Simultaneous Quantification of Diarylheptanoids and Phenolic Compounds in Juglans mandshurica Maxim. by UPLC–TQ-MS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Preparation of Standard and Sample Solutions
2.3. Quantitative Analysis Conditions
2.4. Validation of the Quantitative Method
2.5. Data Analysis
3. Results and Discussion
3.1. Method Validations
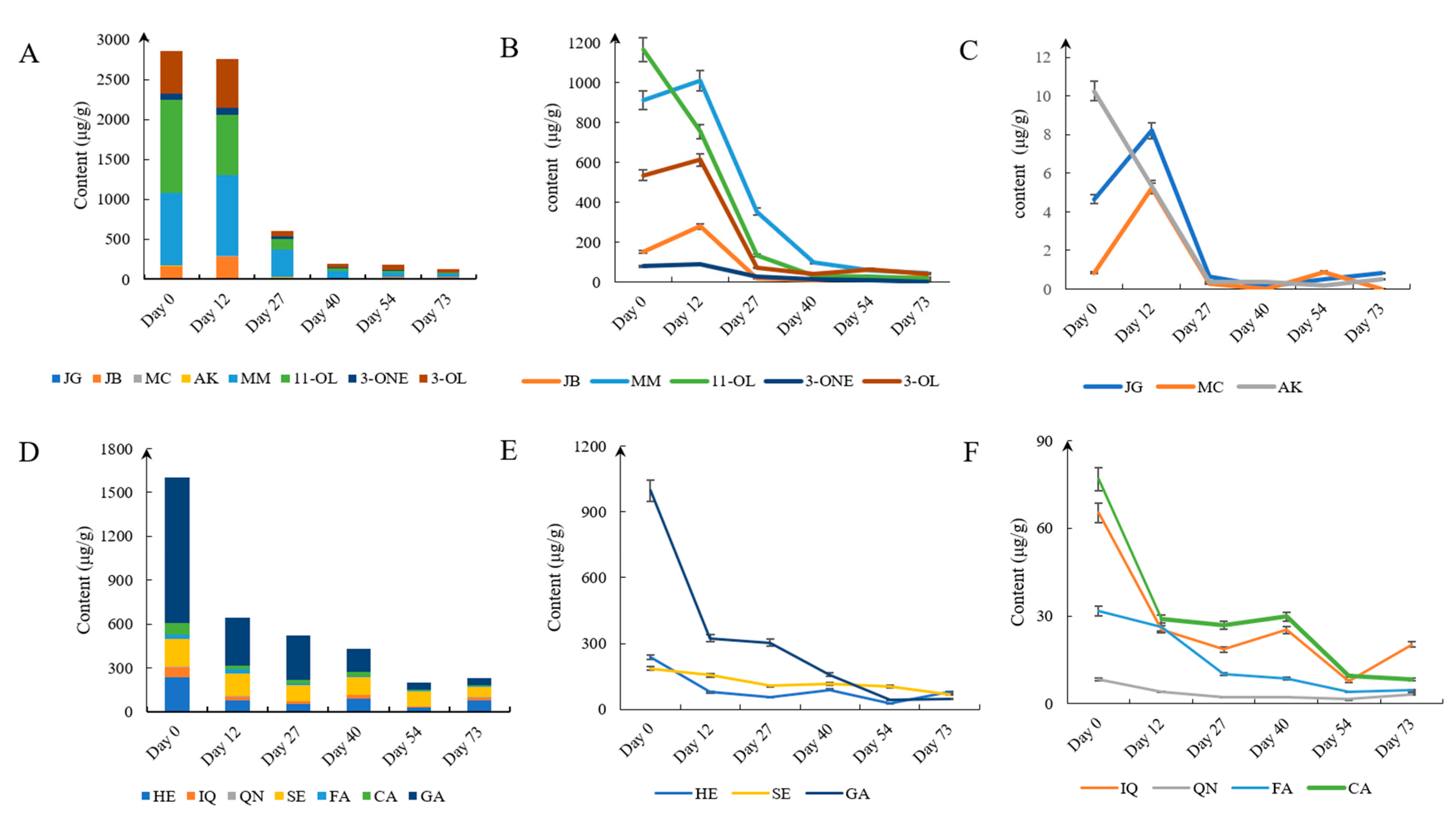
3.2. Quantification of the 15 Compounds in the Epicarps of J. mandshurica
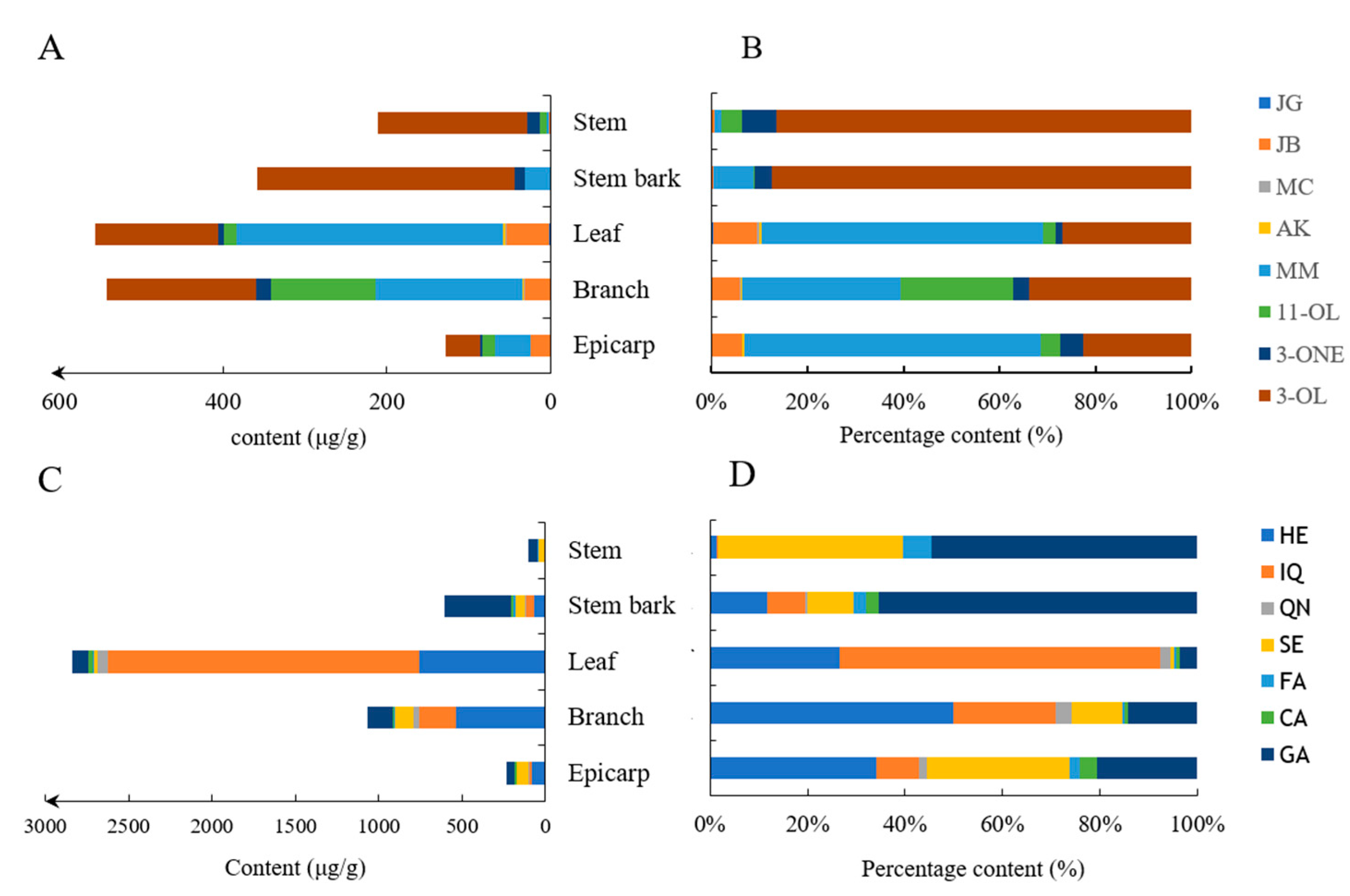
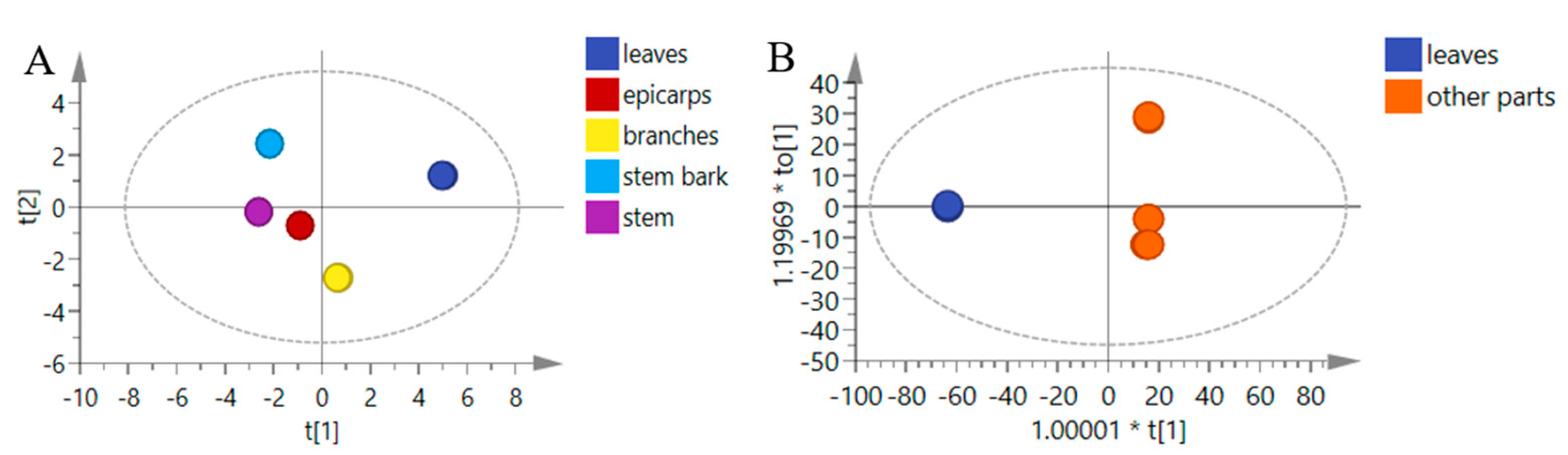
3.3. Quantification of the 15 Compounds in the Different Parts of J. mandshurica
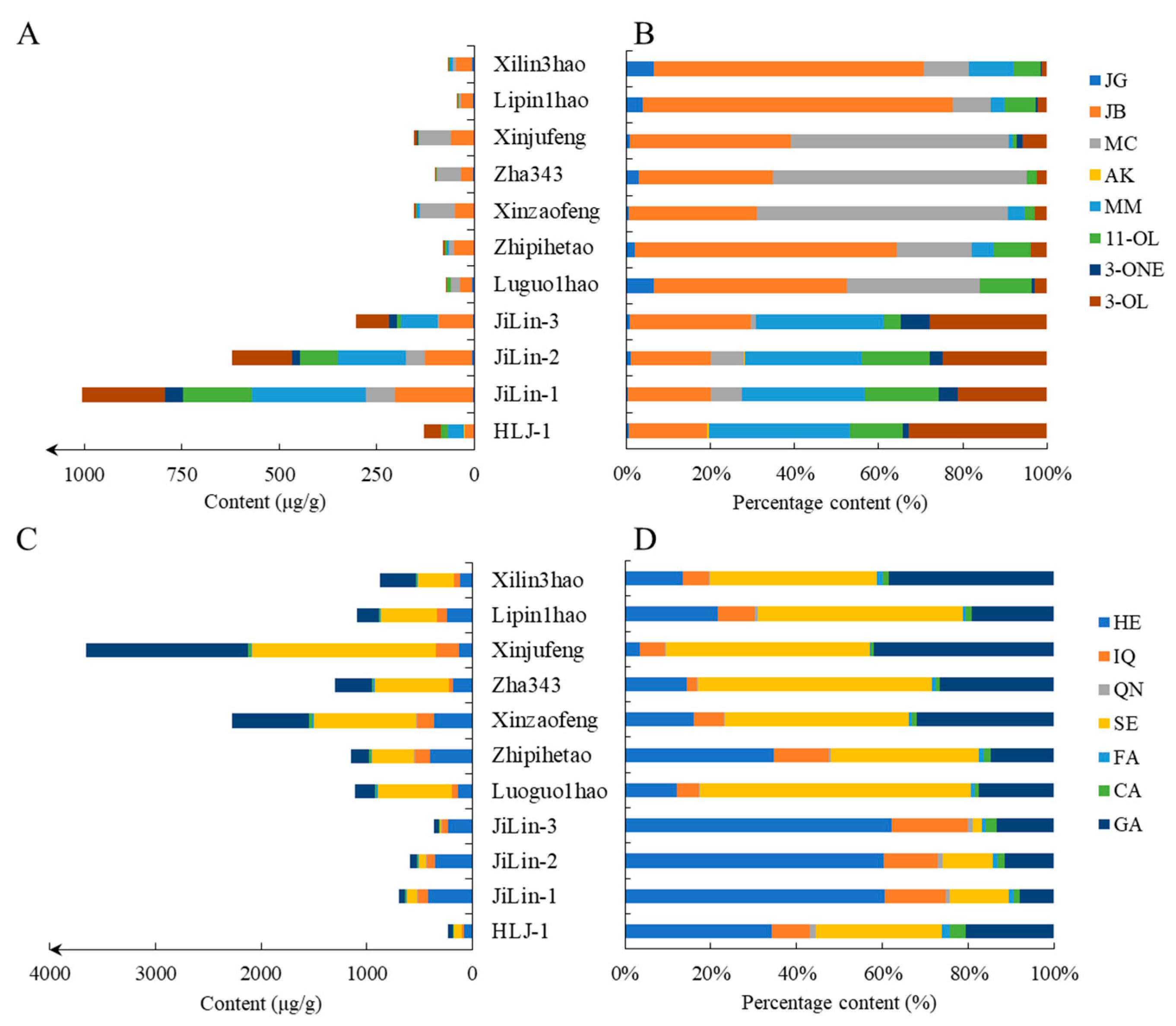
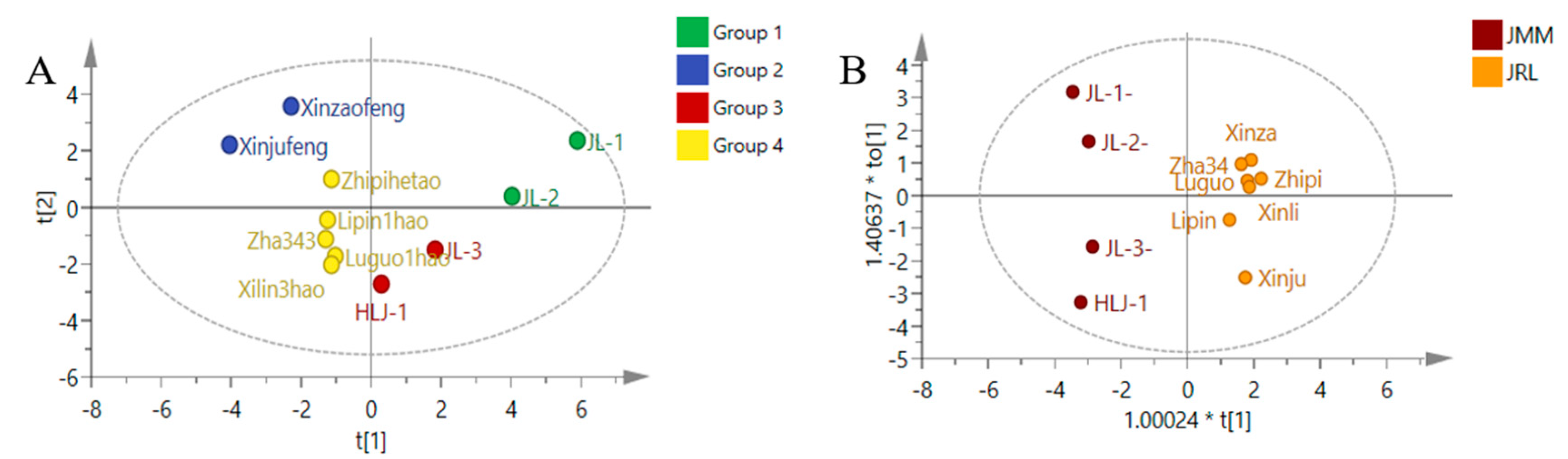
3.4. Quantification of the 15 Compounds in the Epicarps of Different Local Varieties of J. mandshurica and J. regia
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuang, K.R.; Li, P.Q. Flora of China; Scientific Publisher: Beijing, China, 1979. [Google Scholar]
- Lee, S.W.; Lee, K.S.; Son, J.K. New naphthalenyl glycosides from the roots of Juglans mandshurica. Planta Med. 2000, 66, 184–186. [Google Scholar] [CrossRef]
- Liu, L.; Li, W.; Koike, K.; Nikaido, T. Two New Naphthalenyl Glucosides and a New Phenylbutyric Acid Glucoside from the Fruit of Juglans mandshurica. Heterocycles 2004, 63, 1429–1436. [Google Scholar] [CrossRef]
- Diao, S.; Jin, M.; Sun, J.; Zhou, Y.; Ye, C.; Jin, Y.; Zhou, W.; Li, G. A new diarylheptanoid and a new diarylheptanoid glycoside isolated from the roots of Juglans mandshurica and their anti-inflammatory activities. Nat. Prod. Res. 2017, 33, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Regueiro, J.; González, C.S.; Queralt, A.V.; Gándara, J.S.; Raventós, R.L.; Pulido, M.I. Comprehensive identification of walnut polyphenols by liquid chromatography coupled to linear ion trap-Orbitrap mass spectrometry. Food Chem. 2014, 152, 340–348. [Google Scholar] [CrossRef]
- Zhou, Y.Y.; Yang, B.; Liu, Z.; Jiang, Y.; Liu, Y.; Fu, L.; Wang, X.; Kuang, H. Cytotoxicity of Triterpenes from Green Walnut Husks of Juglans mandshurica Maxim in HepG-2 Cancer Cells. Molecules 2015, 20, 19252–19262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, D.L.; Zhang, C.H.; Luo, J.; Jin, M.; Zheng, M.S.; Cui, J.M.; Son, J.K.; Li, G. Chemical constituents from the leaves of Juglans mandshurica. Arch. Pharm. Res. 2015, 38, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.Y.; Yang, B.Y.; Jiang, Y.Q.; Liu, Z.X.; Liu, Y.X.; Wang, X.L.; Kuang, H.X. Studies on Cytotoxic Activity against HepG-2 Cells of Naphthoquinones from Green Walnut Husks of Juglans mandshurica Maxim. Molecules 2015, 20, 15572–15588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Li, W.; Sasaki, T.; Asada, Y.; Koike, K. Juglanone, a novel alpha-tetralonyl derivative with potent antioxidant activity from Juglans mandshurica. J. Nat. Med. 2010, 64, 496–499. [Google Scholar] [CrossRef] [PubMed]
- Pitschmann, A.; Zehl, M.; Atanasov, A.G.; Dirsch, V.M.; Heiss, E.; Glasl, S. Walnut leaf extract inhibits PTP1B and enhances glucose-uptake in vitro. J. Ethnopharmacol. 2014, 152, 599–602. [Google Scholar] [CrossRef]
- Yang, Q.; Yao, Q.S.; Kuang, Y.; Zhang, Y.Z.; Feng, L.L.; Zhang, L.; Guo, L.; Xie, Z.P.; Zhang, S.M. Antimicrobial and cytotoxic juglones from the immature exocarps of Juglans mandshurica. Nat. Prod. Res. 2019, 33, 3203–3209. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Liu, C.; Fang, L.; Min, W. Protein Hydrolyzates from Changbai Mountain Walnut (Juglans mandshurica Maxim.) Boost Mouse Immune System and Exhibit Immunoregulatory Activities. Evid. Based Complementary Altern. Med. 2018, 2018, 4576561. [Google Scholar] [CrossRef]
- Liu, C.; Guo, Y.; Zhao, F.; Qin, H.; Lu, H.; Fang, L.; Wang, J.; Min, W. Potential mechanisms mediating the protective effects of a peptide from walnut (Juglans mandshurica Maxim.) against hydrogen peroxide induced neurotoxicity in PC12 cells. Food Funct. 2019, 10, 3491–3501. [Google Scholar] [CrossRef]
- Luan, F.; Wang, Z.; Yang, Y.; Ji, Y.; Lv, H.; Han, K.; Liu, D.; Shang, X.; He, X.; Zeng, N. Juglans mandshurica Maxim.: A Review of Its Traditional Usages, Phytochemical Constituents, and Pharmacological Properties. Front Pharmacol. 2020, 11, 569800. [Google Scholar] [CrossRef]
- Xu, H.L.; Yu, X.F.; Qu, S.C.; Zhang, R.; Qu, X.R.; Chen, Y.P.; Ma, X.Y.; Sui, D.Y. Anti-proliferative effect of Juglone from Juglans mandshurica Maxim on human leukemia cell HL-60 by inducing apoptosis through the mitochondria-dependent pathway. Eur. J. Pharmacol. 2010, 645, 14–22. [Google Scholar] [CrossRef]
- Park, S.; Kim, N.; Yoo, G.; Kim, S.N.; Kwon, H.J.; Jung, K.; Oh, D.C.; Lee, Y.H.; Kim, S.H. Phenolics and neolignans isolated from the fruits of Juglans mandshurica Maxim. and their effects on lipolysis in adipocytes. Phytochemistry 2017, 137, 87–93. [Google Scholar] [CrossRef]
- Chaudhary, N.; Sasaki, R.; Shuto, T.; Watanabe, M.; Kawahara, T.; Suico, M.; Yokoyama, T.; Mizuguchi, M.; Kai, H.; Devkota, H. Transthyretin Amyloid Fibril Disrupting Activities of Extracts and Fractions from Juglans mandshurica Maxim. var. cordiformis (Makino) Kitam. Molecules 2019, 24, 500. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Gan, C.L.; Guo, Y.P.; Qu, L.Y.; Ma, S.; Ren, Y.B.; Wang, X.W.; Wang, L.B.; Huang, J.; Wang, J.H. Two novel compounds from green walnut husks (Juglans mandshurica Maxim.). Nat. Prod. Res. 2020, 1–9. [Google Scholar] [CrossRef]
- Yang, H.; Ma, Y.; Gao, C.; Wang, B.; Lin, C.; Feng, H.; Wang, L.; Huang, J.; Wang, J.H. Five novel diarylheptanoids from green walnut husks (Juglans regia L.). Fitoterapia 2019, 134, 221–225. [Google Scholar] [CrossRef]
- Akazawa, H.; Akihisa, T.; Taguchi, Y.; Banno, N.; Yoneima, R.; Yasukawa, K. Melanogenesis inhibitory and free radical scavenging activities of diarylheptanoids and other phenolic compounds from the bark of acer nikoense. Bio Pharm. Bull. 2006, 29, 1970–1972. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.S.; Li, G.; Kim, S.H.; Lee, C.S.; Woo, M.H.; Lee, S.H.; Jhang, Y.D.; Son, J.K. Cytotoxic Diarylheptanoids from the Roots of Juglans mandshurica. J. Nat. Prod. 2002, 65, 1707–1708. [Google Scholar] [CrossRef]
- Morikawa, T.; Tao, J.; Ueda, K.; Matsuda, H.; Yoshikawa, M. Medicinal foodstuffs. XXXI. Structures of new aromatic constituents and inhibitors of degranulation in RBL-2H3 cells from a Japanese folk medicine, the stem bark of acer nikoense. Chem. Pharm. Bull. 2003, 51, 62–67. [Google Scholar] [CrossRef] [Green Version]
- Gawlik-Dziki, U.; Durak, A.; Pecio, L.; Kowalska, I. Nutraceutical potential of tinctures from fruits, green husks, and leaves of Juglans regia L. Sci. World J. 2014, 2014, 501392. [Google Scholar] [CrossRef] [Green Version]
- Tsasi, G.; Samara, P.; Tsitsilonis, O.; Jürgenliemk, G.; Skaltsa, H. Isolation, identification and cytotoxic activity of triterpenes and flavonoids from green walnut (Juglans regia L.) pericarps. Rec. Nat. Prod. 2015, 10, 83–92. [Google Scholar]
- Wang, T.M.; Liu, J.; Yi, T.; Zhai, Y.J.; Zhang, H.; Chen, H.B.; Cai, S.Q.; Kang, T.G.; Zhao, Z.Z. Multiconstituent identification in root, branch, and leaf extracts of Juglans mandshurica using ultra high-performance liquid chromatography with quadrupole time-of-flight mass spectrometry. J. Sep. Sci. 2017, 40, 3440–3452. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.-G.; Song, Y.; Liang, J.; Guo, X.-D.; Yang, B.-Y.; Kuang, H.-X. Quality Analysis of American Ginseng Cultivated in Heilongjiang Using UPLC-ESI−-MRM-MS with Chemometric Methods. Molecules 2018, 23, 2396. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.L.; Lai, C.J.; Mao, L.Y.; Yin, B.W.; Tian, M.; Jin, B.L.; Wei, X.Y.; Chen, J.L.; Ge, H.; Zhao, X.; et al. Chemical constituents in different parts of seven species of Aconitum based on UHPLC-Q-TOF/MS. J. Pharm. Biomed. Anal. 2021, 193, 113713. [Google Scholar] [CrossRef]
- Liu, J.; Meng, M.; Li, C.; Huang, X.; Di, D. Simultaneous determination of three diarylheptanoids and an alpha-tetralone derivative in the green walnut husks (Juglans regia L.) by high-performance liquid chromatography with photodiode array detector. J. Chromatogr. A 2008, 1190, 80–85. [Google Scholar] [CrossRef]
- Li, C.; Liu, J.X.; Zhao, L.; Di, D.L.; Meng, M.; Jiang, S.X. Capillary zone electrophoresis for separation and analysis of four diarylheptanoids and an alpha-tetralone derivative in the green walnut husks (Juglans regia L.). J. Pharm. Biomed. Anal. 2008, 48, 749–753. [Google Scholar] [CrossRef]
- Sang, Q.; Jia, Q.; Zhang, H.; Lin, C.; Zhao, X.; Zhang, M.; Wang, Y.; Hu, P. Chemical profiling and quality evaluation of Zhishi-Xiebai-Guizhi Decoction by UPLC-Q-TOF-MS and UPLC fingerprint. J. Pharm. Biomed. Anal. 2021, 194, 113771. [Google Scholar] [CrossRef]
- Tian, T.; Xu, X.; Li, X.; Zhang, W.; Lu, H. Precision-characterization and quantitative determination of main compounds in Si-Ni-San with UHPLC-MS/MS based targeted-profiling method. J. Pharm. Biomed. Anal. 2021, 194, 113816. [Google Scholar] [CrossRef]
- Luo, P.; Dai, W.; Yin, P.; Zeng, Z.; Kong, H.; Zhou, L.; Wang, X.; Chen, S.; Lu, X.; Xu, G. Multiple reaction monitoring-ion pair finder: A systematic approach to transform nontargeted mode to pseudotargeted mode for metabolomics study based on liquid chromatography-mass spectrometry. Anal. Chem. 2015, 87, 5050–5055. [Google Scholar] [CrossRef]
- Britton, E.; Kellogg, J.; Kvalheim, O.; Cech, N. Biochemometrics to Identify Synergists and Additives from Botanical Medicines: A Case Study with Hydrastis canadensis (Goldenseal). J. Nat. Prod. 2018, 81, 484–493. [Google Scholar] [CrossRef] [Green Version]
- Plazas, E.; Casoti, R.; Murillo, M.A.; Da Costa, F.B.; Cuca, L.E. Metabolomic profiling of Zanthoxylum species: Identification of anti-cholinesterase alkaloids candidates. Phytochemistry 2019, 168, 112–128. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.H.; Sun, G.D.; Dong, W.T.; Wang, W.M. Dynamic variation of major effective components in fresh rejuvenated fruit of Juglans mandshurica based on UPLC-Q-TOF-MS. China J. Chin. Mater. Med. 2016, 41, 3379–3388. [Google Scholar]
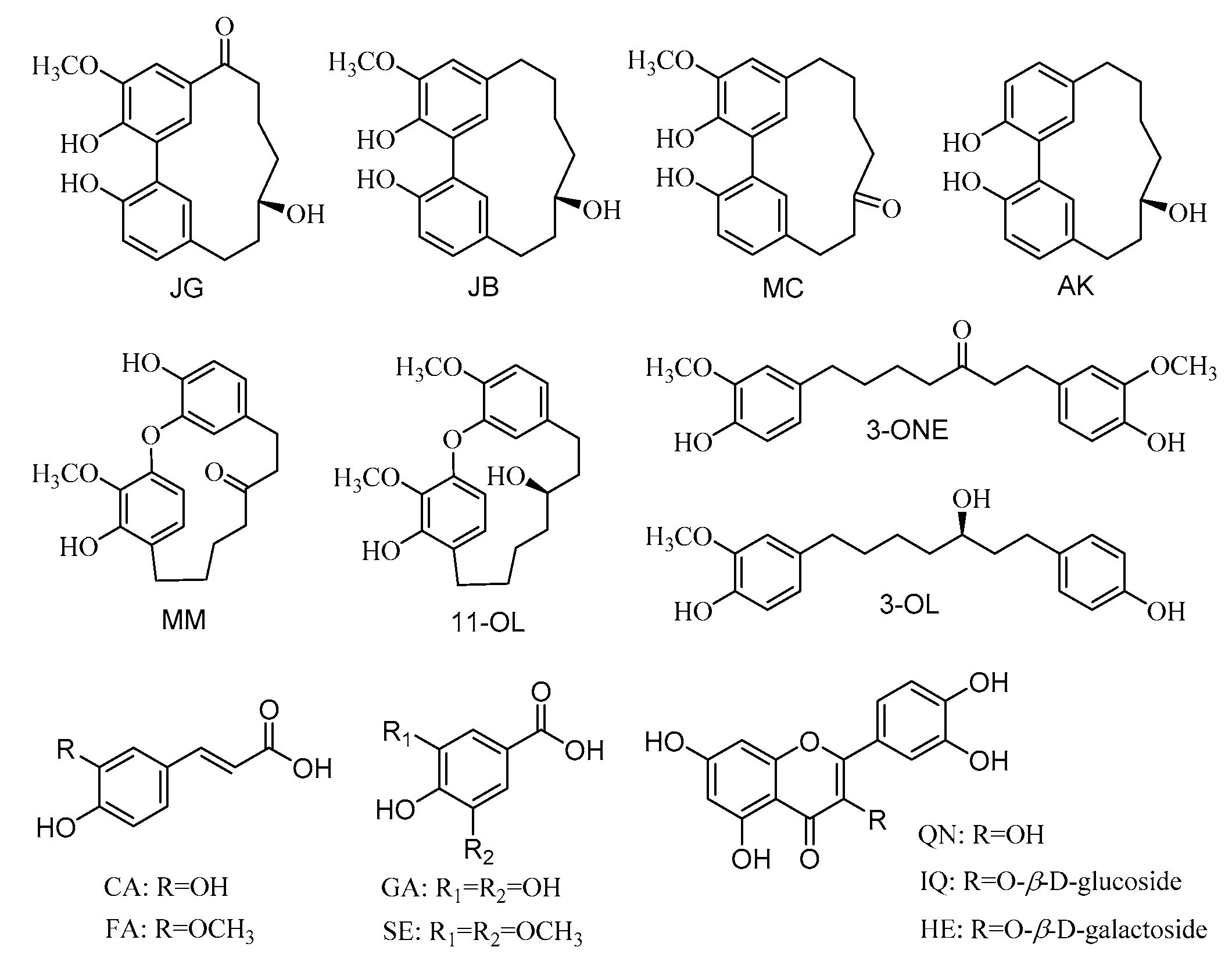


| Analytes | Calibration Curves | R2 | Linear Range (μg/g) | Precision (RSD, %, n = 6) | Repeatability (RSD, %, n = 6) | Stability (RSD, %, n = 6) | Recovery (%, n = 3) | LOD * (μg/g) | LOQ * (μg/g) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | RSD | |||||||||
| JG | y = 114.373x + 52.7865 | 0.9991 | 0.1~500 | 1.95 | 1.60 | 2.72 | 105.56 | 1.55 | 0.07 | 0.1 |
| JB | y = 65.6085x + 45.7752 | 0.9996 | 0.5~1000 | 0.51 | 1.14 | 1.14 | 102.28 | 2.66 | 0.1 | 0.3 |
| MC | y = 4.97847x − 14.7766 | 0.9990 | 1.0~1000 | 0.47 | 1.83 | 1.84 | 108.81 | 2.20 | 0.1 | 0.5 |
| AK | y = 33.5104x − 14.1847 | 0.9995 | 0.1~1000 | 1.18 | 1.27 | 1.63 | 107.04 | 2.36 | 0.05 | 0.1 |
| MM | y = 10.8399x − 68.4462 | 0.9991 | 1.0~1000 | 0.94 | 0.89 | 0.39 | 100.85 | 1.80 | 0.2 | 0.7 |
| 11-OL | y = 44.2525x − 151.669 | 0.9997 | 0.5~1000 | 3.54 | 1.90 | 0.41 | 99.93 | 3.38 | 0.1 | 0.3 |
| 3-ONE | y = 16.7953x + 43.5742 | 0.9993 | 0.5~1000 | 4.11 | 2.28 | 0.34 | 105.19 | 0.13 | 0.2 | 0.3 |
| 3-OL | y = 19.8566x − 24.6636 | 0.9990 | 0.5~1000 | 3.19 | 0.84 | 0.82 | 102.32 | 2.41 | 0.2 | 0.3 |
| HE | y = 24.5306x− 30.5319 | 0.9990 | 0.5~1000 | 3.05 | 0.69 | 0.29 | 99.46 | 3.28 | 0.2 | 0.5 |
| IQ | y = 12.0327x − 38.8435 | 0.9993 | 0.5~1000 | 1.08 | 0.54 | 1.36 | 102.88 | 2.11 | 0.2 | 0.3 |
| QN | y = 18.8688x + 142.158 | 0.9990 | 0.1~500 | 0.77 | 2.79 | 1.86 | 119.04 | 0.44 | 0.05 | 0.1 |
| SE | y = 3.95677x − 123.382 | 0.9990 | 5.0~1000 | 2.56 | 2.22 | 1.72 | 115.45 | 1.52 | 1.0 | 4.0 |
| FA | y = 12.1862x − 104.272 | 0.9991 | 1.0~1000 | 1.61 | 3.91 | 1.00 | 104.22 | 2.41 | 0.5 | 1.0 |
| CA | y = 27.0709x − 241.742 | 0.9990 | 5.0~1000 | 1.92 | 3.27 | 1.06 | 104.84 | 3.33 | 1.0 | 4.0 |
| GA | y = 15.0273x + 85.8018 | 0.9993 | 1.0~1000 | 3.48 | 1.50 | 0.41 | 102.49 | 2.57 | 0.5 | 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Wang, L.-B.; Guo, Y.-P.; Wang, Y.-L.; Chen, X.-X.; Huang, J.; Yang, L.; Zhang, K.; Wang, J.-H. Simultaneous Quantification of Diarylheptanoids and Phenolic Compounds in Juglans mandshurica Maxim. by UPLC–TQ-MS. Separations 2021, 8, 132. https://doi.org/10.3390/separations8090132
Yang H, Wang L-B, Guo Y-P, Wang Y-L, Chen X-X, Huang J, Yang L, Zhang K, Wang J-H. Simultaneous Quantification of Diarylheptanoids and Phenolic Compounds in Juglans mandshurica Maxim. by UPLC–TQ-MS. Separations. 2021; 8(9):132. https://doi.org/10.3390/separations8090132
Chicago/Turabian StyleYang, Hong, Li-Bo Wang, Ya-Ping Guo, Ya-Li Wang, Xiao-Xiang Chen, Jian Huang, Lu Yang, Ke Zhang, and Jin-Hui Wang. 2021. "Simultaneous Quantification of Diarylheptanoids and Phenolic Compounds in Juglans mandshurica Maxim. by UPLC–TQ-MS" Separations 8, no. 9: 132. https://doi.org/10.3390/separations8090132
APA StyleYang, H., Wang, L.-B., Guo, Y.-P., Wang, Y.-L., Chen, X.-X., Huang, J., Yang, L., Zhang, K., & Wang, J.-H. (2021). Simultaneous Quantification of Diarylheptanoids and Phenolic Compounds in Juglans mandshurica Maxim. by UPLC–TQ-MS. Separations, 8(9), 132. https://doi.org/10.3390/separations8090132






