Saline Diffusion Modeling for Sodium Chloride Aqueous Solutions: Freezing for Desalination Purposes
Abstract
:1. Introduction
2. Materials and Methods
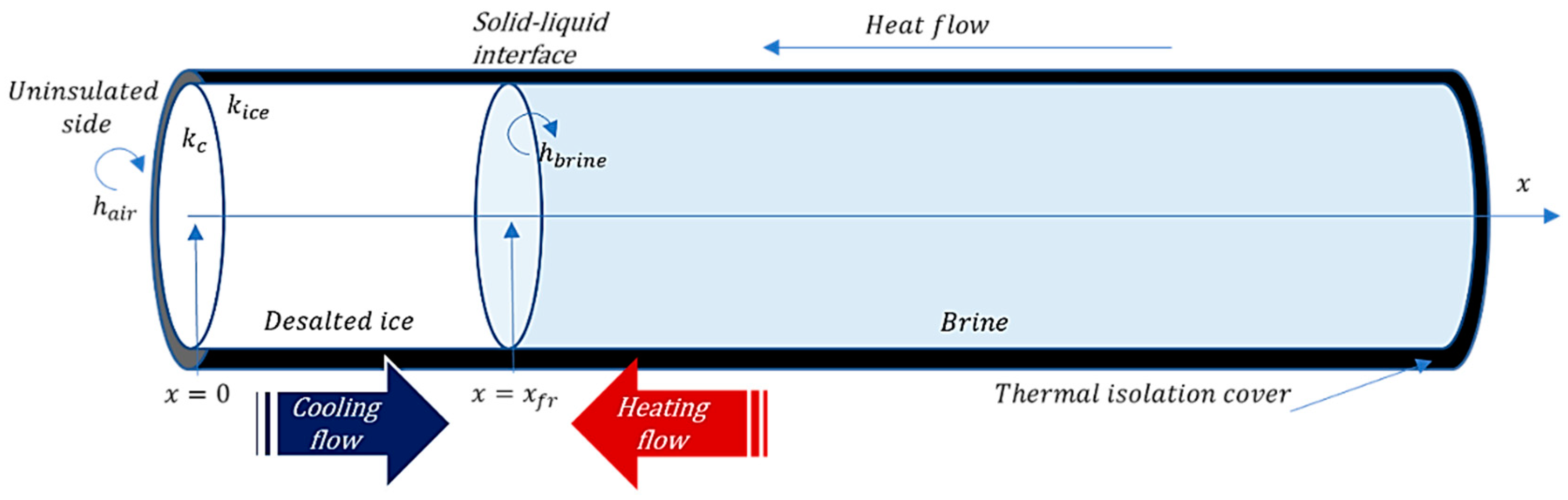
Description of the Separation Process
- Produce ice at the liquid-solid interface, and
- Operate the cooling flow to remove both the latent solidification heat of the water and heat necessary for ice sub-cooling—the correlation between the ice formation kinetics and diffusion time. Ice formation should be performed progressively to allow for the transfer of the ions to the brine.
3. Proposed Model
3.1. Heat Transfer Model Description
3.1.1. Main Equations
3.1.2. Complementary Equations
4. Salt Diffusion Analysis
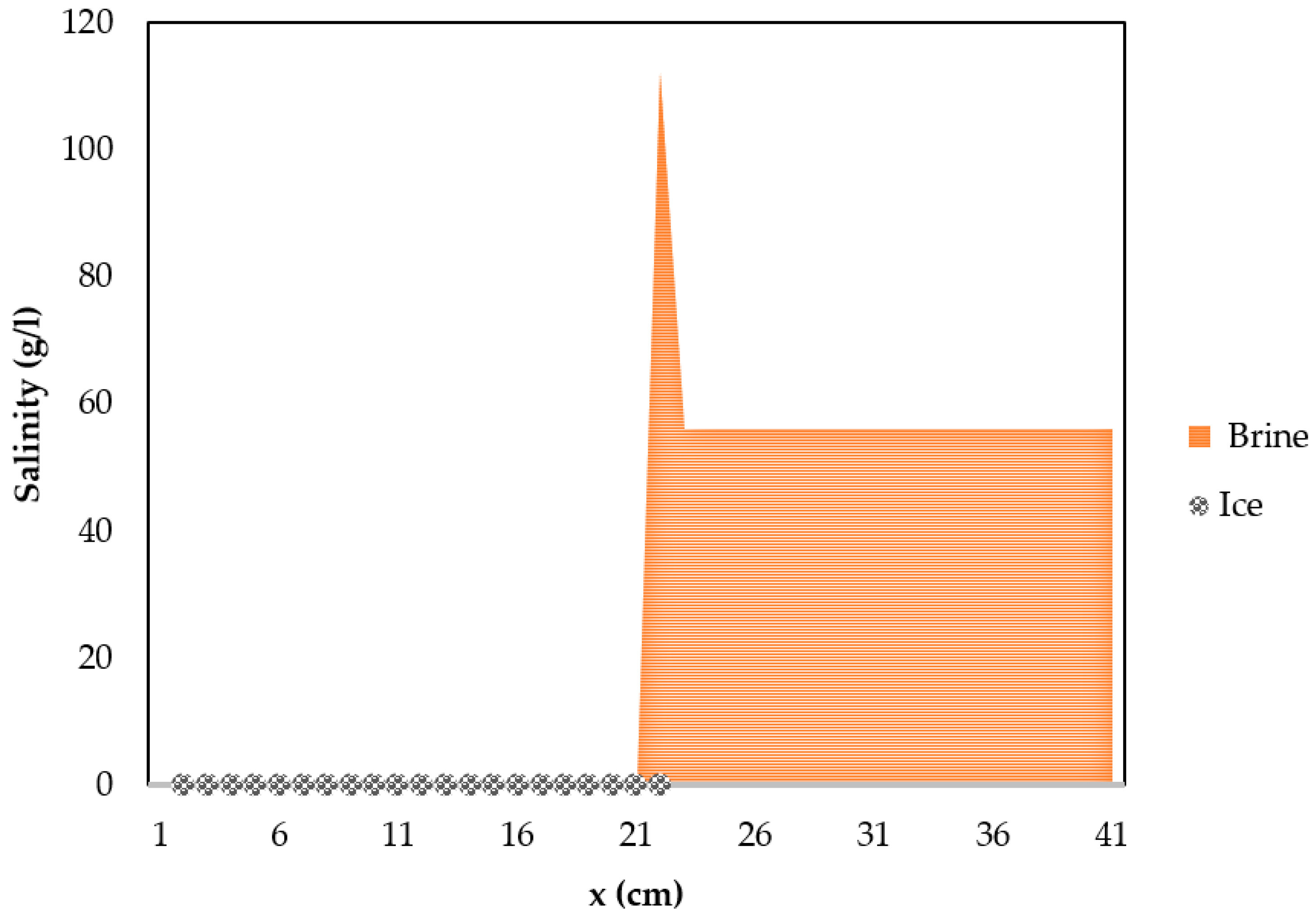
5. Results and Discussions
Conditions for F/M desalination
- A positive evolution of growing ice through thickness x:
- 2.
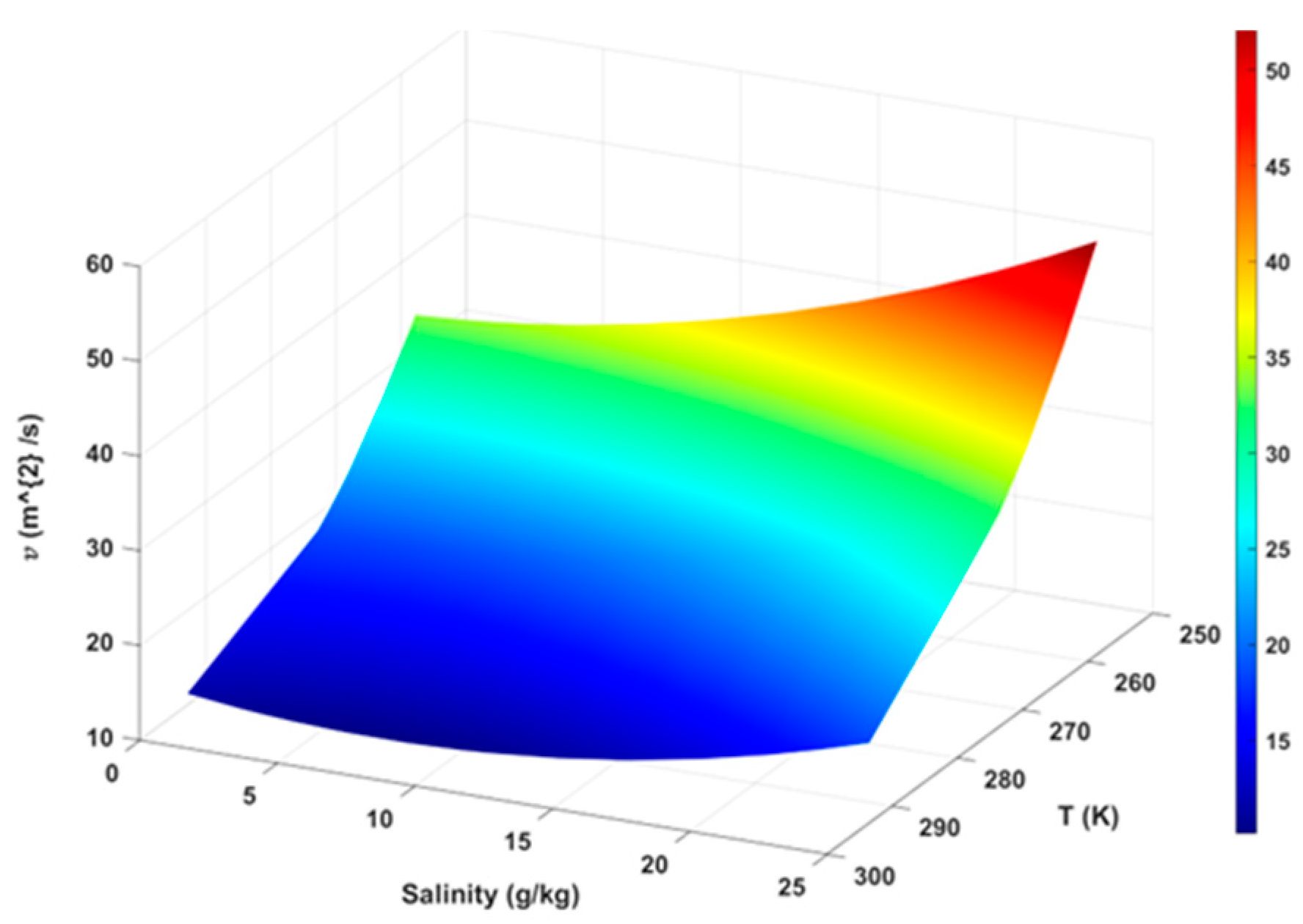
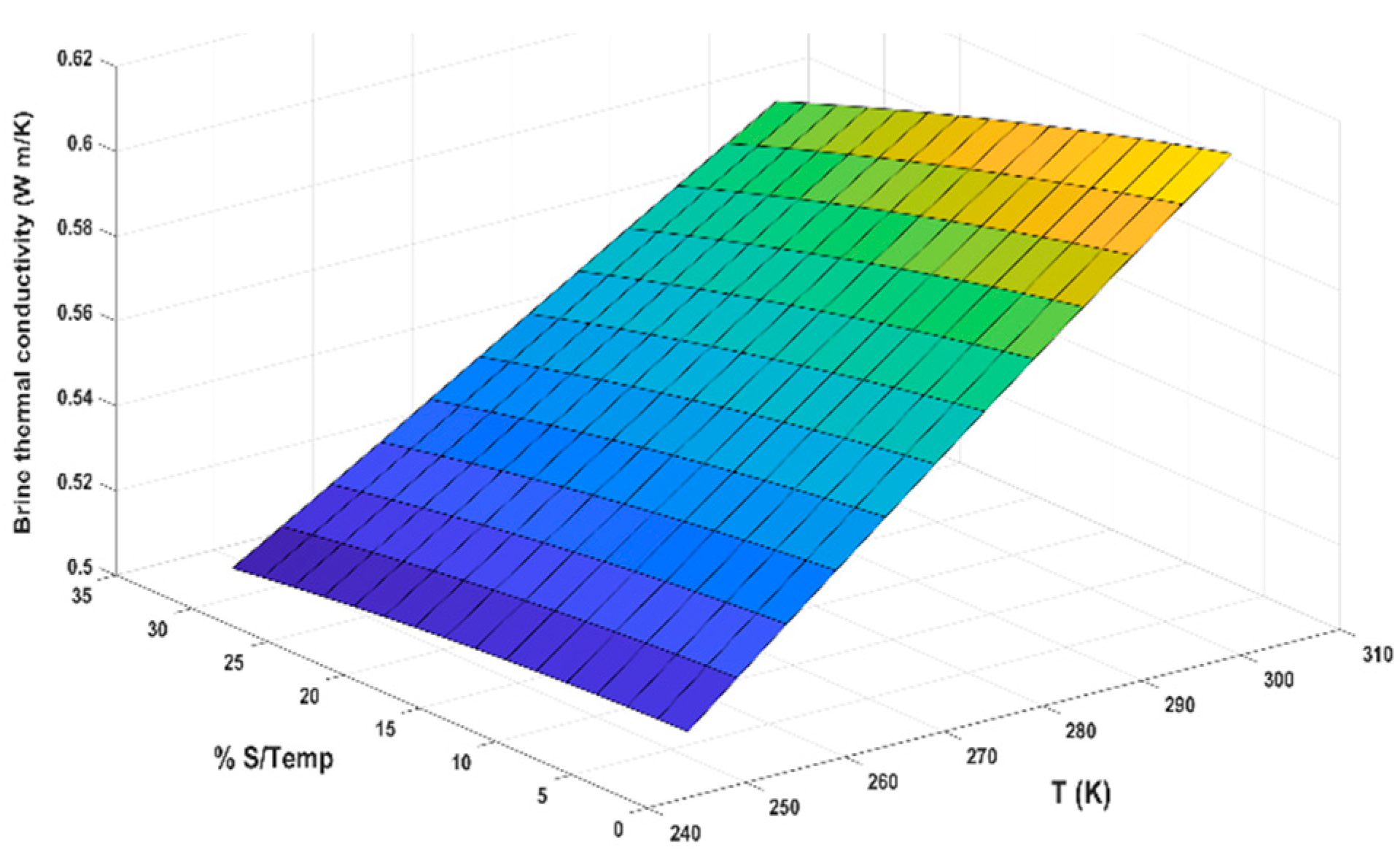
- Since diffusion time depends on the diffusivity, which is a function of salinity as Equation (23) and Table 1 show, the salinity increases while the value of D decreases.
- 3.
- To get salt-free at a diffusion time lower than freezing time:
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| a | ice grow rate | m/s |
| x | specific length (x-axis) | m |
| Lf | water heat fusion | J/kg |
| T | temperature | K |
| h | coefficient of heat convection | W/m K |
| heat flux | W/m 2 | |
| k | thermal conductivity | W/m K |
| diffusion velocity | m/s | |
| saltwater diffusivity | m2/s | |
| l | length | m |
| v | kinematic viscosity | m2/s |
| S | salinity | Kg/m3 |
| Pr | Prandtl number | dimensionless |
| Ra | Rayleigh number | dimensionless |
| molecular weight | g/mol | |
| molar volume | m3 mol | |
| t | time | s |
| α | thermal diffusivity | m2/s |
| density | Kg/m3 | |
| coefficient of volumetric expansion | dimensionless | |
| Sub-indices | ||
| i | liquid-solid interface | |
| s | salt | |
| b | brine | |
| fr | freezing | |
| d | diameter | |
| ∞ | infinity | |
| w | water | |
| c | container | |
| air | air | |
| ice | ice | |
Appendix A
| Temperature (K) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Salinity (g/kg) | 293.15 | 273.15 | 272.15 | 271.15 | 270.15 | 269.15 | 268.15 | 263.15 | 258.15 | |
| 0.1 | 11.486 | 18.471 | 19.180 | 19.928 | 20.717 | 21.547 | 22.419 | 27.438 | 33.645 | |
| 2.0 | 10.838 | 18.396 | 19.134 | 19.911 | 20.729 | 21.589 | 22.490 | 27.661 | 34.026 | |
| 2.9 | 10.614 | 18.426 | 19.177 | 19.969 | 20.800 | 21.674 | 22.589 | 27.833 | 34.272 | |
| 4.0 | 10.397 | 18.519 | 19.288 | 20.096 | 20.945 | 21.835 | 22.768 | 28.099 | 34.630 | |
| 5.6 | 10.195 | 18.768 | 19.561 | 20.393 | 21.267 | 22.182 | 23.140 | 28.599 | 35.263 | |
| 6.0 | 10.165 | 18.851 | 19.650 | 20.489 | 21.368 | 22.290 | 23.254 | 28.745 | 35.442 | |
| 8.0 | 10.140 | 19.391 | 20.220 | 21.089 | 22.000 | 22.952 | 23.948 | 29.599 | 36.462 | |
| 8.3 | 10.155 | 19.490 | 20.323 | 21.197 | 22.112 | 23.070 | 24.070 | 29.745 | 36.633 | |
| 10.0 | 10.324 | 20.138 | 20.998 | 21.898 | 22.839 | 23.823 | 24.849 | 30.661 | 37.691 | |
| 11.0 | 10.494 | 20.590 | 21.465 | 22.380 | 23.337 | 24.336 | 25.378 | 31.270 | 38.383 | |
| 13.6 | 11.179 | 22.008 | 22.922 | 23.878 | 24.874 | 25.914 | 26.997 | 33.096 | 40.425 | |
| 16.2 | 12.215 | 23.778 | 24.732 | 25.727 | 26.763 | 27.843 | 28.967 | 35.274 | 42.819 | |
| 18.8 | 13.603 | 25.899 | 26.892 | 27.927 | 29.004 | 30.124 | 31.289 | 37.804 | 45.565 | |
| 21.2 | 15.196 | 28.169 | 29.199 | 30.270 | 31.384 | 32.542 | 33.744 | 40.451 | 48.412 | |
| 23.1 | 16.670 | 30.179 | 31.237 | 32.338 | 33.481 | 34.668 | 35.900 | 42.759 | 50.877 | |
| 24.9 | 18.239 | 32.256 | 33.341 | 34.469 | 35.640 | 36.855 | 38.115 | 45.118 | 53.387 | |
| Temperature (K) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 272.16 | 272.16 | 272.16 | 272.16 | 272.16 | 272.16 | 272.16 | 272.16 | 272.16 | 272.16 | 272.16 | 272.16 | ||
| Salinity (%) | 2 | 0.559 | 0.559 | 0.559 | 0.559 | 0.559 | 0.559 | 0.559 | 0.559 | 0.559 | 0.559 | 0.559 | 0.559 |
| 4 | 0.558 | 0.558 | 0.558 | 0.558 | 0.558 | 0.558 | 0.558 | 0.558 | 0.558 | 0.558 | 0.558 | 0.558 | |
| 6 | 0.557 | 0.557 | 0.557 | 0.557 | 0.557 | 0.557 | 0.557 | 0.557 | 0.557 | 0.557 | 0.557 | 0.557 | |
| 8 | 0.555 | 0.555 | 0.555 | 0.555 | 0.555 | 0.555 | 0.555 | 0.555 | 0.555 | 0.555 | 0.555 | 0.555 | |
| 10 | 0.554 | 0.554 | 0.554 | 0.554 | 0.554 | 0.554 | 0.554 | 0.554 | 0.554 | 0.554 | 0.554 | 0.554 | |
| 12 | 0.553 | 0.553 | 0.553 | 0.553 | 0.553 | 0.553 | 0.553 | 0.553 | 0.553 | 0.553 | 0.553 | 0.553 | |
| 14 | 0.552 | 0.552 | 0.552 | 0.552 | 0.552 | 0.552 | 0.552 | 0.552 | 0.552 | 0.552 | 0.552 | 0.552 | |
| 16 | 0.550 | 0.550 | 0.550 | 0.550 | 0.550 | 0.550 | 0.550 | 0.550 | 0.550 | 0.550 | 0.550 | 0.550 | |
| 18 | 0.549 | 0.549 | 0.549 | 0.549 | 0.549 | 0.549 | 0.549 | 0.549 | 0.549 | 0.549 | 0.549 | 0.549 | |
| 20 | 0.547 | 0.547 | 0.547 | 0.547 | 0.547 | 0.547 | 0.547 | 0.547 | 0.547 | 0.547 | 0.547 | 0.547 | |
| 22 | 0.546 | 0.546 | 0.546 | 0.546 | 0.546 | 0.546 | 0.546 | 0.546 | 0.546 | 0.546 | 0.546 | 0.546 | |
| 24 | 0.544 | 0.544 | 0.544 | 0.544 | 0.544 | 0.544 | 0.544 | 0.544 | 0.544 | 0.544 | 0.544 | 0.544 | |
| 26 | 0.542 | 0.542 | 0.542 | 0.542 | 0.542 | 0.542 | 0.542 | 0.542 | 0.542 | 0.542 | 0.542 | 0.542 | |
| 28 | 0.541 | 0.541 | 0.541 | 0.541 | 0.541 | 0.541 | 0.541 | 0.541 | 0.541 | 0.541 | 0.541 | 0.541 | |
| 30 | 0.539 | 0.539 | 0.539 | 0.539 | 0.539 | 0.539 | 0.539 | 0.539 | 0.539 | 0.539 | 0.539 | 0.539 | |
| 32 | 0.537 | 0.537 | 0.537 | 0.537 | 0.537 | 0.537 | 0.537 | 0.537 | 0.537 | 0.537 | 0.537 | 0.537 | |
References
- Castillo-Téllez, B.; Pilatowsky Figueroa, I.; Castillo Téllez, M.; Marzoug, R.; Allaf, K. Experimental analysis of saline diffusion during saltwater freezing for desalination purposes. Water Environ. J. 2020, 34, 929–936. [Google Scholar] [CrossRef]
- Erlbeck, L.; Rädle, M.; Nessel, R.; Illner, F.; Müller, W.; Rudolph, K.; Kunz, T.; Methner, F.J. Investigation of the depletion of ions through freeze desalination. Desalination 2017, 407, 93–102. [Google Scholar] [CrossRef]
- Liu, Y.; Ming, T.; Wu, Y.; de Richter, R.; Fang, Y.; Zhou, N. Desalination of seawater by spray freezing in a natural draft tower. Desalination 2020, 496, 114700. [Google Scholar] [CrossRef]
- Ghalavand, Y.; Hatamipour, M.S.; Rahimi, A. A review on energy consumption of desalination processes. Desalin. Water Treat. 2015, 54, 1526–1541. [Google Scholar] [CrossRef]
- Do Thi, H.T.; Pasztor, T.; Fozer, D.; Manenti, F.; Toth, A.J. Comparison of Desalination Technologies Using Renewable Energy Sources with Life Cycle, PESTLE, and Multi-Criteria Decision Analyses. Water 2021, 13, 3023. [Google Scholar] [CrossRef]
- Guo, P.; Li, T.; Wang, Y.; Li, J. Energy and exergy analysis of a spray-evaporation multi-effect distillation desalination system. Desalination 2021, 500, 114890. [Google Scholar] [CrossRef]
- Jayakody, H.; Al-Dadah, R.; Mahmoud, S. Numerical investigation of indirect freeze desalination using an ice maker machine. Energy Convers. Manag. 2018, 168, 407–420. [Google Scholar] [CrossRef]
- Yang, H.; Fu, M.; Zhan, Z.; Wang, R.; Jiang, Y. Study on combined freezing-based desalination processes with microwave treatment. Desalination 2020, 475, 114201. [Google Scholar] [CrossRef]
- Ong, C.W.; Chen, C.L. Technical and economic evaluation of seawater freezing desalination using liquefied natural gas. Energy 2019, 181, 429–439. [Google Scholar] [CrossRef]
- Kalista, B.; Shin, H.; Cho, J.; Jang, A. Current development and future prospect review of freeze desalination. Desalination 2018, 447, 167–181. [Google Scholar] [CrossRef]
- Najim, A. A review of advances in freeze desalination and future prospects. NPJ Clean Water 2022, 5, 1–15. [Google Scholar] [CrossRef]
- El Kadi, K.; Janajreh, I. Desalination by Freeze Crystallization: An Overview. Int. J. Therm. Environ. Eng. 2017, 15, 103–110. [Google Scholar] [CrossRef]
- Badawy, S.M. Laboratory freezing desalination of seawater. Desalin. Water Treat. 2016, 57, 11040–11047. [Google Scholar] [CrossRef]
- Chen, J.; Wu, J.; Xu, J.; Yuan, Q.; Deng, B.; Chen, C.; Li, Z. Experiments and insights of desalination by a freezing/thawing method at low subcooling. Chinese J. Chem. Eng. 2020, 28, 3011–3017. [Google Scholar] [CrossRef]
- Jayakody, H.; Al-Dadah, R.; Mahmoud, S. Computational fluid dynamics investigation on indirect contact freeze desalination. Desalination 2017, 420, 21–33. [Google Scholar] [CrossRef]
- Junkratuek, A.; Srudhiprom, J.; Srinophakun, T. Computational fluid dynamics of a horizontal hydrocyclone for freezing desalination. Asia-Pac. J. Chem. Eng. 2017, 12, 765–774. [Google Scholar] [CrossRef]
- El Kadi, K.; Adeyemi, I.; Janajreh, I. Application of directional freezing for seawater desalination: Parametric analysis using experimental and computational methods. Desalination 2021, 520, 115339. [Google Scholar] [CrossRef]
- Yuan, H.; Sun, K.; Wang, K.; Zhang, J.; Zhang, Z.; Zhang, L.; Li, S.; Li, Y. Ice crystal growth in the freezing desalination process of binary water-NaCl system. Desalination 2020, 496, 114737. [Google Scholar] [CrossRef]
- Najim, A.; Krishnan, S. A similarity solution for heat transfer analysis during progressive freeze-concentration based desalination. Int. J. Therm. Sci. 2022, 172, 107328. [Google Scholar] [CrossRef]
- Kapembwa, M.; Rodríguez-Pascual, M.; Lewis, A.E. Heat and Mass Transfer Effects on Ice Growth Mechanisms in Pure Water and Aqueous Solutions. Cryst. Growth Des. 2014, 14, 389–395. [Google Scholar] [CrossRef]
- Savović, S.; Caldwell, J. Finite difference solution of one-dimensional Stefan problem with periodic boundary conditions. Int. J. Heat Mass Transf. 2003, 46, 2911–2916. [Google Scholar] [CrossRef]
- Caldera, U.; Breyer, C. Learning Curve for Seawater Reverse Osmosis Desalination Plants: Capital Cost Trend of the Past, Present, and Future. Water Resour. Res. 2017, 53, 10523–10538. [Google Scholar] [CrossRef]
- Al-sahali, M.; Ettouney, H. Developments in thermal desalination processes: Design, energy, and costing aspects. Desalination 2007, 214, 227–240. [Google Scholar] [CrossRef]
- Thu, K.; Yanagi, H.; Saha, B.B.; Ng, K.C. Performance analysis of a low-temperature waste heat-driven adsorption desalination prototype. Int. J. Heat Mass Transf. 2013, 65, 662–669. [Google Scholar] [CrossRef]
- Narayan, G.P.; Sharqawy, M.H.; Lienhard V, J.H.; Zubair, S.M. Thermodynamic analysis of humidification dehumidification desalination cycles. Desalin. Water Treat. 2010, 16, 339–353. [Google Scholar] [CrossRef]
- Abid, H.S.; Johnson, D.J.; Hashaikeh, R.; Hilal, N. A review of efforts to reduce membrane fouling by control of feed spacer characteristics. Desalination 2017, 420, 384–402. [Google Scholar] [CrossRef]
- Goh, L.M.; Thong, Z.; Li, W.P.; Ooi, S.T.; Esa, F.; Ng, K.S.; Dhalla, A.; Gudipati, C. Development and Industrial-Scale Fabrication of Next-Generation Low-Energy Membranes for Desalination. Membranes 2022, 12, 540. [Google Scholar] [CrossRef]
- Gu, B.; Adjiman, C.S.; Xu, X.Y. The effect of feed spacer geometry on membrane performance and concentration polarisation based on 3D CFD simulations. J. Memb. Sci. 2017, 527, 78–91. [Google Scholar] [CrossRef]
- Shenvi, S.S.; Isloor, A.M.; Ismail, F.F. A review on RO membrane technology: Developments and challenges. Desalination 2015, 368, 10–26. [Google Scholar] [CrossRef]
- Perrins, J.C.; Cooper, W.J.; van Leeuwen, J.; Herwig, R.P. Ozonation of seawater from different locations: Formation and decay of total residual oxidant-implications for ballast water treatment. Mar. Pollut. Bull. 2006, 52, 1023–1033. [Google Scholar] [CrossRef]
- Cengell, Y.; Afshin, G. Transferencia de Calor y Masa, 4th ed.; McGraw Hill: New York, NY, USA, 2011; Volume 1, ISBN 9786071505408. [Google Scholar]
- Melinder, A.; Ignatowicz, M. Properties of seawater with ice slurry use in focus. Int. J. Refrig. 2015, 52, 51–58. [Google Scholar] [CrossRef]
- Fofonoff, N.P. Physical properties of seawater: A new salinity scale and equation of state for seawater. J. Geophys. Res. 1985, 90, 3332. [Google Scholar] [CrossRef]
- Nayar, K.G.; Sharqawy, M.H.; Banchik, L.D.; Lienhard, J.H. Thermophysical properties of seawater: A review and new correlations that include pressure dependence. Desalination 2016, 390, 1–24. [Google Scholar] [CrossRef]
- Simion, A.I.; Grigoraş, C.; Roșu, A. Mathematical modelling of density and viscosity of nacl aqueous solutions. J. Agroaliment. Process. Technol. 2015, 21, 41–52. [Google Scholar]
- Dreith, F.; Raj, M.; Manglik, M.S. Principles of Heat Transfer, 7th ed.; Cengagle Learning, Inc.: Boston, MA, USA, 2011; Volume 2, ISBN 9780495667704. [Google Scholar]
- Melinder, Å. Thermophysical Properties of Aqueous Solutions Used as Secondary Working Fluids, Royal Institute of Technology. Ph.D. Dissertation, KTH, Stockholm, Sweden, 2007. [Google Scholar]
- Cengel, Y.A.; Boles, M.E. Termodinamica, 7th ed.; McGraw Hill: New York, NY, USA, 2011; ISBN 978-607-15-0743-3. [Google Scholar]
- Allaf, K.; Bouyahy, S.M.N.; Romdhane, M. Drying diffusing model. In Proceedings of the 6th International CIGR Technical Symposium—Towards a Sustainable Food Chain: Food Process, Bioprocessing and Food Quality Management, Nantes, France, 18–20 April 2011. [Google Scholar]
- Allaf, K. Transfer Phenomena and Industrial Applications, 1st ed.; Faculty of Science, Lebanese University: Hadath, Lebanon, 1982. [Google Scholar]
- Fukui, K.; Maeda, K. Direct numerical simulation of solid-layer crystallization from binary melt. J. Cryst. Growth 2002, 235, 633–639. [Google Scholar] [CrossRef]
- Krahn, W.; Schwelger, G.; Lucas, K. Light scattering measurements of mutual diffusion coefficients in binary liquid mixtures. J. Phys. Chem. 1983, 87, 4515–4519. [Google Scholar] [CrossRef]
- Stejskal, E.O.; Tanner, J.E. Spin diffusion measurements: Spin echoes in the presence of a time-dependant field gradient. J. Chem. Phys. 1965, 42, 288–292. [Google Scholar] [CrossRef]
- Atwood, J.G.; Goldstein, J. Measurements of Diffusion Coefficients in Liquids at Atmospheric and Elevated Pressure by the Chromatographic Broadening Technique. J. Phys. Chem. 1984, 88, 1875–1885. [Google Scholar] [CrossRef]
- Ouano, C. Diffusion in Liquid Systems. I. A Simple and Fast Method of Measuring Diffusion Constants. Ind. Eng. Chem. Fundam. 1972, 11, 268–271. [Google Scholar] [CrossRef]
- Hayduk, W.; Laudie, H. Prediction of diffusion coefficients for nonelectrolytes in dilute aqueous solutions. AIChE J. 1974, 20, 611–615. [Google Scholar] [CrossRef]
- Miyabe, K.; Isogai, R. Estimation of molecular diffusivity in liquid phase systems by the Wilke-Chang equation. J. Chromatogr. A 2011, 1218, 6639–6645. [Google Scholar] [CrossRef] [PubMed]
- Wilke, C.R.; Chang, P. Correlation of diffusion coefficients in dilute solutions. AIChE J. 1955, 1, 264–270. [Google Scholar] [CrossRef]
- Poling, B.E.; Prausnitz, J.M.; O’Connell, J.P. Properties of Gases and Liquids, 5th ed.; McGraw-Hill Education: New York, NY, USA, 2001; ISBN 9780070116825. [Google Scholar]


| Salt Diffusivity in Water | Solution Freezing Temperature °C | Brine Salinity g/L |
|---|---|---|
| 8.33 × 10−6 | −1.95 | 35.5 |
| 8.30 × 10−6 | −2.5 | 52.96 |
| 8.2541 × 10−6 | −4.5 | 77.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castillo-Téllez, B.; Romero, R.J.; Allaf, K.; Pilatowsky-Figueroa, I. Saline Diffusion Modeling for Sodium Chloride Aqueous Solutions: Freezing for Desalination Purposes. Separations 2022, 9, 272. https://doi.org/10.3390/separations9100272
Castillo-Téllez B, Romero RJ, Allaf K, Pilatowsky-Figueroa I. Saline Diffusion Modeling for Sodium Chloride Aqueous Solutions: Freezing for Desalination Purposes. Separations. 2022; 9(10):272. https://doi.org/10.3390/separations9100272
Chicago/Turabian StyleCastillo-Téllez, Beatriz, Rosenberg J. Romero, Karim Allaf, and Isaac Pilatowsky-Figueroa. 2022. "Saline Diffusion Modeling for Sodium Chloride Aqueous Solutions: Freezing for Desalination Purposes" Separations 9, no. 10: 272. https://doi.org/10.3390/separations9100272
APA StyleCastillo-Téllez, B., Romero, R. J., Allaf, K., & Pilatowsky-Figueroa, I. (2022). Saline Diffusion Modeling for Sodium Chloride Aqueous Solutions: Freezing for Desalination Purposes. Separations, 9(10), 272. https://doi.org/10.3390/separations9100272









