Abstract
The objectives of this research were to carry out GC–MS and LC–MS-based phytochemical profiling of Barleria hochstetteri, as well as flow cytometry-based mechanistic investigations of the cytotoxic effect of its extracts against breast and lung cancer cell lines. This preclinical in vitro study was carried out in Saudi Arabia and India, from 11 August to 15 January 2022. Barleria hochstetteri was sequentially extracted using the Soxhlet extraction technique. Utilizing LC–MS and GC–MS methods, the phytochemical profiling was performed. Additionally, the total phenolic compounds and flavonoids were quantified in the plant extract using spectrophotometric techniques. In this study, we first examined the cytotoxicity of the plant extract on non-malignant L929 cells and on the carcinogenic MCF-7 and A549 cell lines. Then, we studied the underlying molecular pathways by means of Anti-Bcl-2, caspase-3, and DNA fragmentation (TUNEL) assays, using flow cytometry. The results revealed phenolic compounds and flavonoids to be the two major components in the methanolic extract of B. hochstetteri, with concentrations of 3210 µg GAE/g dwt and 1863 µg QE/g dwt, respectively. Results from GC–MS and LC–MS analyses revealed the presence of bioactive phytochemicals with known cytotoxicity. From the MTT assay on cell viability, the IC50 of the methanol extract for the MCF-7 and A549 cell lines were 219.67 and 144.30 µg/mL, respectively. With IC50 values of 324.24 and 266.66 µg/mL, respectively, the aqueous and methanol extracts were less toxic when tested against the non-cancerous L929 cell line. The extract caused early and late apoptosis in the tested breast and lung cancer cells by activating caspase-3 and inhibiting Bcl-2 protein, and it also caused cell death via DNA damage, based on flow cytometric and molecular marker analyses. These findings indicate that the methanol extract of B. hochstetteri was cytotoxic on breast cancer and lung cancer cell lines. To uncover cancer-fighting chemicals, there is a need for further research on B. hochstetteri, as it is a promising source of anti-cancer chemotherapeutic drugs.
1. Introduction
Cancer kills millions of people around the world every year. Increases in cancer rates are predicted as a result of a number of socioeconomic and demographic shifts, including but not limited to larger populations, more sedentary lifestyles, greater exposure to potential carcinogens, longer life expectancies, and urbanization [1].
In Saudi Arabia, breast, colorectal, prostate, brain, lymphoma, kidney, and thyroid cancers are the most prevalent types. While medical advancements have made great strides in cancer treatment, there are still numerous difficulties that need to be addressed in order to enhance cancer therapy. Treatments for newly-diagnosed or recurring cancers, and the emergence of resistance to currently used medications, are also other challenges [2,3]. As a result, oncological research is actively geared towards discovering novel and effective therapies that may mitigate the negative effects of current treatments. There are a variety of technologies and novel lead compounds which are either currently being tested in preclinical and clinical trials, or are already in use in clinical settings [2].
There are currently no viable treatments for newly-diagnosed or recurring cancers, and the emergence of resistance to currently used medications is a grave and immediate worry [3]. The plant kingdom contains a wide range of highly bioactive phytochemicals that are beneficial in cancer treatment [4]. Due to their availability, versatility, and lower side effects, plant-derived natural products are a valuable therapeutic option for the treatment of cancer. Natural products, along with their synthetic counterparts, have a significant impact on cancer therapy by altering the tumor microenvironment and a variety of signaling pathways. Paclitaxel, vincristine, and vinblastine are only a few of the well-known anticancer medicines that originate in plants. Many phytochemicals have been demonstrated to have cytotoxic and antitumor properties, according to recent studies. Curcumin, honokiol, ursolic acid, hesperidin, gossypol, resveratrol, caffeic acid phenethyl ester, apigenin, and lycopene, to name a few, have all demonstrated promising therapeutic promise in the management of cancer [5]. The WHO traditional medicine (TM) strategy (2014–2023), encourages the easy access and rational use of TM and complementary medicine to increase the availability and affordability of TM, with an emphasis on access for poor populations, and also promotes the therapeutically sound use of appropriate TM by practitioners and consumers [6].
In this study, the plant used was Barleria hochstetteri (Acanthaceae), which is native to the Sahara, South Asia, and the Arabian Peninsula. Previous studies have shown that species of Barleria have immunomodulatory [7], anti-respiratory syncytial viral [8], antiarthritic, anti-inflammatory, and hepatoprotective activities [9]; inhibitory effects on glutathione S-transferase and acetylcholinesterase activities; and antibacterial effects [10]. Several species from the Barleria genus have been reported to exert cytotoxic effects against human hepatocellular carcinoma (HepG2), human breast adenocarcinoma (MCF7), human oral epidermoid carcinoma (KB), human colon adenocarcinoma (HT29), murine lymphocytic leukemia (P388), and human cervical carcinoma (HeLa), as well as two normal cell lines, i.e., African green monkey kidney (Vero) cells and mouse subcutaneous connective tissues (L929) [11]. However, not much is known about the cytotoxic potential of Barleria hochstetteri against cancer cell lines, even though it is rich in polyphenolic compounds and flavonoids [12].
The present study was carried out using the MTT assay to investigate an indigenously-grown Barleria hochstetteri (Southern Saudi Arabian) plant with respect to its qualitative and quantitative phytochemical composition, as well as the cytotoxic properties of its different fractions against non-cancerous fibroblast cells (L929), and cancerous breast cancer (MCF7) and lung cancer (A549) cell lines.
2. Methodology
2.1. Plant Collection, Extraction, and Phytochemical Analysis
Dry samples of B. hochstetteri were coarsely pulverized, and 100 g portions were sequentially extracted with a series of solvents using a Soxhlet extractor device to obtain a crude extract. A total of 70 to 75 Soxhlet extraction cycles were performed. The extraction procedure lasted for 24 h, or until the solvent in the siphon tube became colorless. The solvents used for the extraction were hexane, ethyl acetate, methanol, and distilled water, in ascending order of polarity. After each extraction, the dried plant material was re-extracted using a different solvent. All the extracts were concentrated and dried using a rotating vacuum evaporator. The crude hexane, ethyl acetate, methanol, and distilled water extracts of B. hochstetteri were screened for the presence and absence of different phytochemical constituents using standard phytochemical tests [13].
2.2. GC–MS Analysis
A split injector was used to inject 1 µL of methanol extract of B. hochstetteri into the instrument. The column temperature was initially set at 80 °C, while the injector temperature was set at 260 °C, and the temperature flow was set to increase at a rate of 10 °C/min throughout the process. The specifications were as follows—pressure: 65.0 kPa, column flow: 1.00 mL/min, total flow: 24.0 mL/min, purge flow: 3.0 mL/min, linear velocity: 36.8 cm/s. The final temperature was set at 280 °C, and the process was operated for 6 min [14].
2.3. Liquid Chromatography–Mass Spectrometry (LC–MS) Analyses
The chemical components of the methanol extract of B. hochstetteri were identified using a binary pump equipped with LC–MS-8040 (Shimadzu). A mass spectrometer with an ESI source was connected to the HPLC. For the analysis, a C18 HPLC column was employed. The solvent had an overall flow rate of 0.2 mL/min and was composed of methanol and water in a volume ratio of 80:20. The positive ion mode was used to acquire the MS spectra. It made use of the electron spray ionization (ESI) probe. For analysis, a total of 3 µL of sample was injected [15].
2.4. Estimation of Total Phenolic and Flavonoid Contents
Spectrophotometry was used to determine the total phenolics in the plant extract as per the Folin–Ciocalteau method [16]. Calibration curves for gallic acid (GA) were made in the range of 20–100 µg/mL. The concentrations of phenolic compounds were converted to μg gallic acid equivalents (μg GAE) per g of dry weight of the extract (μg GAE/g dwt). The standard calibration curve (regression equation: y = 0.001x + 0.113) was used to obtain the total amount of phenolics.
In addition, a colorimetric assay with aluminum chloride was used to obtain the total flavonoid content [17]. Standard quercetin concentrations of 20–100 µg/mL were created in methanol. Then, 1 mL of each quercetin concentration in methanol was pipetted into a 10 mL volumetric flask containing 4 mL of double-distilled water. At time zero, 0.3 mL of 5% sodium nitrite, 0.3 mL of 10% AlCl3, and 2 mL of 1 M sodium hydroxide were added. Immediately, 2.4 mL of double-distilled water was added to the mixture, with thorough stirring. The absorbance of the pink-colored mixture was measured at 510 nm against a quercetin-free blank. The average quercetin absorbance values were used to draw a calibration curve (regression equation: y = 0.265x − 0.152) from which the flavonoid concentrations were extrapolated and expressed as μg quercetin equivalents per gram of dry weight of the extract (μg QE/g dwt).
2.5. In Vitro Cytotoxicity of B. hochstetteri Extracts
A cytotoxicity assay was performed using the standard MTT assay procedure. The non-cancerous cell line, as well as the lung cancer (A549) and breast cancer (MCF-7) cell lines, were treated with graded concentrations of different solvent extracts of B. hochstetteri (50, 100, 150, 200, and 250 µg/mL). Untreated cells served as the negative control group, while cells treated with the standard drug cisplatin served as the positive control group. Extract-treated cells were taken as the test group. IC50 value was calculated from the Y = mx + c equation derived from the graph of the concentration of samples versus the percentage of cell viability. The IC50 values were determined on the basis of percentage of cell viability calculated using the formula below [18].
2.6. Determination of Early and Late Apoptosis through Binding of Annexin V to Phosphatidylserine
In the present study, early and late apoptosis were determined after treating MCF-7 and A549 cells with the IC50 concentration of methanol extract of B. hochstetteri for 24 h. Flow cytometric analysis was performed after 24 h incubation with the extract using FlowJoX 10.0.7 software (10.0.7, Becton Dickinson, Oregon, USA), in accordance with the kit instructions [19].
2.7. Caspase Activation Assay
Caspase-3 activity was measured using a commercial kit (TACS kit), following the instructions from the manufacturer. Briefly, the MCF-7 and A549 cancer cell lines were pre-treated with caspase-3 inhibitor Z-DEVD-FMK (20 μM, BD Biosciences, Franklin Lakes, NJ, USA) for 2 h, followed by treatment with the required concentration of methanol extract of B. hochstetteri for 24 h. Caspase-3 activity was determined using a flow cytometer (Acquisition: 7.Cytomics FC500 Flow cytometer, Beckman Coulter, Brea, CA, USA) [20].
2.8. Anti Bcl-2 Activity
Flow cytometry was used to quantify the level of Bcl-2 protein in the MCF-7 and A549 cancer cell lines treated with methanolic extract of B. hochstetteri, according to the procedure described by Moraes et al. [21]. After treatment with the test sample at its IC50 concentration, the cell preparations were analyzed flow cytometrically within 30 min using FlowJo X 10.0.7 software.
2.9. DNA Fragmentation Analysis
Apoptosis-induced DNA fragmentation due to the methanol extract of B. hochstetteri was determined with a TUNEL assay kit (The APO-DIRECT™ Kit) [22,23]. The percentage of TUNEL-positive cells was calculated flow cytometrically using Flowzo 10.1 Acquisition: 7 Cytomics FC500 Flow cytometer, (Beckman Coulter, Brea, CA, USA).
2.10. Statistical Analysis
The results are presented as mean ± standard deviation (SD; n = 3). GraphPad Prism version 5 was used to carry out one-way analysis of variance (ANOVA), followed by Dunnett’s test. The level of statistical significance was set at p ˂ 0.05.
3. Results
3.1. Phytochemical Analysis; Total Phenolic and Flavonoid Contents
The preliminary phytochemical study revealed the presence of various secondary metabolites in the solvent extracts, which included glycosides, flavonoids, terpenoids, phenols, and saponins (Table 1). The phenolic and terpenoid compounds were the major phytochemical constituents in the methanol and aqueous extracts. However, these phytochemicals were absent in the hexane and ethyl acetate extracts. The methanolic and aqueous fractions were also rich in flavonoids. Furthermore, quantitative analysis of the total phenolic and flavonoid contents of the methanol and aqueous extract revealed that the methanolic fraction exhibited a higher phenolic content (3210 µg GAE/g of dwt) and flavonoid content (1863 µg QE/g dwt), when compared to the aqueous extract, which had phenolic content of 2552 µg GAE/g dwt and a flavonoid content of 972 µg QE/g dwt (Table 2).

Table 1.
Phytochemical analysis of different solvent extracts of B. hochstetteri.

Table 2.
Total phenolic and flavonoid contents of B. hochstetteri fractions.
Because the methanolic fraction revealed the presence of higher contents, as well as encouraging results in cytotoxic activity against breast and lung cancer cell lines, it was subjected to phytochemical profiling by GC–MS and LC–MS.
3.2. GC–MS and LC–MS Analysis
Results of GC–MS revealed the presence of 9 major compounds, i.e., beta-caryophyllen, 9-aristolene, methyl palmitate, linolenic acid methyl ester, iso-phytol acetate, methyl iso-stearate, squalene, tetraprenol, and solanesol. Moreover, LC–MS profiling revealed two major compounds, i.e., 8-oxo-pseudopalmatine, which is a methyl ester used in detergents, and N-methyl-metacryloyl-lupinine, which is an alkaloid with anti-inflammatory, antimicrobial, and anticancer properties. These results are shown in Table 3, and spectral graphs are depicted in Figures S1 and S2 (Supplementary File).

Table 3.
GC–MS and LC–MS profiling of methanol extract of B. hochstetteri.
3.3. In Vitro Cytotoxicity of B. hochstetteri Extracts
The results from the MTT assay on the viability of the L929 cell line showed that, with lower percentage cell viability values of 39.79 ± 0.014 and 26.30 ± 0.014, respectively, the ethyl acetate and hexane extracts were more toxic than the aqueous and methanol extracts, which exhibited better percentage of viability values of 52.73 ± 0.015 and 60.41 ± 0.004, respectively, at a higher concentration. The effects of all tested extracts on the viability of the L929 cell line are presented in Figure S3 (Supplementary File). The morphological effects of each solvent extract as revealed through the MTT assay are provided as Supplementary Files (Figures S4–S7). Based on the calibration curve made from the percentage of cell viability values, IC50 values were calculated for the tested extracts. The results showed that methanol and the aqueous extracts had higher IC50 values, i.e., 266.66 µg and 324.24 µg, respectively, than the other extracts. Overall toxicity studies revealed that the methanol and aqueous extracts were less toxic to the non-cancerous L929 cell lines than were the other extracts.
After the MTT-based toxicity study, the different solvent extracts were screened for cytotoxicity against breast cancer MCF-7 and lung cancer A549 cells. The results revealed that all tested samples exerted toxicity against A549 in a dose-dependent manner. In lung cancer A549 cells, methanol extract showed higher toxicity and lower percentage of cell viability, i.e., 69.52 ± 0.019 at the low level of 50 µg, than the other extracts. The aqueous, methanol and hexane extracts showed good toxicity against breast cancer MCF-7 cells at higher level of 250 µg. These results for A549 and MCF-7 are presented in Figures S8 and S9, respectively (Supplementary File). The hexane, aqueous, and methanol extracts exhibited significant IC50 results, with values of 180.24, 185.65, and 219.67 µg, respectively, against the MCF-7 cell line (Table 4).

Table 4.
IC50 values of different solvent extracts of B. hochstetteri against different cell lines.
3.4. Effects of Extracts on Early and Late Apoptosis; Flow Cytometry-Based Mechanistic Pathway Study
The morphological observations due to apoptosis correlated with the results of the MTT assay. In treated MCF-7 and A549 cell lines, the test extract samples produced changes in cellular morphology when compared to the untreated cells. The morphological changes produced by each solvent extract on MCF-7 and A549 cells, as seen in MTT assay, are shown in Figures S10–S17 (Supplementary Files).
Flow cytometry-based pathway studies comprised assays of apoptosis, caspase-3, anti Bcl-2, and DNA damage. In apoptosis study, the IC50 concentration of the methanol extract of B. hochstetteri was taken as the treatment dose used on lung cancer A549 cells. The results clearly showed that, compared to the untreated cells, the methanol extract, as well as the standard drug cisplatin, induced early and late apoptosis in lung cancer cells. There was 4.24% apoptosis in untreated cells, whereas for the standard drug cisplatin and methanol extract, the corresponding degrees of apoptosis were 50.30 and 36.20%, respectively. These results are shown in Figure 1.
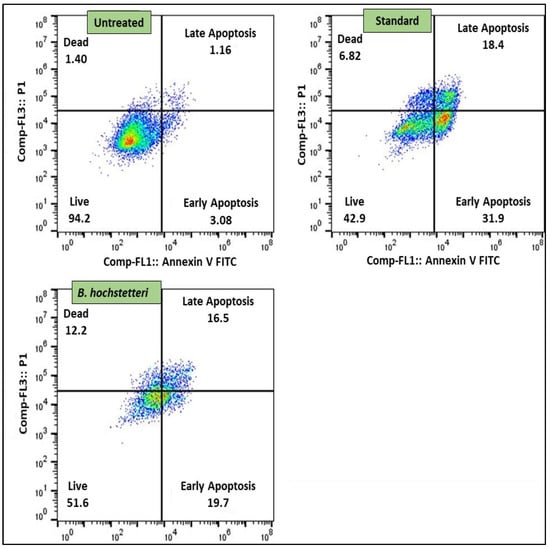
Figure 1.
The methanol extract of B. hochstetteri induced early and late apoptosis in the lung cancer A549 cell line.
Flow cytometry-based caspase-3 assay results showed that in methanol extract-treated cells, about 16.50% cells were positive for the release of caspase-3, whereas in cells treated with the standard drug cisplatin and untreated cells, the corresponding degrees of caspase-3 release were 51.1 and 6.24%, respectively. The caspase-3 assay results are shown in Figure 2.
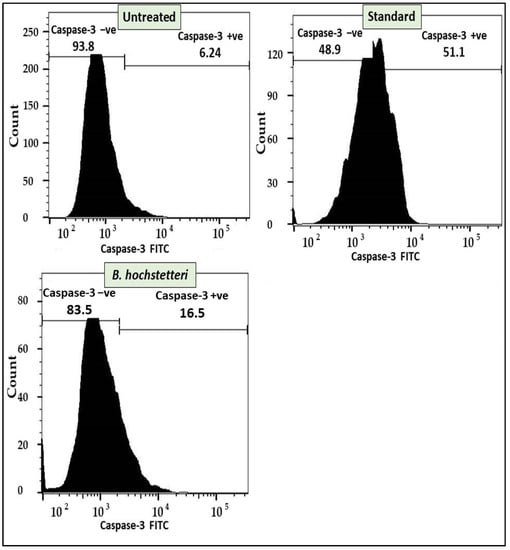
Figure 2.
Apoptosis induction by the methanol extract of B. hochstetteri in lung cancer A549 cell line through the caspase-3 assay.
Flow cytometry-based anti-Bcl-2 protein was studied in extract sample-treated A549 cells, along with untreated cells and cells treated with the standard drug cisplatin. The results revealed that in untreated cells, there were more cells positive for high expression of Bcl-2 protein, i.e., 98.5%. In contrast, in cells treated with methanol extract and the standard drug cisplatin, there were lower percentages of Bcl-2 positive cells, i.e., 63.7 and 59.15%, respectively. In addition, the standard drug cisplatin and the methanol extract resulted in 40.9% and 36.3% expressions of Bcl-2, respectively. Thus, the methanol extract and standard drug cisplatin inhibited the expression of BCl-2 protein. The Bcl-2 protein assay results are shown in Figure 3.
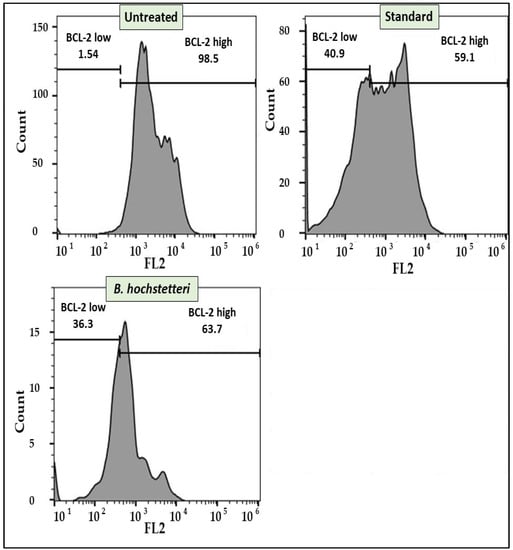
Figure 3.
Flow cytometry showing the inhibitory effect of methanol extract of B. hochstetteri on the anti-apoptotic protein Bcl-2 in the lung cancer A549 cell line.
Furthermore, flow cytometry was used to determine DNA damage caused by the methanol extract of B. hochstetteri and the standard drug cisplatin via TUNEL assay. The results revealed that 90.70% of cisplatin-treated cells showed DNA damage, whereas in the methanol extract-treated and untreated cells, only 32.70 and 0.69% of cells, respectively, exhibited DNA damage. The results of the TUNEL assay are presented in Figure 4.
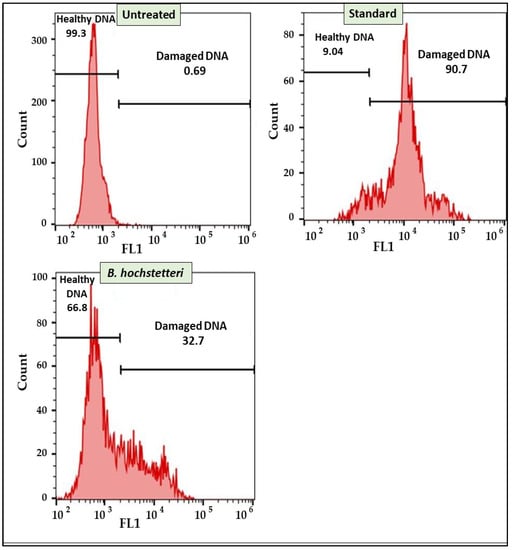
Figure 4.
Effect of methanol extract of B. hochstetteri on DNA damage in the lung cancer A549 cell line.
4. Discussion
Although modern synthetic drugs are accessible and very effective in the treatment of a wide range of disorders, some populations still prefer old folk remedies because they produce fewer adverse effects. The prevention and treatment of cancer may benefit immensely from the application of herbal medicine [35]. Traditional remedies rely mainly on phytocompounds. Cumulative pharmacognostic investigations may be used to identify and standardize these substances for improved therapeutic effects. Phytochemical and pharmacological evaluations are necessary to ascertain the underlying potency of crude medicinal preparations exhibiting antioxidant properties.
In the present study, the phytochemical analysis of four separate solvent extracts of B. hochstetteri (hexane, ethyl acetate, methanol, and aqueous extracts) was carried using standard biochemical tests. Phenolics are considered the most important phytochemical compounds, with significant antioxidant activities due to the fact that their chemical structures contain numerous hydroxyl groups that function as scavengers and chelating agents [36]. Flavonoids exert a wide range of pharmacological effects—anti-tumor, anti-microbial, and anti-inflammatory—and are therefore used to control blood pressure, support brain function, and protect the skin. In the present study, the B. hochstetteri extract contained flavonoids and phenolic compounds to which its cytotoxic potential on the tested cancer cell lines may be attributed to [17]. The findings of the present study are in line with previous studies; for example, the isoflavones genistein and daidzein have been shown to significantly inhibit cancer growth and proliferation [37,38,39].
Due to their chemical structures, which are rich in hydroxyl groups, secondary metabolites are regarded as the primary phytochemical compounds that serve essential roles as antioxidants and metal chelating agents [40,41,42,43]. About half of all modern medications are derived from plants. Thus, plants are crucial to the pharmaceutical industry. Results from GC–MS analysis of the potent methanol extract of B. hochstetteri revealed the presence of compounds belonging to terpenoids and alkaloids. These compounds are known to have several biological and medicinal effects, including anti-inflammatory, antioxidant, antimicrobial, and anticancer properties. Furthermore, LC–MS was used to identify the bioactive molecules present in the selected methanol extract. Due to the very complex biochemistry of plants, LC–MS-based approaches are of special importance in the identification of the phytochemical constituents of extracts. This is because plants contain a wide range of semi-polar molecules, such as crucial secondary metabolite groups, which can best be separated and detected using LC–MS methods [44]. Both of the two major compounds—8-Oxypseudopalmatine and N-methyl-metacryloyl-lupinin—detected in the LC–MS have shown cytotoxic activity in previous studies [33,34]. These findings are consistent with previous studies that have reported the cytotoxic potential of secondary metabolites against various cancers, e.g., liriodenine against ovarian cancer and laryngocarcinoma [45,46]; cryptolepine against osteosarcoma, T-cell leukemia, multiple myeloma, and renal adenocarcinoma [47,48,49]; brucine against lung, colon, and hepatocellular carcinoma [36,37,38]; and hirsutine against human breast and mouse mammary carcinoma [50,51].
Results from phytochemical, toxicity, and cytotoxic studies showed that the methanol extract was the most potent. Hence, the methanol extract was selected for flow cytometry-based studies on lung cancer A549 to determine the mechanism involved in its cytotoxic effect on lung cancer cells. The combination of bioactive compounds in herbal medications may have a synergistic action against disease by acting on several routes, minimizing negative side effects, and changing kinetics and elimination [8,42,43,44].
Once apoptosis has occurred, the classical morphological and cellular changes associated with it, such as cell shrinkage, blebbing of the plasma membrane, cellular detachment, nuclear condensation, nuclear DNA fragmentation, and externalization of phosphatidylserine (PS) on the outer cellular membrane, can be used to assess the viability of the cells [40,41]. In the present study, in the untreated group, the morphology of the cells was clear, and they were more numerous, without any intracellular spaces. In contrast, in test sample-treated cells, as the concentration was increased, the number of cells was gradually decreased, and there were intracellular spaces among the cells. Apart from this observation, there were unique morphological features, including abnormalities such as apoptotic bodies, cell shrinkage, membrane blabbing, and cell turgidity, all of which are typical features of cells undergoing apoptosis. In addition to the production of apoptotic bodies, the treated cells displayed apoptotic characteristics such as nuclear condensation and fragmentation into segregated bodies [41]. These findings corroborate the findings previous studies, which reported several secondary metabolites with apoptotic mechanism, for example, subditine, a monoterpenoid indole alkaloid from N. subdita bark, triggers apoptosis dose-dependently in human prostate cancer cells [52]. Similarly, scutebarbatine A, an alkaloid found in Scutellaria barbata, was reported to have a dose-dependent anti-proliferative effect against human lung cancer cells via caspase-3 and -9 cleavage and downregulation of Bcl-2 protein expression [53]. Furthermore, two alkaloids isolated from Tabernaemontana elegans Stapf, i.e., tabernaelegantine C and tabernaelegantinine B, were discovered to activate caspase-8 in colon cells [54].
The chemical pathways involved in the cytotoxicity of the extract were determined using flow cytometric analysis. Flow cytometry is a widely used method for profiling the expression of proteins in heterogeneous populations of cells in suspension without the need to physically separate the cells [55]. During the course of development, apoptosis evolved as a vital homeostatic process for the removal of surplus cells that would otherwise accumulate [56]. Several well-defined aspects define apoptosis, or programmed cell death, such as chromatin condensation and fragmentation, DNA cleavage in the inter-nucleosomes, membrane blebbing, caspase activation, and the translocation of phosphatidylserine from the inner to outer lining of the plasma membrane [57]. Annexin V-FITC binds and targets the externalized phosphatidylserine (apoptotic cell surface marker) of the plasma membrane, making it a highly specific marker for early apoptosis. Phospholipids on the plasma membrane move from the inner to the outer leaflet during the early stages of apoptosis, thereby exposing phosphatidylserine.
Aspartate-specific cysteine proteases, known as caspases, exist in the cell cytoplasm in inactive forms known as procaspases, and are activated either by autoproteolysis induced by interactions with adapter proteins (death effector domains or caspase recruitment domains) or by cleavage by other proteases [58]. Executioner caspases, such as caspase-3 and caspase-7, and initiator caspases, including caspase-8 and caspase-9, are two types of caspases [59]. The initiator caspases activate caspase-3 or caspase-7, which induces apoptosis [60]. The inability of injured cells to be purged by apoptosis is a feature of an unbalanced cell proliferation-apoptosis cycle. One of the most important cancer treatment strategies is to trigger apoptotic pathways in tumor cells, a process which leads to tumor cell death. Apoptosis is a mechanism by which several natural compounds that have been identified as potential anticancer medicines exert their antitumor effects [61]. Cleavage of caspases is the key to apoptosis. Researchers learn more about cell death and other biological processes by determining what happens when caspase cleavage occurs [62].
Both pro-apoptotic (BH3-only proteins and multidomain proteins) and anti-apoptotic (Bcl-2-like proteins) members of the Bcl-2 protein family exist. These proteins interact to regulate the release of cytochrome-c from the mitochondrion, which modifies the susceptibility to cell death signals [63]. Cancer cells frequently upregulate anti-apoptotic factors such as Bcl-2 in an effort to alter these death signals, becoming reliant on this anti-apoptotic protein [64,65]. Real-time cell death activation may occur swiftly or gradually. To thoroughly assess the mechanism(s) involved in the initiation of apoptosis, it is important to comprehend the quantitative abundance of Bcl-2 family proteins as a function of time and therapy. The survival or death of cells is greatly regulated by members of the Bcl-2 family. The proteins Bcl-2 and Bcl-xL prevent cells from going into apoptosis.
Recent research has shown the critical functions that apoptosis plays in the creation of therapeutic medicines and the management of cancer [66]. Cancer therapy is aimed at hastening the demise of cancer cells without significantly harming healthy cells [67,68]. Cell death in the apoptotic cells might be due to DNA damage.
Plant-based compounds are less harmful and more selective in their targeting than their synthetic counterparts used in chemotherapies. Therefore, it is crucial to investigate the possibility of using medicinal plants for cancer treatments. Several studies have reported the potent effects of extracts and isolated phytochemicals against tumor cell lines, suggesting that the Barleria species has considerable potential for anticancer effects. Human breast adenocarcinoma (MCF7), human colon adenocarcinoma (HT29), murine lymphocytic leukaemia (P388), human cervical carcinoma (HeLa), and two normal cell lines—African green monkey kidney (Vero) and mouse subcutaneous connective tissue (SCT)—have all been shown to be susceptible to B. prionitis [69], B. cristata [70], and B. strigosa [71].
The methanol extract of B. hochstetteri exhibited strong cytotoxic effects on human lung cancer cells. In light of this, it is possible that nutraceuticals extracted from B. hochstetteri may be further clinically developed as novel anticancer medicines.
5. Conclusions
This study has demonstrated that the methanol extract of B. hochstetteri exerted a significant cytotoxic effect on breast cancer MCF-7 and lung cancer A549 cells. The results of flow cytometry revealed that the molecular mechanism of action was similar to that of the standard drug cisplatin. The methanol extract of B. hochstetteri exhibited its cytotoxic action through the induction of apoptosis via DNA damage. Therefore, B. hochstetteri is a medicinally interesting plant which should be investigated further in order to determine lead compounds with potential to act as chemotherapeutics for cancer. However, it is necessary to carry out more thorough investigations before extrapolating the findings of the current study to drug development.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/separations9100298/s1, Figure S1. GC-MS Profiling of Methanol extract of B. hochstetteri. Figure S2. LC-MS analysis peaks of Methanol extract of B. hochstetteri. Figure S3. Cytotoxicity of different solvent extracts of B. hochstetteri against non-cancerous transformed fibroblast L929 cell line. Figure S4. Morphological effects of Hexane extract of B. hochstetteri on non-cancerous transformed fibroblast L929 cell line. Figure S5. Morphological effects of Ethyl acetate extract of B. hochstetteri on non-cancerous transformed fibroblast L929 cell line. Figure S6. Morphological effects of Methanol extract of B. hochstetteri on non-cancerous transformed fibroblast L929 cell line. Figure S7. Morphological effects of aqueous extract of B. hochstetteri on non-cancerous transformed fibroblast L929 cell line. Figure S8. Cytotoxic activity of different solvent extracts of B. hochstetteri against Lung cancer A549 cell line. Figure S9. Cytotoxic activity of different solvent extracts of B. hochstetteri against Breast cancer MCF-7 cell line. Figure S10. Morphological effects of Hexane extract of B. hochstetteri on Lung cancer A549 cell line. Figure S11. Morphological effects of Ethyl acetate extract of B. hochstetteri on Lung cancer A549 cell line. Figure S12. Morphological effects of Methanol extract of B. hochstetteri on Lung cancer A549 cell line. Figure S13. Morphological effects of Aqueous extract of B. hochstetteri on Lung cancer A549 cell line. Figure S14. Morphological effects of Hexane extract of B. hochstetteri on Breast cancer MCF-7 cell line. Figure S15. Morphological effects of Ethyl acetate extract of B. hochstetteri on Breast cancer MCF-7 cell line. Figure S16. Morphological effects of Methanol extract of B. hochstetteri on Breast cancer MCF-7 cell line. Figure S17. Morphological effects of Aqueous extract of B. hochstetteri on Breast cancer MCF-7 cell line.
Author Contributions
Conceptualization, S.A.A., I.A.S. and A.M.A.; methodology, M.A.A.O., A.K.S., B.A.A.-W. and J.H.H.; software, I.A.W., M.S.H. and M.M.K.; validation, B.A.A.-W. and J.H.H.; formal analysis, J.H.H.; investigation, S.A.A., I.A.S., J.H.H., and A.M.A.; resources, M.A.A.O., A.K.S., B.A.A.-W. and J.H.H.; data curation, A.K.S.; writing—original draft preparation, I.A.S., A.K.S., A.M.A. and M.M.K.; writing—review and editing, I.A.S., A.K.S., M.A.A.O., and A.M.A.; supervision, A.K.S.; project administration, I.A.S., and A.K.S.; funding acquisition, A.M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
All data has been included in the manuscript and supplementary file.
Acknowledgments
The authors would like to acknowledge the support of the Deputy for Research and Innovation-Ministry of Education, Kingdom of Saudi Arabia, for a grant (NU/IFC/ENT/01/006) under the Institutional Funding Committee at Najran University, Kingdom of Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Al-Shamsi, H.O.; Abu-Gheida, I.H.; Iqbal, F.; Al-Awadhi, A. (Eds.) Cancer in the Arab World, 1st ed.; Springer: Singapore, 2022; ISBN 9789811679476. [Google Scholar]
- Pucci, C.; Martinelli, C.; Ciofani, G. Innovative Approaches for Cancer Treatment: Current Perspectives and New Challenges. Ecancermedicalscience 2019, 13, 961. [Google Scholar] [CrossRef] [PubMed]
- Nikolaou, M.; Pavlopoulou, A.; Georgakilas, A.G.; Kyrodimos, E. The Challenge of Drug Resistance in Cancer Treatment: A Current Overview. Clin. Exp. Metastasis 2018, 35, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Talhouk, R.S.; Karam, C.; Fostok, S.; El-Jouni, W.; Barbour, E.K. Anti-Inflammatory Bioactivities in Plant Extracts. J. Med. Food 2007, 10, 1–10. [Google Scholar] [CrossRef]
- Hashem, S.; Ali, T.A.; Akhtar, S.; Nisar, S.; Sageena, G.; Ali, S.; Al-Mannai, S.; Therachiyil, L.; Mir, R.; Elfaki, I.; et al. Targeting Cancer Signaling Pathways by Natural Products: Exploring Promising Anti-Cancer Agents. Biomed. Pharmacother. 2022, 150, 113054. [Google Scholar] [CrossRef] [PubMed]
- WHO Traditional Medicine Strategy: 2014–2023. Available online: https://apps.who.int/iris/bitstream/handle/10665/92455/9789241506090_eng.pdf (accessed on 28 September 2022).
- Ghule, B.V.; Yeole, P.G. In Vitro and in Vivo Immunomodulatory Activities of Iridoids Fraction from Barleria Prionitis Linn. J. Ethnopharmacol. 2012, 141, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-H.; Cheng, Y.-C. Old Formula, New Rx: The Journey of PHY906 as Cancer Adjuvant Therapy. J. Ethnopharmacol. 2012, 140, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Chandan, B.K.; Prabhakar, A.; Taneja, S.C.; Singh, J.; Qazi, G.N. Chemistry and Hepatoprotective Activity of an Active Fraction from Barleria Prionitis Linn. in Experimental Animals. Phytother. Res. 2005, 19, 391–404. [Google Scholar] [CrossRef]
- Kosmulalage, K.S.; Zahid, S.; Udenigwe, C.C.; Akhtar, S.; Ata, A.; Samarasekera, R. Glutathione S-Transferase, Acetylcholinesterase Inhibitory and Antibacterial Activities of Chemical Constituents of Barleria Prionitis. Z. Naturforsch. B J. Chem. Sci. 2007, 62, 580–586. [Google Scholar] [CrossRef]
- Gangaram, S.; Naidoo, Y.; Dewir, Y.H.; El-Hendawy, S. Phytochemicals and Biological Activities of Barleria (Acanthaceae). Plants 2021, 11, 82. [Google Scholar] [CrossRef]
- Lekhak, M.M.; Patil, S.S.; Deshmukh, P.V.; Lekhak, U.M.; Kumar, V.; Rastogi, A. Genus Barleria L. (Acanthaceae): A Review of Its Taxonomy, Cytogenetics, Phytochemistry and Pharmacological Potential. J. Pharm. Pharmacol. 2022, 74, 812–842. [Google Scholar] [CrossRef]
- Agyare, C.; Obiri, D.D.; Boakye, Y.D.; Osafo, N. Anti-Inflammatory and Analgesic Activities of African Medicinal Plants. In Medicinal Plant Research in Africa; Elsevier: Amsterdam, The Netherlands, 2013; pp. 725–752. ISBN 9780124059276. [Google Scholar]
- Davies, N.W. Gas Chromatographic Retention Indices of Monoterpenes and Sesquiterpenes on Methyl Silicon and Carbowax 20M Phases. J. Chromatogr. A 1990, 503, 1–24. [Google Scholar] [CrossRef]
- Hanafi, H.H.; Irawan, C.; Rochaeni, H.; Sulistiawaty, L.; Roziafanto, A.N.; Supriyono, S.S. Phytochemical Screening, LC-MS Studies and Antidiabetic Potential of Methanol Extracts of Seed Shells of Archidendron Bubalinum (Jack) I.c. Nielson (Julang Jaling) from Lampung, Indonesia. Pharmacogn. J. 2018, 10, s77–s82. [Google Scholar] [CrossRef]
- Selvin, A.S.M.; Ismael, M.F.; Ricardo, S.A.; Jhunior, A.M.F.; da María, C.C.F. Determination of Total Phenolic Compounds, Antioxidant Activity and Nutrients in Brazil Nuts (Bertholletia Excelsa H. B. K.). J. Med. Plant Res. 2020, 14, 373–376. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The Determination of Flavonoid Contents in Mulberry and Their Scavenging Effects on Superoxide Radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- van Meerloo, J.; Kaspers, G.J.L.; Cloos, J. Cell Sensitivity Assays: The MTT Assay. Methods Mol. Biol. 2011, 731, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Crowley, L.C.; Marfell, B.J.; Scott, A.P.; Waterhouse, N.J. Quantitation of Apoptosis and Necrosis by Annexin V Binding, Propidium Iodide Uptake, and Flow Cytometry. Cold Spring Harb. Protoc. 2016, 2016, pdb-prot087288. [Google Scholar] [CrossRef]
- Ravid, T.; Tsaba, A.; Gee, P.; Rasooly, R.; Medina, E.A.; Goldkorn, T. Ceramide Accumulation Precedes Caspase-3 Activation during Apoptosis of A549 Human Lung Adenocarcinoma Cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003, 284, L1082–L1092. [Google Scholar] [CrossRef]
- Moraes, V.W.R.; Caires, A.C.F.; Paredes-Gamero, E.J.; Rodrigues, T. Organopalladium Compound 7b Targets Mitochondrial Thiols and Induces Caspase-Dependent Apoptosis in Human Myeloid Leukemia Cells. Cell Death Dis. 2013, 4, e658. [Google Scholar] [CrossRef]
- Kyrylkova, K.; Kyryachenko, S.; Leid, M.; Kioussi, C. Detection of Apoptosis by TUNEL Assay. Methods Mol. Biol. 2012, 887, 41–47. [Google Scholar] [CrossRef]
- Ros, M.; Pernice, M.; Le Guillou, S.; Doblin, M.A.; Schrameyer, V.; Laczka, O. Colorimetric Detection of Caspase 3 Activity and Reactive Oxygen Derivatives: Potential Early Indicators of Thermal Stress in Corals. J. Mar. Biol. 2016, 2016, 6825949. [Google Scholar] [CrossRef]
- Sharma, C.; Al Kaabi, J.M.; Nurulain, S.M.; Goyal, S.N.; Kamal, M.A.; Ojha, S. Polypharmacological Properties and Therapeutic Potential of β-Caryophyllene: A Dietary Phytocannabinoid of Pharmaceutical Promise. Curr. Pharm. Des. 2016, 22, 3237–3264. [Google Scholar] [CrossRef] [PubMed]
- Paciana, I.; Butnariu, M. Highlighting the Compounds with Pharmacological Activity from Some Medicinal Plants from the Area of Romania. Med. Aromat. Plants 2021, 10, 370. [Google Scholar]
- Breeta, R.D.I.E.; Grace, V.M.B.; Wilson, D.D. Methyl Palmitate-A Suitable Adjuvant for Sorafenib Therapy to Reduce in Vivo Toxicity and to Enhance Anti-Cancer Effects on Hepatocellular Carcinoma Cells. Basic Clin. Pharmacol. Toxicol. 2021, 128, 366–378. [Google Scholar] [CrossRef] [PubMed]
- Letawe, C.; Boone, M.; Piérard, G.E. Digital Image Analysis of the Effect of Topically Applied Linoleic Acid on Acne Microcomedones. Clin. Exp. Dermatol. 1998, 23, 56–58. [Google Scholar] [CrossRef] [PubMed]
- McGinty, D.; Letizia, C.S.; Api, A.M. Fragrance Material Review on Phytol. Food Chem. Toxicol. 2010, 48 (Suppl. 3), S59–S63. [Google Scholar] [CrossRef]
- Abraham, T.W.; Höfer, R. Lipid-Based Polymer Building Blocks and Polymers. In Polymer Science: A Comprehensive Reference; Elsevier: Amsterdam, The Netherlands, 2012; pp. 15–58. [Google Scholar]
- Cárdeno, A.; Aparicio-Soto, M.; Montserrat-de la Paz, S.; Bermudez, B.; Muriana, F.J.G.; Alarcón-de-la-Lastra, C. Squalene Targets Pro- and Anti-Inflammatory Mediators and Pathways to Modulate over-Activation of Neutrophils, Monocytes and Macrophages. J. Funct. Foods 2015, 14, 779–790. [Google Scholar] [CrossRef]
- Rangel, M.L.; Guerrero-Analco, J.A.; Monribot-Villanueva, J.L.; Kiel-Martínez, A.L.; Avendaño-Reyes, S.; Díaz Abad, J.P.; Bonilla-Landa, I.; Dávalos-Sotelo, R.; Olivares-Romero, J.L.; Angeles, G. Anatomical and Chemical Characteristics of Leaves and Branches of Juniperus Deppeana Var. Deppeana (Cupressaceae): A Potential Source of Raw Materials for the Perfume and Sweet Candies Industries. Ind. Crops Prod. 2018, 113, 50–54. [Google Scholar] [CrossRef]
- Yan, N.; Liu, Y.; Liu, L.; Du, Y.; Liu, X.; Zhang, H.; Zhang, Z. Bioactivities and Medicinal Value of Solanesol and Its Accumulation, Extraction Technology, and Determination Methods. Biomolecules 2019, 9, 334. [Google Scholar] [CrossRef]
- Van, H.T.M.; Yang, S.H.; Khadka, D.B.; Kim, Y.-C.; Cho, W.-J. Total Synthesis of 8-Oxypseudopalmatine and 8-Oxypseudoberberine via Ring-Closing Metathesis. Tetrahedron 2009, 65, 10142–10148. [Google Scholar] [CrossRef]
- Comprehensive Natural Products Chemistry; Meth-Cohn, O., Barton, D., Eds.; Pergamon, an Elsevier Science Imprint: Oxford, UK, 1999; ISBN 9780080912837. [Google Scholar]
- Xiong, Y.; Wu, X.; Rao, L. Tetrastigma Hemsleyanum (Sanyeqing) Root Tuber Extracts Induces Apoptosis in Human Cervical Carcinoma HeLa Cells. J. Ethnopharmacol. 2015, 165, 46–53. [Google Scholar] [CrossRef]
- López, M.; Martínez, F.; Del Valle, C.; Ferrit, M.; Luque, R. Study of Phenolic Compounds as Natural Antioxidants by a Fluorescence Method. Talanta 2003, 60, 609–616. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Kim, H.S.; Song, Y.-S. Genistein as a Potential Anticancer Agent against Ovarian Cancer. J. Tradit. Complement. Med. 2012, 2, 96–104. [Google Scholar] [CrossRef]
- Adjakly, M.; Ngollo, M.; Boiteux, J.-P.; Bignon, Y.-J.; Guy, L.; Bernard-Gallon, D. Genistein and Daidzein: Different Molecular Effects on Prostate Cancer. Anticancer Res. 2013, 33, 39–44. [Google Scholar]
- Hwang, K.-A.; Choi, K.-C. Anticarcinogenic Effects of Dietary Phytoestrogens and Their Chemopreventive Mechanisms. Nutr. Cancer 2015, 67, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.C.; Cullen, S.P.; Martin, S.J. Apoptosis: Controlled Demolition at the Cellular Level. Nat. Rev. Mol. Cell Biol. 2008, 9, 231–241. [Google Scholar] [CrossRef]
- Moongkarndi, P.; Kosem, N.; Kaslungka, S.; Luanratana, O.; Pongpan, N.; Neungton, N. Antiproliferation, Antioxidation and Induction of Apoptosis by Garcinia Mangostana (Mangosteen) on SKBR3 Human Breast Cancer Cell Line. J. Ethnopharmacol. 2004, 90, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.; Cai, X.; Lu, W.; Zhou, F.; Huo, J. Growth Inhibition and Induction of Apoptosis in SHG-44 Glioma Cells by Chinese Medicine Formula “Pingliu Keli”. Evid. Based. Complement. Alternat. Med. 2011, 2011, 958243. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, İ.; Gören, A.C.; Taslimi, P.; Alwasel, S.H.; Kılıc, O.; Bursal, E. Anticholinergic, Antidiabetic and Antioxidant Activities of Anatolian Pennyroyal (Mentha Pulegium)-Analysis of Its Polyphenol Contents by LC-MS/MS. Biocatal. Agric. Biotechnol. 2020, 23, 101441. [Google Scholar] [CrossRef]
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K.M.; Yoga Latha, L. Extraction, Isolation and Characterization of Bioactive Compounds from Plants’ Extracts. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 1–10. [Google Scholar] [CrossRef]
- Nordin, N.; Majid, N.A.; Hashim, N.M.; Rahman, M.A.; Hassan, Z.; Ali, H.M. Liriodenine, an Aporphine Alkaloid from Enicosanthellum Pulchrum, Inhibits Proliferation of Human Ovarian Cancer Cells through Induction of Apoptosis via the Mitochondrial Signaling Pathway and Blocking Cell Cycle Progression. Drug Des. Dev. 2015, 9, 1437–1448. [Google Scholar]
- Li, L.; Xu, Y.; Wang, B. Liriodenine Induces the Apoptosis of Human Laryngocarcinoma Cells via the Upregulation of P53 Expression. Oncol. Lett. 2015, 9, 1121–1127. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Gooderham, N.J. Mechanisms of Induction of Cell Cycle Arrest and Cell Death by Cryptolepine in Human Lung Adenocarcinoma A549 Cells. Toxicol. Sci. 2006, 91, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.-A.; Sowa, Y.; Murata, H.; Takagi, K.; Nakanishi, R.; Aoki, S.; Yoshikawa, M.; Kobayashi, M.; Sakabe, T.; Kubo, T.; et al. The Plant Alkaloid Cryptolepine Induces P21WAF1/CIP1 and Cell Cycle Arrest in a Human Osteosarcoma Cell Line. Int. J. Oncol. 2007, 31, 915–922. [Google Scholar] [CrossRef]
- Laryea, D.; Isaksson, A.; Wright, C.W.; Larsson, R.; Nygren, P. Characterization of the Cytotoxic Activity of the Indoloquinoline Alkaloid Cryptolepine in Human Tumour Cell Lines and Primary Cultures of Tumour Cells from Patients. Investig. New Drugs 2009, 27, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Lou, C.; Yokoyama, S.; Saiki, I.; Hayakawa, Y. Selective Anticancer Activity of Hirsutine against HER2positive Breast Cancer Cells by Inducing DNA Damage. Oncol. Rep 2015, 33, 2072–2076. [Google Scholar] [CrossRef]
- Lou, C.; Takahashi, K.; Irimura, T.; Saiki, I.; Hayakawa, Y. Identification of Hirsutine as an Anti-Metastatic Phytochemical by Targeting NF-ΚB Activation. Int. J. Oncol. 2014, 45, 2085–2091. [Google Scholar] [CrossRef]
- Liew, S.Y.; Looi, C.Y.; Paydar, M.; Cheah, F.K.; Leong, K.H.; Wong, W.F.; Mustafa, M.R.; Litaudon, M.; Awang, K. Subditine, a New Monoterpenoid Indole Alkaloid from Bark of Nauclea Subdita (Korth.) Steud. Induces Apoptosis in Human Prostate Cancer Cells. PLoS ONE 2014, 9, e87286. [Google Scholar] [CrossRef]
- Yang, X.-K.; Xu, M.-Y.; Xu, G.-S.; Zhang, Y.-L.; Xu, Z.-X. In Vitro and in Vivo Antitumor Activity of Scutebarbatine A on Human Lung Carcinoma A549 Cell Lines. Molecules 2014, 19, 8740–8751. [Google Scholar] [CrossRef]
- Mansoor, T.A.; Borralho, P.M.; Dewanjee, S.; Mulhovo, S.; Rodrigues, C.M.P.; Ferreira, M.-J.U. Monoterpene Bisindole Alkaloids, from the African Medicinal Plant Tabernaemontana Elegans, Induce Apoptosis in HCT116 Human Colon Carcinoma Cells. J. Ethnopharmacol. 2013, 149, 463–470. [Google Scholar] [CrossRef]
- Adan, A.; Alizada, G.; Kiraz, Y.; Baran, Y.; Nalbant, A. Flow Cytometry: Basic Principles and Applications. Crit. Rev. Biotechnol. 2017, 37, 163–176. [Google Scholar] [CrossRef]
- Czabotar, P.E.; Lessene, G.; Strasser, A.; Adams, J.M. Control of Apoptosis by the BCL-2 Protein Family: Implications for Physiology and Therapy. Nat. Rev. Mol. Cell Biol. 2014, 15, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Talib, W.H.; Mahasneh, A.M. Antiproliferative Activity of Plant Extracts Used against Cancer in Traditional Medicine. Sci. Pharm. 2010, 78, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Krysko, D.V.; Vanden Berghe, T.; D’Herde, K.; Vandenabeele, P. Apoptosis and Necrosis: Detection, Discrimination and Phagocytosis. Methods 2008, 44, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S. Regulation of Caspase Activation in Apoptosis: Implications in Pathogenesis and Treatment of Disease. Clin. Exp. Pharmacol. Physiol. 1999, 26, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.J.; Jun, D.Y.; Lee, J.Y.; Woo, M.H.; Yang, C.H.; Kim, Y.H. Apoptogenic Activity of 2α,3α-Dihydroxyurs-12-Ene-28-Oic Acid from Prunella Vulgaris Var. Lilacina Is Mediated via Mitochondria-Dependent Activation of Caspase Cascade Regulated by Bcl-2 in Human Acute Leukemia Jurkat T Cells. J. Ethnopharmacol. 2011, 135, 626–635. [Google Scholar] [CrossRef]
- Elkady, A.I.; Abuzinadah, O.A.; Baeshen, N.A.; Rahmy, T.R. Differential Control of Growth, Apoptotic Activity, and Gene Expression in Human Breast Cancer Cells by Extracts Derived from Medicinal Herbs Zingiber Officinale. J. Biomed. Biotechnol. 2012, 2012, 614356. [Google Scholar] [CrossRef]
- Shao, W.; Yeretssian, G.; Doiron, K.; Hussain, S.N.; Saleh, M. The Caspase-1 Digestome Identifies the Glycolysis Pathway as a Target during Infection and Septic Shock. J. Biol. Chem. 2007, 282, 36321–36329. [Google Scholar] [CrossRef]
- Letai, A.; Bassik, M.C.; Walensky, L.D.; Sorcinelli, M.D.; Weiler, S.; Korsmeyer, S.J. Distinct BH3 Domains Either Sensitize or Activate Mitochondrial Apoptosis, Serving as Prototype Cancer Therapeutics. Cancer Cell 2002, 2, 183–192. [Google Scholar] [CrossRef]
- Letai, A.; Sorcinelli, M.D.; Beard, C.; Korsmeyer, S.J. Antiapoptotic BCL-2 Is Required for Maintenance of a Model Leukemia. Cancer Cell 2004, 6, 241–249. [Google Scholar] [CrossRef]
- Certo, M.; Del Gaizo Moore, V.; Nishino, M.; Wei, G.; Korsmeyer, S.; Armstrong, S.A.; Letai, A. Mitochondria Primed by Death Signals Determine Cellular Addiction to Antiapoptotic BCL-2 Family Members. Cancer Cell 2006, 9, 351–365. [Google Scholar] [CrossRef]
- Hassan, M.; Watari, H.; AbuAlmaaty, A.; Ohba, Y.; Sakuragi, N. Apoptosis and Molecular Targeting Therapy in Cancer. Biomed Res. Int. 2014, 2014, 150845. [Google Scholar] [CrossRef]
- Gerl, R.; Vaux, D.L. Apoptosis in the Development and Treatment of Cancer. Carcinogenesis 2005, 26, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Alshabi, A.M.; Alkahtani, S.A.; Shaikh, I.A.; Orabi, M.A.A.; Abdel-Wahab, B.A.; Walbi, I.A.; Habeeb, M.S.; Khateeb, M.M.; Hoskeri, J.H.; Shettar, A.K.; et al. Phytochemicals from Corchorus Olitorius Methanolic Extract Induce Apoptotic Cell Death via Activation of Caspase-3, Anti-Bcl-2 Activity, and DNA Degradation in Breast and Lung Cancer Cell Lines. J. King Saud Univ. Sci. 2022, 34, 102238. [Google Scholar] [CrossRef]
- Sawarkar, H.A.; Kashyap, P.P.; Pandey, A.K.; Singh, M.K.; Kaur, C.D. Antimicrobial and Cytotoxic Activities of Barleria Prionitis and Barleria Grandiflora: A Comparative Study. Bangladesh J. Pharmacol. 2016, 11, 802. [Google Scholar] [CrossRef]
- El-Halawany, A.M.; Abdallah, H.M.; Hamed, A.R.; Khalil, H.E.; Almohammadi, A.M. Phenolics from Barleria Cristata Var. Alba as Carcinogenesis Blockers against Menadione Cytotoxicity through Induction and Protection of Quinone Reductase. BMC Complement. Altern. Med. 2018, 18, 163. [Google Scholar] [CrossRef] [PubMed]
- Manapradit, N.; Poeaim, S.; Charoenying, P. Cytotoxicity and Antimicrobial Activities of Leaf Extracts from Barleria Strigosa. Int. J. Agric. Technol 2015, 11, 551–561. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).

