
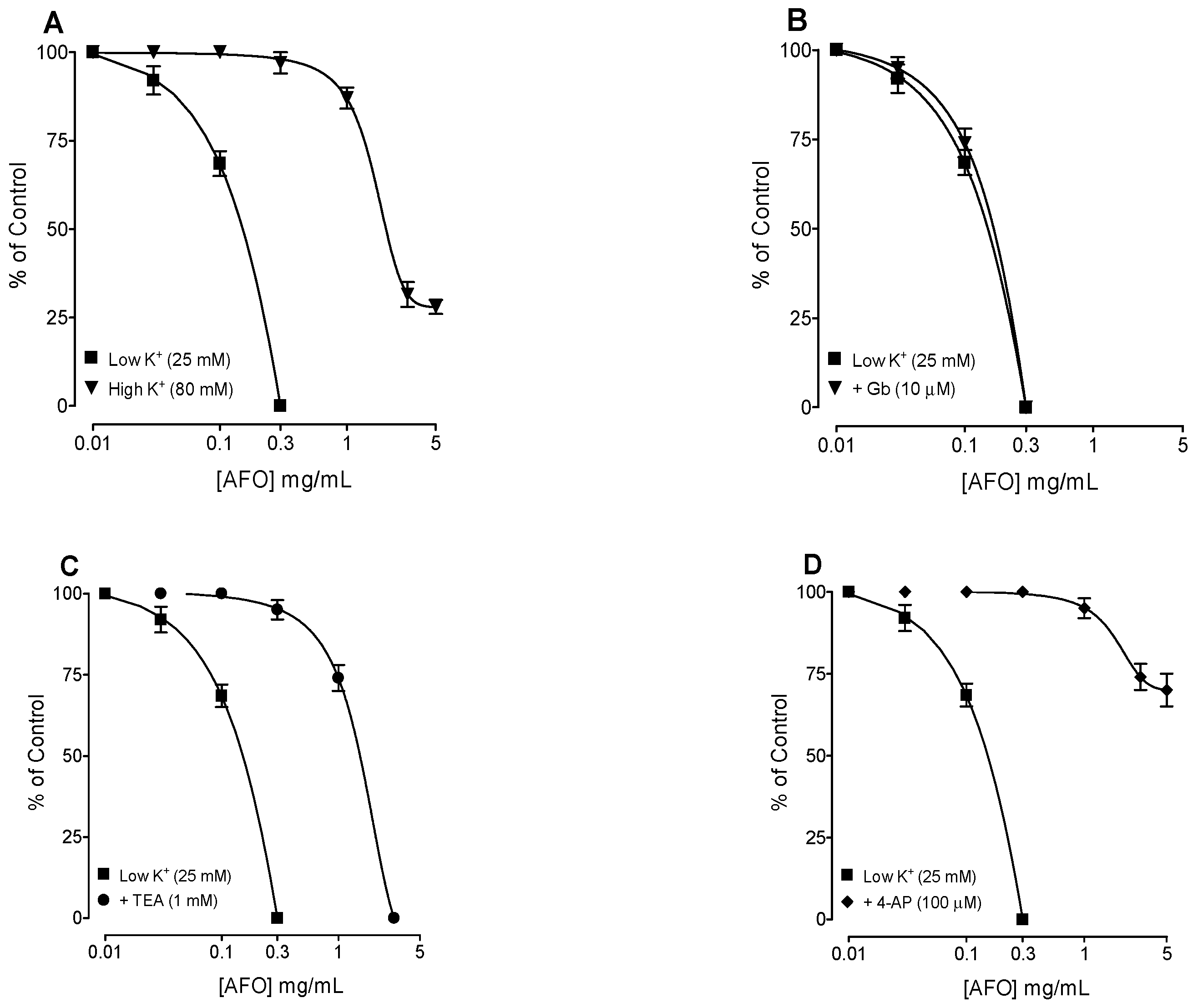
3.2. Bronchodilator Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vasisht, K.; Kumar, V. Compendium of Medicinal and Aromatic Plants; ICS-UNIDO: Trieste, Italy, 2004; Volume 1, p. 124. [Google Scholar]
- Saeidnia, S.; Gohari, A.; Mokhber-Dezfuli, N.; Kiuchi, F. A review on phytochemistry and medicinal properties of the genus Achillea. DARU J. Fac. Pharm. Tehran Univ. Med. Sci. 2011, 19, 173–186. [Google Scholar]
- Abdel-Azim, N.S.; Shams, K.A.; Shahat, A.A.; El Missiry, M.M.; Ismail, S.I.; Hammouda, F.M. Egyptian herbal drug industry: Challenges and future prospects. Res. J. Med. Plant. 2011, 5, 136–144. [Google Scholar] [CrossRef]
- Rawashdeh, I.M. Genetic diversity analysis of Achillea fragrantissima (Forskal) Schultz Bip. Populations collected from different regions of Jordan using RAPD Markers. Jordan J. Biol. Sci. 2011, 4, 21–28. [Google Scholar]
- Eissa, T.A.F.; Palomino, O.M.; Carretero, M.E.; Gómez-Serranillos, M.P. Ethnopharmacological study of medicinal plants used in the treatment of CNS disorders in Sinai Peninsula, Egypt. J. Ethnopharmacol. 2014, 151, 317–332. [Google Scholar] [CrossRef]
- Available online: https://www.mosoah.com/health/alternative-and-natural-medicine (accessed on 10 October 2022).
- Awad, B.M.; Abd-Alhaseeb, M.M.; Habib, E.S.; Ibrahim, A.K.; Ahmed, S.A. Antitumor activity of methoxylated flavonoids separated from Achillea fragrantissima extract in Ehrlich’s ascites carcinoma model in mice. J. Herbmed. Pharmacol. 2020, 9, 28–34. [Google Scholar] [CrossRef]
- Ahmed, W.; Aburjai, T.; Hudaib, M.; Al-Karablieh, N. Chemical Composition of Essential Oils Hydrodistilled from Aerial Parts of Achillea fragrantissima (Forssk.) Sch. Bip. and Achillea santolina L. (Asteraceae) Growing in Jordan. J. Essent. Oil Bear. Plants 2020, 23, 15–25. [Google Scholar] [CrossRef]
- Harris, B. Technology, and Application. In Handbook of Essential Oils; Can Baser, K.H., Buchbauer, G., Eds.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: New York, NY, USA, 2010; pp. 315–351. [Google Scholar]
- Horváth, G.; Ács, K. Essential oils in the treatment of respiratory tract diseases highlighting their role in bacterial infections and their anti-inflammatory action: A review. Flavour Fragr. J. 2015, 30, 331–341. [Google Scholar] [CrossRef]
- European Pharmacopoea, 5th ed.; Directorate for the Quality of Medicines of the Council of Europe: Strasburg, France, 2004; Volume 2, pp. 1004, 1108, 1570, 2206, 2534, 2569.
- NRC (National Research Council). Guide for the Care and Use of Laboratory Animals; National Academy Press: Washington, DC, USA, 1996; pp. 1–7. [Google Scholar]
- Venkatasamy, R.; Spina, D. Novel relaxant effects of RPL554 on guinea pig tracheal smooth muscle contractility. Br. J. Pharmacol. 2016, 173, 2335–2351. [Google Scholar] [CrossRef]
- Holroyde, M. The influence of epithelium on the responsiveness of guinea-pig isolated trachea. Br. J. Pharmacol. 1986, 87, 501–507. [Google Scholar] [CrossRef]
- Van-Rossum, J.M. Cumulative dose response curves. II. Technique for making of dose-response curves in isolated organs and the evaluation of drug parameters. Arch. Int. Pharmacodyn. Ther. 1963, 143, 299–330. [Google Scholar]
- Goldberg, L.A.; Rucker, F.J. Opposing effects of atropine and timolol on the color and luminance emmetropization mechanisms in chicks. Vis. Res. 2016, 122, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Kader, M.S.; Rehman, N.U.; Alghafis, M.A.; Al-Matri, M.A. Brochodilator Phenylpropanoid Glycosides from the Seeds of Prunus mahaleb L. Rec. Nat. Prod. 2022, 5, 443–453. [Google Scholar] [CrossRef]
- Rehman, N.U.; Ansari, M.N.; Ahmad, W.; Ahamad, S.R. Dual inhibition of phosphodiesterase and Ca++ channels explain the medicinal use of Balanites aegyptiaca (L.) in hyperactive gut disorders. Plants 2022, 11, 1183. [Google Scholar] [CrossRef] [PubMed]
- Palande, N.V.; Bhoyar, R.C.; Biswas, S.P.; Jadhao, A.G. Short-term exposure to L-type calcium channel blocker, verapamil, alters the expression pattern of calcium-binding proteins in the brain of goldfish, Carassius auratus. Comp. Biochem. Physiol. C: Toxicol. Pharmacol. 2015, 176–177, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Rehman, N.U.; Ansari, M.N.; Ahmad, W.; Samad, A. In silico and ex vivo studies on the spasmolytic activities of Fenchone using isolated guinea-pig trachea. Molecules 2022, 27, 1360. [Google Scholar] [CrossRef]
- Cook, N.S. The pharmacology of potassium channels and their therapeutic potential. Trend Pharmacol. Sci. 1988, 9, 21–28. [Google Scholar] [CrossRef]
- Satake, N.; Shibata, M.; Shibata, S. The inhibitory effects of iberiotoxin and 4-aminopyridine on the relaxation induced by beta1- and beta2-adrenoceptor activation in rat aortic rings. Br. J. Pharmacol. 1996, 119, 505–510. [Google Scholar] [CrossRef]
- Frank, H.; Puschmann, A.; Schusdziarra, V.; Allescher, H.D. Functional evidence for a glibenclamide-sensitive K+ channel in rat ileal smooth muscle. Eur. J. Pharmacol. 1994, 271, 379–386. [Google Scholar] [CrossRef]
- Alqarni, M.H.; Salkini, A.A.; Abujheisha, K.Y.; Daghar, M.F.; Al-khuraif, F.A.; Abdel-Kader, M.S. Qualitative, quantitative and antimicrobial activity variations of the essential oils isolated from Thymus Vulgaris and Micromeria Fruticosa Samples Subjected to Different Drying Conditions. Arab. J. Sci. Eng. 2022, 47, 6861–6867. [Google Scholar] [CrossRef]
- Althurwi, H.N.; Salkini, M.A.; Soliman, G.A.; Ansari, M.N.; Ibnouf, E.O.; Abdel-Kader, M.S. Wound Healing Potential of Commiphora gileadensis Stems Essential Oil and Chloroform Extract. Separations 2022, 9, 254. [Google Scholar] [CrossRef]
- Dharmage, S.C.; Perret, J.L.; Custovic, A. Epidemiology of Asthma in Children and Adults. Front. Pediatr. 2019, 7, 246. [Google Scholar] [CrossRef] [PubMed]
- Canning, B.J. Animal model of asthma and chronic obstructive pulmonary diseases. Pulm. Pharmacol. Ther. 2008, 21, 695. [Google Scholar] [CrossRef]
- Mathewson, H.S. Anti-asthmatic properties of calcium antagonists. Respir. Care 1985, 30, 779–781. [Google Scholar]
- Thirstrup, S. Control of airway tone: II-Pharmacology of relaxation. Respir. Med. 2000, 94, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Clement, Y.N.; Williams, A.F.; Aranda, D.; Chase, R.; Watson, N.; Mohammed, R.; Stubbs, O.; Williamson, D. Medicinal herb use among asthmatic patients attending a specialty care facility in Trinidad. BMC Complement. Altern. Med. 2005, 5, 3. [Google Scholar] [CrossRef]
- Rehman, N.U.; Ansari, M.N.; Hailea, T.; Karim, A.; Abujheisha, K.Y.; Ahamad, S.R.; Imam, F. Possible tracheal relaxant and antimicrobial effects of the essential oil of Ethiopian Thyme specie (Thymus serrulatus Hoschst. Ex Benth.): A multiple mechanistic approach. Front. Pharmacol. 2021, 12, 615228. [Google Scholar] [CrossRef]
- Arunlakhshana, O.; Schild, H.O. Some quantitative uses of drug antagonists. Br. J. Pharmacol. 1959, 14, 48–58. [Google Scholar] [CrossRef]
- Gilani, A.H.; Khan, A.U.; Ali, T.; Ajmal, S. Mechanisms underlying the antispasmodic and bronchodilatory properties of Terminalia bellerica fruit. J. Ethnopharmacol. 2008, 116, 528–538. [Google Scholar] [CrossRef]
- Twiss, M.A.; Harman, E.; Chesrown, S.; Hendeles, L. Efficacy of calcium channel blockers as maintenance therapy for asthma. Br. J. Clin. Pharmacol. 2002, 53, 243–249. [Google Scholar] [CrossRef]
- Barnes, P.J. Drugs for asthma. Br. J. Pharmacol. 2006, 147 (Suppl. 1), S297–S303. [Google Scholar] [CrossRef]
- Rehman, N.U.; Khan, A.U.; Alkharfy, K.M.; Gilani, A.H. Pharmacological basis for the medicinal use of Lepidium sativum in airways disorders. Evid.-Based Complement. Altern. Med. 2012, 2021, 596524. [Google Scholar]
- Gilani, A.H.; Khan, A.U.; Ghayur, M.N.; Ali, S.F.; Herzig, J.W. Antispasmodic effects of Rooibos tea (Aspalathus linearis) is mediated predominantly through K+-channel activation. Basic Clin. Pharmacol. Toxicol. 2006, 99, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Rehman, N.U.; AlKharfy, K.M.; Gilani, A.H. Antidiarrheal and antispasmodic activities of Salvia officinalis are mediated through activation of K+ channels. Bangladesh J. Pharmacol. 2011, 6, 111–116. [Google Scholar] [CrossRef]
- Kishii, K.; Morimoto, T.; Nakajima, N.; Yamazaki, K.; Tsujitani, M.; Takayanagi, I. Effect of LP-805, a novel vasorelaxant agent, a potassium channel opener on rat thoracic aorta. Gen. Pharmacol. 1992, 23, 347–353. [Google Scholar] [CrossRef]
- Okabe, K.; Kitamura, K.; Kuriyama, H. Feature of 4-aminopyridine sensitive outward current observed in single smooth muscle cells from the rabbit pulmonary artery. Pflug. Arch. 1987, 409, 561–568. [Google Scholar] [CrossRef]
- Quest, U. Potassium channel openers: Pharmacological and clinical aspects. Fund. Clin. Pharmacol. 1992, 6, 279–293. [Google Scholar] [CrossRef]
- Cunha, J.; Campestrini, F.; Calixto, J.; Scremin, A.; Paulino, N. The mechanism of gentisic acid-induced relaxation of the guinea pig isolated trachea: The role of potassium channels and vasoactive intestinal peptide receptors. Braz. J. Med. Biol. Res. 2001, 34, 381–388. [Google Scholar] [CrossRef]
- Lenz, T.; Wagner, G. Potential role of potassium channel openers for the treatment of cardiovascular disease. In Hypertension: Pathophysiology, Diagnosis and Management; Laragh, J.H., Brenner, B.M., Eds.; Raven Press: New York, NY, USA, 1995; pp. 2953–2968. [Google Scholar]
| Condition | Weight (g) | Weight of Oil (g) | % w/w |
|---|---|---|---|
| Fresh | 250 | 0.694 ± 0.021 | 0.28 |
| Dried | 100 | 0.545 ± 0.018 | 0.218 ** |
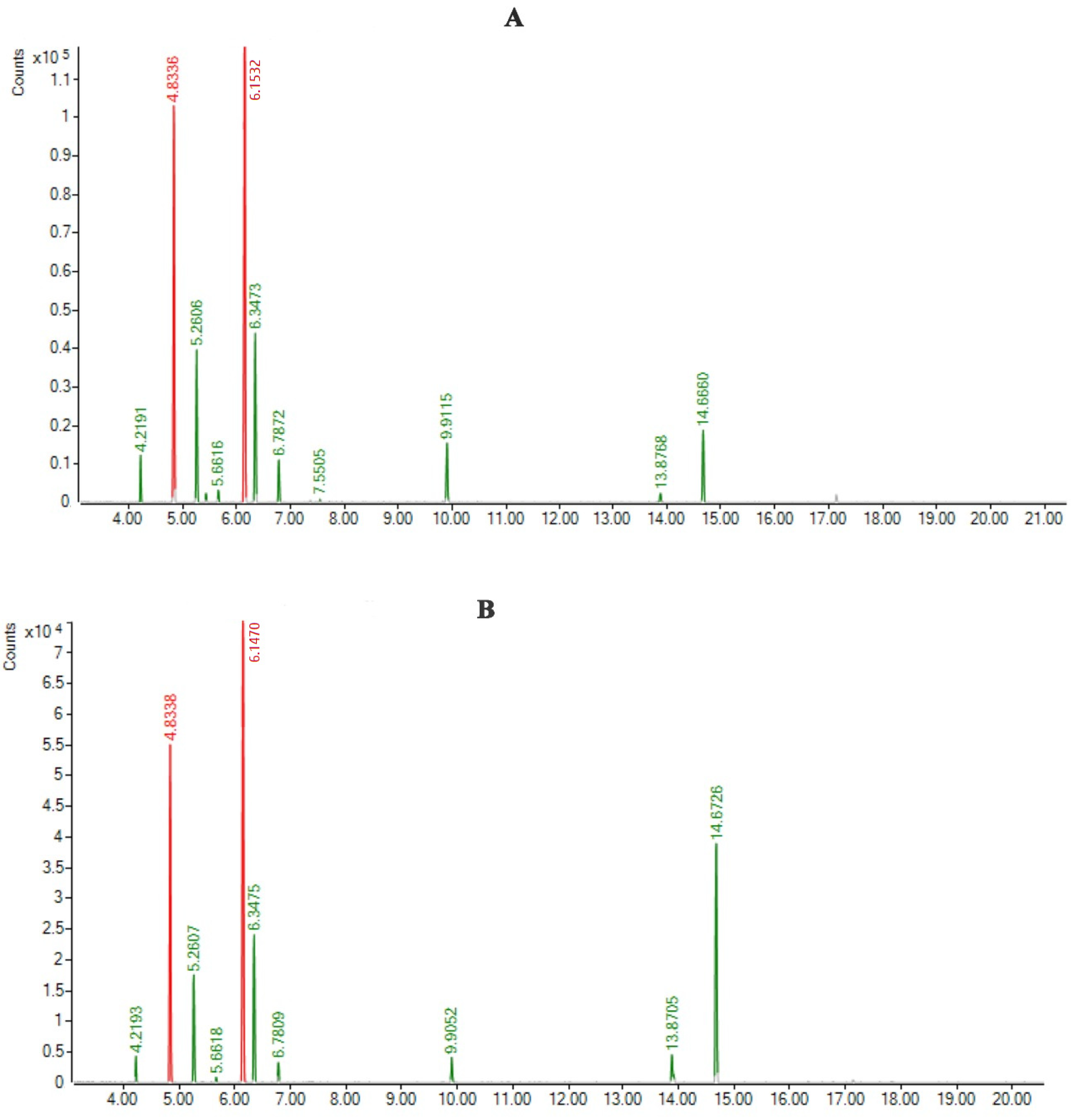
| Name of Components | RT | RRI | Area% | |
|---|---|---|---|---|
| Fresh | Dry | |||
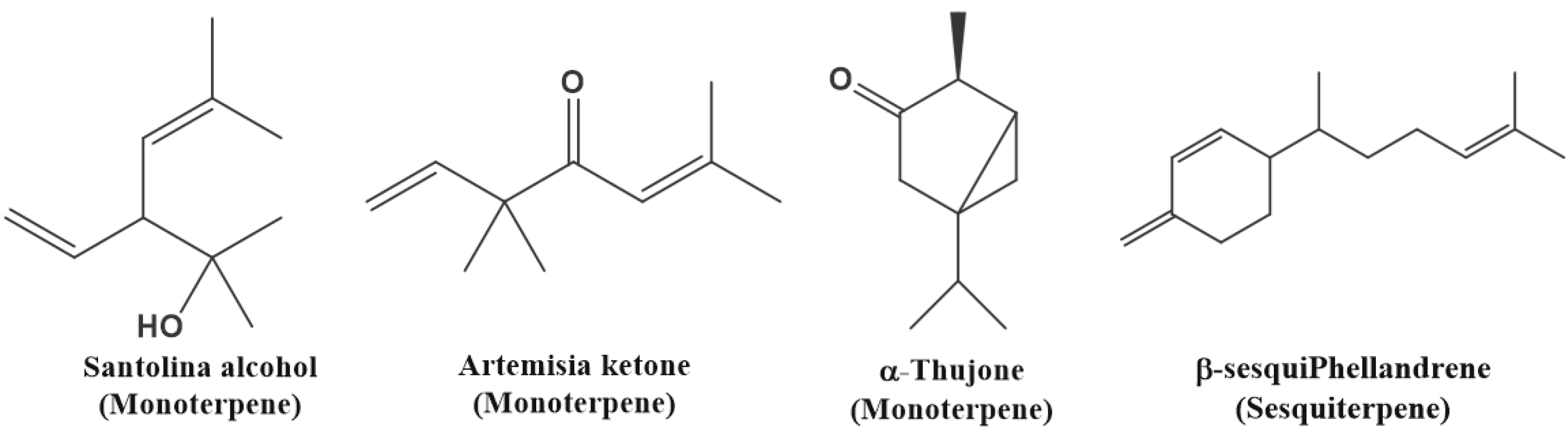
| Yomogi alcohol | 4.2191 | 1000 | 2.458 | 1.228 |
| (+)-Santolina alcohol | 4.8336 | 1038 | 25.643 | 21.711 |
| artemisia ketone | 5.2606 | 1061 | 9.949 | 7.209 |
| α-Thujone | 6.1470 | 1102 | 34.471 | 33.397 |
| β-Thujone | 6.3475 | 1110 | 11.939 | 10.704 |
| Sabinol | 6.7872 | 1143 | 3.052 | 1.268 |
| Sabinyl acetate | 9.9115 | 1291 | 4.582 | 1.585 |
| Bicyclosesquiphellandrene | 13.8768 | 1498 | 0.614 | 2.562 |
| β-sesquiphellandrene | 14.666 | 1525 | 5.511 | 20.068 |
| Monoterpenes% | 92.094 | 77.102 | ||
| Sesquiterpenes | 6.125 | 22.63 | ||
| Total | 98.219 | 99.732 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rehman, N.U.; Salkini, M.A.A.; Alanizi, H.M.K.; Alharbi, A.G.; Alqarni, M.H.; Abdel-Kader, M.S. Achillea fragrantissima Essential Oil: Composition and Detailed Pharmacodynamics Study of the Bronchodilator Activity. Separations 2022, 9, 334. https://doi.org/10.3390/separations9110334
Rehman NU, Salkini MAA, Alanizi HMK, Alharbi AG, Alqarni MH, Abdel-Kader MS. Achillea fragrantissima Essential Oil: Composition and Detailed Pharmacodynamics Study of the Bronchodilator Activity. Separations. 2022; 9(11):334. https://doi.org/10.3390/separations9110334
Chicago/Turabian StyleRehman, Najeeb Ur, Mohammad Ayman A. Salkini, Hatem M. K. Alanizi, Abdulrahman G. Alharbi, Mohammed H. Alqarni, and Maged S. Abdel-Kader. 2022. "Achillea fragrantissima Essential Oil: Composition and Detailed Pharmacodynamics Study of the Bronchodilator Activity" Separations 9, no. 11: 334. https://doi.org/10.3390/separations9110334
APA StyleRehman, N. U., Salkini, M. A. A., Alanizi, H. M. K., Alharbi, A. G., Alqarni, M. H., & Abdel-Kader, M. S. (2022). Achillea fragrantissima Essential Oil: Composition and Detailed Pharmacodynamics Study of the Bronchodilator Activity. Separations, 9(11), 334. https://doi.org/10.3390/separations9110334








