Standards-Based UPLC-Q-Exactive Orbitrap MS Systematically Identifies 36 Bioactive Compounds in Ampelopsis grossedentata (Vine Tea)
Abstract
:1. Introduction
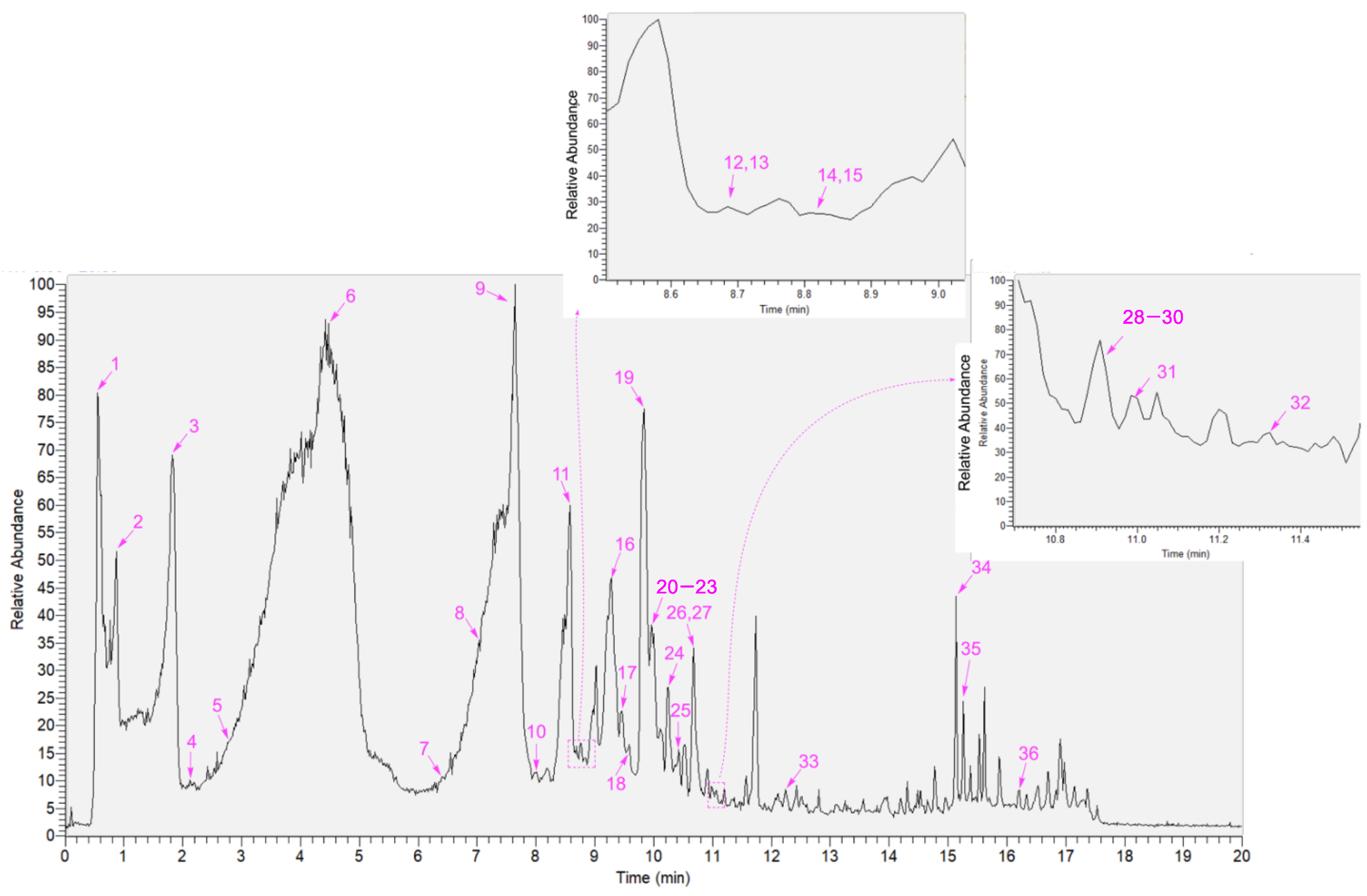
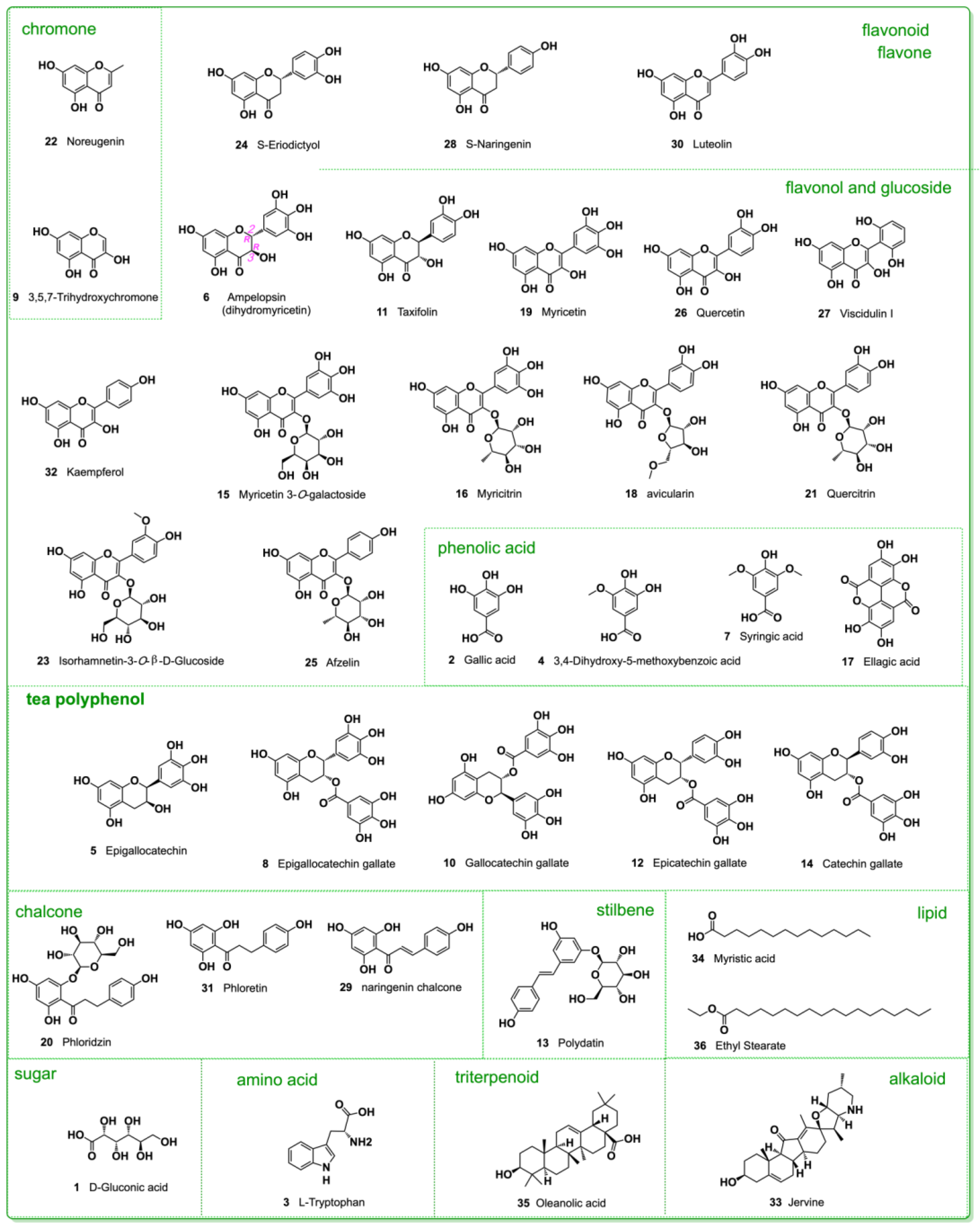
2. Results and Discussion
| No | Name | R.T. min | Formula | [M-H] m/z | Fragments m/z | Error (ppm) | Activity or Application |
|---|---|---|---|---|---|---|---|
| 1 | D-gluconic acid | 0.51 | C6H12O7 | 195.0501 | 177.0395, 159.0287, 129.0182, 111.0079, 99.0075, 75.0075 | −4.25 | metabolism [16] |
| 2 | gallic acid | 0.87 | C7H6O5 | 169.0132 | 125.0232, 107.0127, 97.0283, 83.1264, 79.0176, 69.0333 | −4.68 | anticancer, antioxidant [17,18], |
| 3 | L-tryptophan | 1.75 | C11H12N2O2 | 203.0817 | 186.0554, 159.0917, 142.0652, 130.0647, 116.0494, 74.0234 | −3.33 | metabolism [19] |
| 4 | 3,4-dihydroxy-5-methoxybenzoic acid | 2.19 | C8H8O5 | 183.0289 | 168.0053, 139.0389, 124.0154, 107.0128, 95.0127 | −4.88 | anticancer [20] |
| 5 | epigallocatechin | 2.75 | C15H14O7 | 305.0663 | 219.0663, 179.0334, 167.0336, 147.0441, 137.0229, 125.0232, 109.0282, 96.9587, 81.0332 | −0.41 | nutrition [21] |
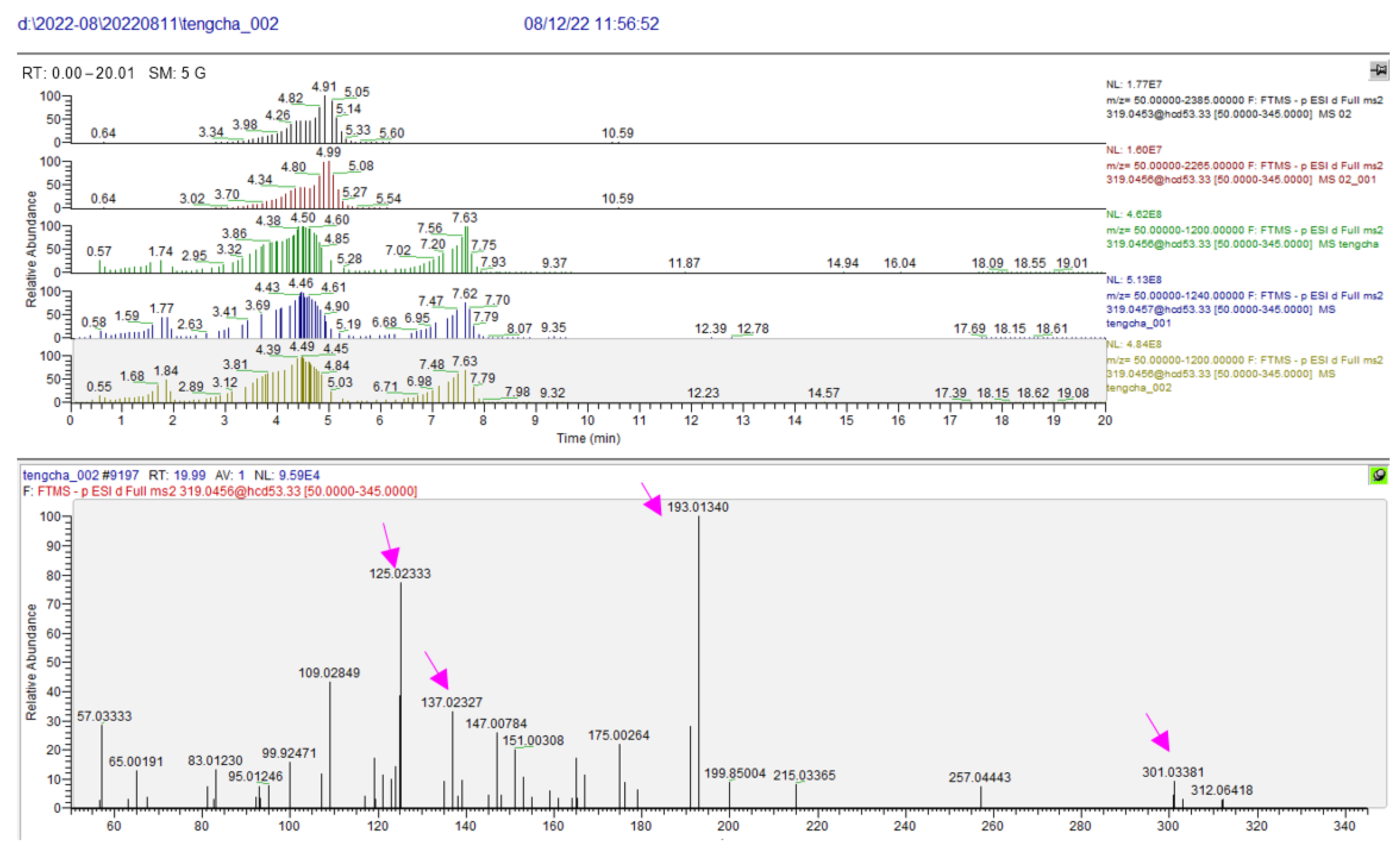
| 6 | ampelopsin | 4.46 | C15H12O8 | 319.0457 | 301.0346, 257.0448, 193.0134, 175.0027, 151.0056, 137.0223, 125.0233 | −1.88 | cytoprotection [3,22,23] |
| 7 | syringic acid | 6.55 | C9H10O5 | 198.1730 | 182.0210, 166.9976, 153.0545, 138.0311, 123.0076, 106.0051, 95.0126, 78.9576, 67.0176 | −3.57 | antioxidant [24] |
| 8 | epigallocatechin gallate | 7.98 | C22H18O11 | 457.0768 | 331.0469, 305.0669, 287.0564, 219.0647, 193.0135, 169.0132, 125.0233, 109.0284 | −0.45 | antioxidant [12,18] |
| 9 | 3,5,7-trihydroxychromone | 7.64 | C9H6O5 | 193.0100 | 175.0026, 165.0183, 147.0076, 137.0233, 121.0283, 109.0283, 91.0177, 67.0177 | −4.34 | antioxidant [25] |
| 10 | gallocatechin gallate | 7.99 | C22H18O11 | 457.0761 | 331.0454, 305.06659, 251.0342, 193.0135, 169.0131, 137.0234, 125.0232 | −0.25 | antioxidant [12] |
| 11 | taxifolin | 8.57 | C15H12O7 | 303.0511 | 285.0403, 259.0609, 241.0499, 217.0498, 199.0396, 175.0391, 150.0313, 125.0232 | −0.42 | cytoprotection [26,27] |
| 12 | epicatechin gallate | 8.64 | C22H18O10 | 441.0821 | 289.0716, 203.0705, 169.0132, 137.0231, 125.0233, 109.0283, 97.0281 | −0.25 | antioxidant [28,29] |
| 13 | polydatin | 8.70 | C20H22O8 | 389.1719 | 253.6727, 227.0705, 185.0597, 159.0806, 143.0491, 115.0541 | −0.43 | nutrition [30] |
| 14 | catechin gallate | 8.85 | C22H18O10 | 441.0837 | 331.0442, 289.0718, 245.0455, 203.0705, 169.0132, 125.0233, 109.0284 | −0.39 | cytoprotection [12] |
| 15 | myricetin 3-O-galactoside | 8.85 | C21H20O13 | 479.0821 | 316.0218, 287.0194, 271.0244, 242.0214, 214.0264, 185.0235, 151.0025, 124.0154 | −0,72 | osteomodulation [31] |
| 16 | myricitrin | 9.30 | C21H20O12 | 463.0877 | 316.0218, 287.0195, 271.0244, 259.0245, 242.0211, 214.0264, 185.0235, 151.0025, 124.0154 | −0.34 | anti-inflammation [32,33] |
| 17 | ellagic acid | 9.45 | C14H6O8 | 300.9986 | 283.9958, 257.0088, 229.0133, 200.0107, 172.0155, 145.0284, 133.0283, 117.0334 | −0.59 | antioxidant [34] |
| 18 | avicularin | 9.72 | C20H18O11 | 433.0772 | 300.0271, 271.0244, 255.0293, 243.0293, 227.0345, 199.0393, 171.0441, 135.0077 | −1.25 | antidiabetic [35] |
| 19 | myricetin | 9.83 | C15H10O8 | 317.0296 | 288.0235, 271.0247, 227.0345, 178.9976, 151.0026, 137.0233, 117.0333, 109.0283, 83.0125, 65.0020 | −1.11 | antioxidant [36,37] |
| 20 | phloridzin | 9.95 | C21H24O10 | 435.1314 | 273.0766, 229.0867, 179.0339, 167.0398, 151.0024, 123.0440, 93.0333, 81.0333 | −0.63 | anti-inflammation [38] |
| 21 | quercitrin | 10.01 | C21H20O11 | 447.0926 | 300.0269, 271.0244, 255.0293, 243.0290, 199.0391, 187.0390, 171.0441, 151.0026, 121.0284, 109.0283 | −0.59 | anti-inflammation [39,40,41] |
| 22 | noreugenin | 10.05 | C10H8O4 | 191.0341 | 176.0106, 163.0386, 151.0021, 147.0439, 132.0205, 119.0490, 105.0333, 81.0333, 63.0227 | −4.19 | healthcare [42] |
| 23 | isorhamnetin-3-O-β-D-Glucoside | 10.07 | C22H22O12 | 477.1029 | 315.0144, 299.0193, 271.0246, 243.0293, 215.0340, 187.0392, 143.0491, 131.0489 | −0.89 | metabolomics [43] |
| 24 | S-eriodictyol | 10.28 | C15H12O6 | 287.0561 | 259.0245, 203.0341, 151.0026, 135.0441, 125.0232, 117.0332, 107.0127, 83.0126, 65.0021 | −0.28 | antiobesity [44] |
| 25 | afzelin | 10.52 | C21H20O10 | 431.0976 | 285.0401, 255.0294, 227.0342, 211.0392, 199.0392, 183.0441, 167.0492, 155.0492, 107.0126 | −0.59 | renoprotection [45] |
| 26 | quercetin | 10.68 | C15H10O7 | 301.0347 | 245.0460, 227.0339, 211.0396, 187.0395, 178.9976, 151.0026, 145.0283, 139.0391, 121.0283, 107.01262 | −0.74 | antiferroptosis [46] |
| 27 | viscidulin I | 10.69 | C15H10O7 | 301.0347 | 273.0392, 227.0339, 211.0396, 178.9976, 151.0029, 121.0283, 107.0126, 93.0333, 83.0125, 65.0020 | −0.74 | glucosidase inhibitor [47] |
| 28 | S-naringenin | 10.90 | C15H12O5 | 271.0609 | 187.0388, 177.0185, 151.0026, 119.0491, 107.0126, 93.0333, 83.0126 | −0.27 | antioxidant [48,49] |
| 29 | naringenin chalcone | 10.90 | C15H12O5 | 271.0609 | 227.0706, 187.0388, 177.0185, 165.0188, 151.0026, 119.0491, 107.0126, 83.0126 | −0.27 | anti-inflammatory [50] |
| 30 | luteolin | 10.92 | C15H10O6 | 285.0401 | 267.0288, 241.0497, 199.0397, 175.0390, 151.0026, 133.0283, 121.0284, 107.0125 | −0.51 | cytoprotection [51,52] |
| 31 | phloretin | 11.06 | C15H14O5 | 273.0766 | 229.0863, 189.0551, 179.0337, 167.0339, 151.0027, 119.0491, 107.0490, 81.0332 | 0.31 | antimicrobial [53] |
| 32 | kaempferol | 11.33 | C15H10O6 | 285.0402 | 255.0307, 239.0342, 211.0390, 183.0441, 159.0442, 143.0491, 117.0334, 93.0333 | −0.19 | anti-inflammatory [54,55] |
| 33 | jervine | 12.33 | C27H39NO3 | 424.2853 | 248.1651, 179.1072, 163.1118, 147.0803, 133.1012, 117.0693 | 0.18 | anti-inflammatory [56,57] |
| 34 | myristic acid | 15.12 | C14H28O2 | 227.2012 | 190.4671, 176.1224, 100.2251, 92.1639, 70.3649, 62.0709 | −2.51 | anti-# apoptosis [58] |
| 35 | oleanolic acid | 15.20 | C30H48O3 | 455.3530 | 407.3330, 128.5021, 84.7155, 75.6721 | 0.58 | anticancer [59] |
| 36 | ethyl stearate | 16.19 | C20H40O2 | 311.1685 | 293.2842, 197.0269, 183.0112, 155.9873, 133.0649, 119.0491, 79.9561 | −0.55 | neuroprotection [60] |
3. Materials and Methods
3.1. Plants, Materials and Chemicals
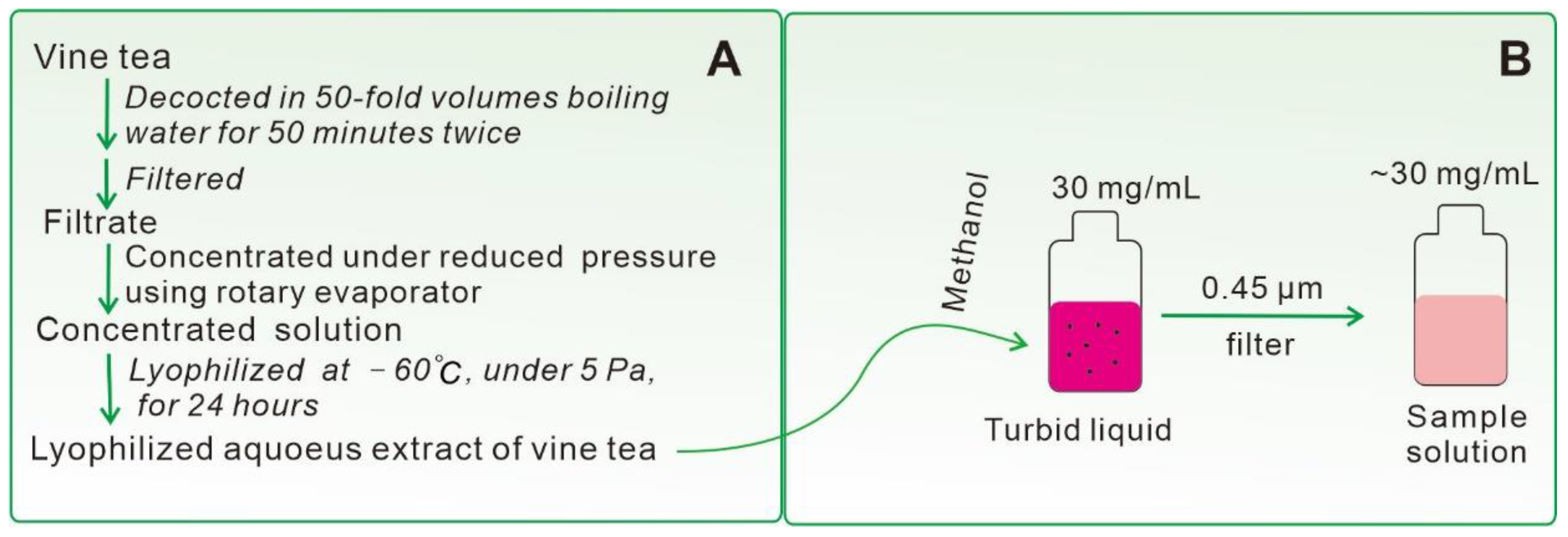
3.2. Preparation of Lyophilized Aqueous Extract of Vine Tea
3.3. UPLC-Q-Exactive Orbitrap MS Analysis and Data Acquisition
3.3.1. Analysis Apparatus and Conditions
3.3.2. Software, Data Acquisition and Putative Identification
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Murakami, T.; Miyakoshi, M.; Araho, D.; Mizutani, K.; Kambara, T.; Ikeda, T.; Chou, W.H.; Inukai, M.; Takenaka, A.; Igarashi, K. Hepatoprotective activity of tocha, the stems and leaves of Ampelopsis grossedentata, and ampelopsin. Biofactors 2004, 21, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.; He, C.; Ma, Y.; Shen, J.; Zhang, L.H.; Peng, Y.; Xiao, P. Investigation of free amino acid, total phenolics, antioxidant activity and purine alkaloids to assess the health properties of non-Camellia tea. Acta Pharm. Sin. B 2016, 6, 170–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Liu, J.; Lin, J.; Wang, T.; Huang, J.; Lin, Y.; Chen, D. Protective Effects of Dihydromyricetin against •OH-Induced Mesenchymal Stem Cells Damage and Mechanistic Chemistry. Molecules 2016, 21, 604. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, R.C.; Ye, L.; Baek, N.; Teixeira, G.H.; O’Keefe, S.F. Vine tea (Ampelopsis grossedentata): A review of chemical composition, functional properties, and potential food applications. J. Funct. Foods 2021, 76, 104317. [Google Scholar] [CrossRef]
- Ma, Q.; Cai, S.; Jia, Y.; Sun, X.; Yi, J.; Du, J. Effects of Hot-Water Extract from Vine Tea (Ampelopsis grossedentata) on Acrylamide Formation, Quality and Consumer Acceptability of Bread. Foods 2020, 9, 373. [Google Scholar] [CrossRef] [Green Version]
- Xiong, Y.; Zhu, G.; Zhang, Y.; Hu, Q.; Wang, H.; Yu, H.; Qin, X.; Guan, X.; Xiang, Y.; Ge, G. Flavonoids in Ampelopsis grossedentata as covalent inhibitors of SARS-CoV-23CLpro: Inhibition potentials, covalent binding sites and inhibitory mechanisms. Int. J. Biol. Macromol. 2021, 187, 976–987. [Google Scholar] [CrossRef]
- Hu, H.; Luo, F.; Wang, M.; Fu, Z.; Shu, X. New Method for Extracting and Purifying Dihydromyricetin from Ampelopsis grossedentata. ACS Omega 2020, 5, 13955–13962. [Google Scholar] [CrossRef]
- Xie, K.; He, X.; Chen, K.; Chen, J.; Sakao, K.; Hou, D.X. Antioxidant Properties of a Traditional Vine Tea, Ampelopsis grossedentata. Antioxidants 2019, 8, 295. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Yin, X.; Wang, X.; Li, X. Determination of dihydromyricetin in rat plasma by LC-MS/MS and its application to a pharmacokinetic study. Pharm. Biol. 2017, 55, 657–662. [Google Scholar] [CrossRef] [Green Version]
- Umair, M.; Sultana, T.; Xiaoyu, Z.; Senan, A.M.; Jabbar, S.; Khan, L.; Abid, M.; Murtaza, M.A.; Kuldeep, D.; Al-Areqi, N.A.S.; et al. LC-ESI-QTOF/MS characterization of antimicrobial compounds with their action mode extracted from vine tea (Ampelopsis grossedentata) leaves. Food Sci. Nutr. 2022, 10, 422–435. [Google Scholar] [CrossRef]
- Li, X.C. Improved pyrogallol autoxidation method: A reliable and cheap superoxide-scavenging assay suitable for all antioxidants. J. Agric. Food Chem. 2012, 60, 6418–6424. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Li, X.C.; Ren, Z.X.; Qiu, W.M.; Chen, J.L.; Jiang, Q.; Chen, B.; Chen, D.F. Antioxidant and Cytoprotective Effects of Tibetan Tea and Its Phenolic Components. Molecules 2018, 23, 179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Huai, W.; Zhang, Y.; Shen, J.; Tang, X.; Xie, X.; Wang, K.; Fan, H. Ultra-Performance Liquid Chromatography Hyphenated with Quadrupole-Orbitrap Mass Spectrometry for Simultaneous Determination of Necine-Core-Structure Pyrrolizidine Alkaloids in Crotalaria sessiliflora L. without all Corresponding Standards. Phytochem. Anal. 2017, 28, 365–373. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, Y.; Xue, H.; Jiang, G.C.; Liu, X.J. Antioxidant activity of vine tea (Ampelopsis grossedentata) extract on lipid and protein oxidation in cooked mixed pork patties during refrigerated storage. Food Sci. Nutr. 2019, 7, 1735–1745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, L.; Wang, H.; Duncan, S.E.; Eigel, W.N.; O’Keefe, S.F. Antioxidant activities of Vine Tea (Ampelopsis grossedentata) extract and its major component dihydromyricetin in soybean oil and cooked ground beef. Food Chem. 2015, 172, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Sainz, F.; Navarro, D.; Mateo, E.; Torija, M.J.; Mas, A. Comparison of D-gluconic acid production in selected strains of acetic acid bacteria. Int. J. Food Microbiol. 2016, 222, 40–47. [Google Scholar] [CrossRef]
- Kang, D.Y.; Sp, N.; Jo, E.S.; Rugamba, A.; Hong, D.Y.; Lee, H.G.; Yoo, J.-S.; Liu, Q.; Jang, K.-J.; Yang, Y.M. The inhibitory mechanisms of tumor PD-L1 expression by natural bioactive gallic acid in non-small-cell lung cancer (NSCLC) cells. Cancers 2020, 12, 727. [Google Scholar] [CrossRef] [Green Version]
- Li, X.C. 2-Phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide (PTIO•) Radical Scavenging: A New and Simple Antioxidant Assay in vitro. J. Agric. Food Chem. 2017, 65, 6288–6297. [Google Scholar] [CrossRef]
- Hou, E.; Zhao, Y.; Hang, J.; Qiao, J. Metabolomics and correlation network analysis of follicular fluid reveals associations between l-tryptophan, l-tyrosine and polycystic ovary syndrome. Biomed. Chromatogr. 2021, 35, e4993. [Google Scholar] [CrossRef]
- Henning, S.M.; Wang, P.; Abgaryan, N.; Vicinanza, R.; de Oliveira, D.M.; Zhang, Y.; Lee, R.P.; Carpenter, C.L.; Aronson, W.J.; Heber, D. Phenolic acid concentrations in plasma and urine from men consuming green or black tea and potential chemopreventive properties for colon cancer. Mol. Nutr. Food Res. 2013, 57, 483–493. [Google Scholar] [CrossRef]
- Ma, J.; Yao, Q.; Chen, X.; Lv, C.; Zang, J.; Zhao, G. Weak Binding of Epigallocatechin to α-Lactalbumin Greatly Improves Its Stability and Uptake by Caco-2 Cells. J. Agric. Food Chem. 2021, 69, 8482–8491. [Google Scholar] [CrossRef] [PubMed]
- Al Omran, A.J.; Shao, A.S.; Watanabe, S.; Zhang, Z.; Zhang, J.; Xue, C.; Watanabe, J.; Davies, D.L.; Shao, X.M.; Liang, J. Social isolation induces neuroinflammation and microglia overactivation, while dihydromyricetin prevents and improves them. J. Neuroinflamm. 2022, 19, 2. [Google Scholar] [CrossRef] [PubMed]
- Ai, J.; Han, X.; Zhan, J.; Huang, W.; You, Y. Study on effects of processing technology and storage on the composition of Ampelopsis grossedentata by untargeted metabolomics. Food Res. Int. 2022, 161, 111867. [Google Scholar] [CrossRef]
- Vo, Q.V.; Bay, M.V.; Nam, P.C.; Quang, D.T.; Flavel, M.; Hoa, N.T.; Mechler, A. Theoretical and experimental studies of the antioxidant and antinitrosant activity of syringic acid. J. Org. Chem. 2020, 85, 15514–15520. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, X.; Li, X.; Lu, W.; Zhao, X.; Chen, D. A null B-ring improves the antioxidant levels of Flavonol: A comparative study between Galangin and 3, 5, 7-Trihydroxychromone. Molecules 2018, 23, 3083. [Google Scholar] [CrossRef] [Green Version]
- Su, H.; Ruan, Y.-T.; Li, Y.; Chen, J.-G.; Yin, Z.-P.; Zhang, Q.-F. In vitro and in vivo inhibitory activity of taxifolin on three digestive enzymes. Int. J. Biol. Macromol. 2020, 150, 31–37. [Google Scholar] [CrossRef]
- Li, X.; Xie, H.; Jiang, Q.; Wei, G.; Lin, L.; Li, C.; Ou, X.; Yang, L.; Xie, Y.; Fu, Z.; et al. The mechanism of (+) taxifolin’s protective antioxidant effect for •OH-treated bone marrow-derived mesenchymal stem cells. Cell. Mol. Biol. Lett. 2017, 22, 231. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.-Q.; Gao, Y.; Granato, D. Effects of epigallocatechin gallate, epigallocatechin and epicatechin gallate on the chemical and cell-based antioxidant activity, sensory properties, and cytotoxicity of a catechin-free model beverage. Food Chem. 2021, 339, 128060. [Google Scholar] [CrossRef]
- Ji, M.; Li, C.; Li, Q. Rapid separation and identification of phenolics in crude red grape skin extracts by high performance liquid chromatography coupled to diode array detection and tandem mass spectrometry. J. Chromatogr. A 2015, 1414, 138–146. [Google Scholar] [CrossRef]
- Oliviero, F.; Galozzi, P.; Scanu, A.; Galuppini, F.; Lazzarin, V.; Brocco, S.; Ravagnan, G.; Sfriso, P.; Ramonda, R.; Spinella, P. Polydatin Prevents Calcium Pyrophosphate Crystal-Induced Arthritis in Mice. Nutrients 2021, 13, 929. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhao, L.; Yi, J.; Cai, S. Effects and Mechanisms of Rhus chinensis Mill. Fruits on Suppressing RANKL-Induced Osteoclastogenesis by Network Pharmacology and Validation in RAW264. 7 Cells. Nutrients 2022, 14, 1020. [Google Scholar] [CrossRef] [PubMed]
- Domitrović, R.; Rashed, K.; Cvijanović, O.; Vladimir-Knežević, S.; Škoda, M.; Višnić, A. Myricitrin exhibits antioxidant, anti-inflammatory and antifibrotic activity in carbon tetrachloride-intoxicated mice. Chem.-Biol. Interact. 2015, 230, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Du, L.-Y.; Zhao, M.; Xu, J.; Qian, D.-W.; Jiang, S.; Shang, E.-X.; Guo, J.-M.; Liu, P.; Su, S.-L.; Duan, J.-A. Identification of the metabolites of myricitrin produced by human intestinal bacteria in vitro using ultra-performance liquid chromatography/quadrupole time-of-flight mass spectrometry. Expert Opin. Drug Metab. Toxicol. 2014, 10, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Huang, R.; Wang, N.; Deng, Y.; Tan, B.; Yin, Y.; Qi, M.; Wang, J. Ellagic Acid Alleviates Oxidative Stress by Mediating Nrf2 Signaling Pathways and Protects against Paraquat-Induced Intestinal Injury in Piglets. Antioxidants 2022, 11, 252. [Google Scholar] [CrossRef]
- Zhu, X.; Qiu, Z.; Ouyang, W.; Miao, J.; Xiong, P.; Mao, D.; Feng, K.; Li, M.; Luo, M.; Xiao, H. Hepatic transcriptome and proteome analyses provide new insights into the regulator mechanism of dietary avicularin in diabetic mice. Food Res. Int. 2019, 125, 108570. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Zhu, M.; Wang, L.; Yu, S. Anti-tumor effects and associated molecular mechanisms of myricetin. Biomed. Pharmacother. 2019, 120, 109506. [Google Scholar] [CrossRef]
- Li, X.; Mai, W.; Chen, D.J.J.W.; Sons, L. Chemical Study on Protective Effect Against Hydroxyl-induced DNA Damage and Antioxidant Mechanism of Myricitrin. J. Chin. Chem. Soc. 2014, 61, 383–390. [Google Scholar] [CrossRef]
- Un, H.; Ugan, R.A.; Gurbuz, M.A.; Bayir, Y.; Kahramanlar, A.; Kaya, G.; Cadirci, E.; Halici, Z. Phloretin and phloridzin guard against cisplatin-induced nephrotoxicity in mice through inhibiting oxidative stress and inflammation. Life Sci. 2021, 266, 118869. [Google Scholar] [CrossRef]
- Chen, J.; Li, G.; Sun, C.; Peng, F.; Yu, L.; Chen, Y.; Tan, Y.; Cao, X.; Tang, Y.; Xie, X. Chemistry, pharmacokinetics, pharmacological activities, and toxicity of Quercitrin. Phytother. Res. 2022, 36, 1545–1575. [Google Scholar] [CrossRef]
- Li, X.; Jiang, Q.; Wang, T.; Liu, J.; Chen, D.J.M. Comparison of the Antioxidant Effects of Quercitrin and Isoquercitrin: Understanding the Role of the 6″-OH Group. Molecules 2016, 21, 1246. [Google Scholar] [CrossRef]
- Jiang, S.; Yang, J.; Qian, D.; Guo, J.; Shang, E.-X.; Duan, J.-A.; Xu, J. Rapid screening and identification of metabolites of quercitrin produced by the human intestinal bacteria using ultra performance liquid chromatography/quadrupole-time-of-flight mass spectrometry. Arch. Pharm. Res. 2014, 37, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Semwal, R.B.; Semwal, D.K.; Combrinck, S.; Viljoen, A. Health benefits of chromones: Common ingredients of our daily diet. Phytochem. Rev. 2020, 19, 761–785. [Google Scholar] [CrossRef]
- Wang, X.; Bai, J.; Wang, W.; Zhang, G.; Yin, S.; Wang, D. A comparative metabolomics analysis of the halophyte Suaeda salsa and Salicornia europaea. Environ. Geochem. Health 2021, 43, 1109–1122. [Google Scholar] [CrossRef] [PubMed]
- Kwon, E.-Y.; Do, E.; Lee, D.; Kim, J.; Choi, M.-S. Elucidation of anti-obesity and anti-diabetic function of eriodictyol in diet-induced obese mice. Clin. Nutr. 2018, 37, S146. [Google Scholar] [CrossRef]
- Cechinel-Zanchett, C.C.; Bolda Mariano, L.N.; Boeing, T.; da Costa, J.D.C.; Da Silva, L.M.; Bastos, J.K.; Cechinel-Filho, V.; de Souza, P. Diuretic and renal protective effect of kaempferol 3-O-alpha-l-rhamnoside (afzelin) in normotensive and hypertensive rats. J. Nat. Prod. 2020, 83, 1980–1989. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zeng, J.; Liu, Y.; Liang, M.; Liu, Q.; Li, Z.; Zhao, X.; Chen, D. Inhibitory Effect and Mechanism of Action of Quercetin and Quercetin Diels-Alder anti-Dimer on Erastin-Induced Ferroptosis in Bone Marrow-Derived Mesenchymal Stem Cells. Antioxidants 2020, 9, 205. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Tan, N.; Wang, H.; Hu, J.; Diwu, W.; Wang, X. A systematic analysis of natural alpha-glucosidase inhibitors from flavonoids of Radix scutellariae using ultrafiltration UPLC-TripleTOF-MS/MS and network pharmacology. BMC Complement. Med. Ther. 2020, 20, 72. [Google Scholar] [CrossRef] [Green Version]
- Cai, R.; Li, X.; Chen, B.; Xie, Y.; Xie, H.; Chen, D. Antioxidant Change in Biosynthesis from Naringenin Chalcone to Flavonoid Apingenin. ChemistrySelect 2019, 4, 5155–5159. [Google Scholar] [CrossRef]
- Ng, K.R.; Lyu, X.; Mark, R.; Chen, W.N. Antimicrobial and antioxidant activities of phenolic metabolites from flavonoid-producing yeast: Potential as natural food preservatives. Food Chem. 2019, 270, 123–129. [Google Scholar] [CrossRef]
- Escribano-Ferrer, E.; Queralt Regué, J.; Garcia-Sala, X.; Boix Montañés, A.; Lamuela-Raventos, R.M. In vivo anti-inflammatory and antiallergic activity of pure naringenin, naringenin chalcone, and quercetin in mice. J. Nat. Prod. 2019, 82, 177–182. [Google Scholar] [CrossRef]
- Lin, L.-C.; Pai, Y.-F.; Tsai, T.-H. Isolation of luteolin and luteolin-7-O-glucoside from Dendranthema morifolium Ramat Tzvel and their pharmacokinetics in rats. J. Agric. Food Chem. 2015, 63, 7700–7706. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Li, X.C.; Tian, Y.G.; Lin, Q.Q.; Xie, H.; Lu, W.B.; Chi, Y.G.; Chen, D.F. Lyophilized aqueous extracts of Mori Fructus and Mori Ramulus protect Mesenchymal stem cells from *OH-treated damage: Bioassay and antioxidant mechanism. BMC Complement. Altern. Med. 2017, 16, 423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barreca, D.; Bellocco, E.; Laganà, G.; Ginestra, G.; Bisignano, C. Biochemical and antimicrobial activity of phloretin and its glycosilated derivatives present in apple and kumquat. Food Chem. 2014, 160, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Devi, K.P.; Malar, D.S.; Nabavi, S.F.; Sureda, A.; Xiao, J.; Nabavi, S.M.; Daglia, M. Kaempferol and inflammation: From chemistry to medicine. Pharmacol. Res. 2015, 99, 1–10. [Google Scholar] [CrossRef]
- Li, X.; Tian, Y.; Wang, T.; Lin, Q.; Feng, X.; Jiang, Q.; Liu, Y.; Chen, D. Role of the p-Coumaroyl Moiety in the Antioxidant and Cytoprotective Effects of Flavonoid Glycosides: Comparison of Astragalin and Tiliroside. Molecules 2017, 22, 1165. [Google Scholar] [CrossRef] [Green Version]
- Dumlu, F.A.; Aydin, T.; Odabasoglu, F.; Berktas, O.A.; Kutlu, Z.; Erol, H.S.; Halici, M.B.; Cadirci, E.; Cakir, A. Anti-inflammatory and antioxidant properties of jervine, a sterodial alkaloid from rhizomes of Veratrum album. Phytomedicine 2019, 55, 191–199. [Google Scholar] [CrossRef]
- Melnik, E.V.; Belova, M.V.; Potskhveriya, M.M.; Simonova, A.Y.; Tyurin, I.A.; Ramenskaya, G.V. Veratrum alkaloid determination in four cases of Veratrum Aqua poisonings. J. Anal. Toxicol. 2022, 46, e42–e47. [Google Scholar] [CrossRef]
- Khalil, A.S.M.; Giribabu, N.; Yelumalai, S.; Shahzad, H.; Kilari, E.K.; Salleh, N. Myristic acid defends against testicular oxidative stress, inflammation, apoptosis: Restoration of spermatogenesis, steroidogenesis in diabetic rats. Life Sci. 2021, 278, 119605. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Dai, X.; Kumar, A.P.; Tan, B.K.; Sethi, G.; Bishayee, A. Oleanolic acid and its synthetic derivatives for the prevention and therapy of cancer: Preclinical and clinical evidence. Cancer Lett. 2014, 346, 206–216. [Google Scholar] [CrossRef] [Green Version]
- Ye, S.; Zhong, J.; Huang, J.; Chen, L.; Yi, L.; Li, X.; Lv, J.; Miao, J.; Li, H.; Chen, D. Protective effect of plastrum testudinis extract on dopaminergic neurons in a Parkinson’s disease model through DNMT1 nuclear translocation and SNCA’s methylation. Biomed. Pharmacother. 2021, 141, 111832. [Google Scholar] [CrossRef]
- Tian, Y.; Li, X.; Xie, H.; Wang, X.; Xie, Y.; Chen, C.; Chen, D. Protective Mechanism of the Antioxidant Baicalein toward Hydroxyl Radical-Treated Bone Marrow-Derived Mesenchymal Stem Cells. Molecules 2018, 23, 223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Cui, J.; Zhao, G.; Liu, H.; Wang, J. Pharmacokinetics of Veratramine and Jervine from Alcohol Extracts of Radix Veratri. Comput. Math. Methods Med. 2022, 2022, 8289548. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wen, B.; Wang, Y.; Wu, S.; Zhang, X.; Gu, Y.; Wang, Z.; Wang, J.; Zhang, W.; Yong, J. Jervine exhibits anticancer effects on nasopharyngeal carcinoma through promoting autophagic apoptosis via the blockage of Hedgehog signaling. Biomed. Pharm. 2020, 132, 110898. [Google Scholar] [CrossRef]
- Xie, Y.; Li, X.; Xu, J.; Jiang, Q.; Xie, H.; He, J.; Chen, D. Two phenolic antioxidants in Suoyang enhance viability of •OH-damaged mesenchymal stem cells: Comparison and mechanistic chemistry. Chem. Cent. J. 2017, 11, 81. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, X.C.; Zeng, H.P. Synthesis, antioxidation activity of (E)-9-p-Tolyl-3-[2-(8-hydroxy-quinol-2-yl)vinyl]-carbazole and (E)-9-(p-Anisyl)-3-[2-(8-hydroxy-quinol-2-yl)vinyl]-carbazole and their induction proliferation of mesenchymal stem cells. Acta Chim. Sin. 2009, 67, 974–982. [Google Scholar]
- Hua, Y.; Li, X.C.; Zhang, W.; Chen, B.; Liu, Y.; Zhao, X.; Xie, H.; Chen, D. Antioxidant product analysis of Folium Hibisci Mutabilis. J. Saudi Chem. 2021, 25, 101272. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.; Hua, Y.; Li, Z.; Chen, B.; Liu, A.; Lu, W.B.; Zhao, X.; Diao, Y.; Chen, D. Antioxidant product analysis of Hulu Tea (Tadehagi triquetrum). New J. Chem. 2021, 45, 20257–20265. [Google Scholar] [CrossRef]
- Gross, J.H. Mass Spectrometry, 2nd ed.; Science Press: Beijing, China, 2013. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, R.; Li, X.; Li, C.; Zhu, J.; Zeng, J.; Li, J.; Tang, B.; Li, Z.; Liu, S.; Yan, Y. Standards-Based UPLC-Q-Exactive Orbitrap MS Systematically Identifies 36 Bioactive Compounds in Ampelopsis grossedentata (Vine Tea). Separations 2022, 9, 329. https://doi.org/10.3390/separations9110329
Cai R, Li X, Li C, Zhu J, Zeng J, Li J, Tang B, Li Z, Liu S, Yan Y. Standards-Based UPLC-Q-Exactive Orbitrap MS Systematically Identifies 36 Bioactive Compounds in Ampelopsis grossedentata (Vine Tea). Separations. 2022; 9(11):329. https://doi.org/10.3390/separations9110329
Chicago/Turabian StyleCai, Rongxin, Xican Li, Chunhou Li, Jiayi Zhu, Jingyuan Zeng, Jianwu Li, Boxu Tang, Zheng Li, Shuqin Liu, and Yan Yan. 2022. "Standards-Based UPLC-Q-Exactive Orbitrap MS Systematically Identifies 36 Bioactive Compounds in Ampelopsis grossedentata (Vine Tea)" Separations 9, no. 11: 329. https://doi.org/10.3390/separations9110329
APA StyleCai, R., Li, X., Li, C., Zhu, J., Zeng, J., Li, J., Tang, B., Li, Z., Liu, S., & Yan, Y. (2022). Standards-Based UPLC-Q-Exactive Orbitrap MS Systematically Identifies 36 Bioactive Compounds in Ampelopsis grossedentata (Vine Tea). Separations, 9(11), 329. https://doi.org/10.3390/separations9110329







