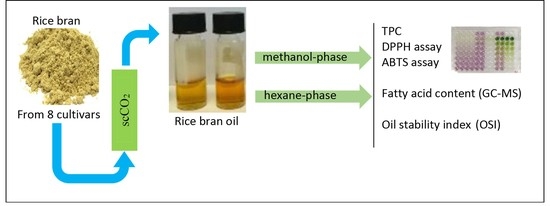
Supercritical Carbon Dioxide Extraction, Antioxidant Activity, and Fatty Acid Composition of Bran Oil from Rice Varieties Cultivated in Portugal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Reagents
2.3. Supercritical Fluid Extraction Apparatus and Procedure
2.4. Antioxidant Activities of Bran Oil Fractions
2.4.1. Total Phenolic Content
2.4.2. DPPH• Scavenging Activity
2.4.3. ABTS•+ Scavenging Activity
2.5. Determination of the Fatty Acids Profile
2.6. Oxidation Stability Index Determination
2.7. Statistical Analysis
3. Results
3.1. Influence of SFE Conditions on the Extraction Yield of Bran Oil
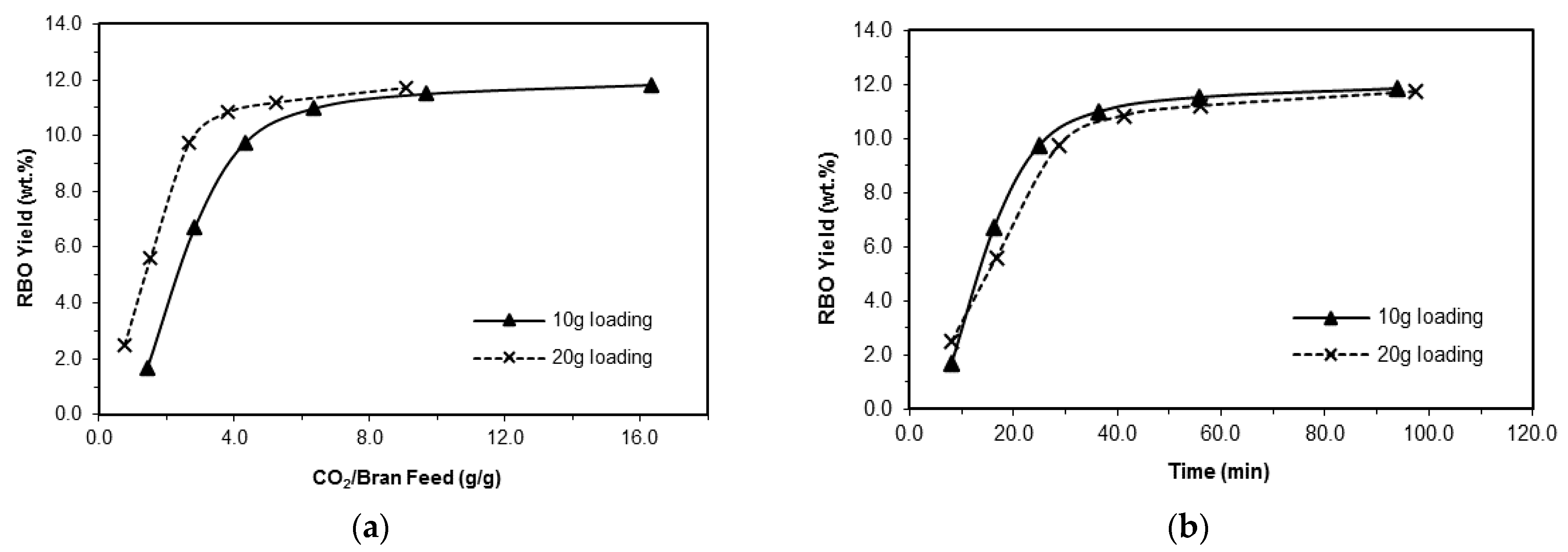
3.1.1. Influence of Plant Loading
3.1.2. Influence of the Solvent Flow Rate
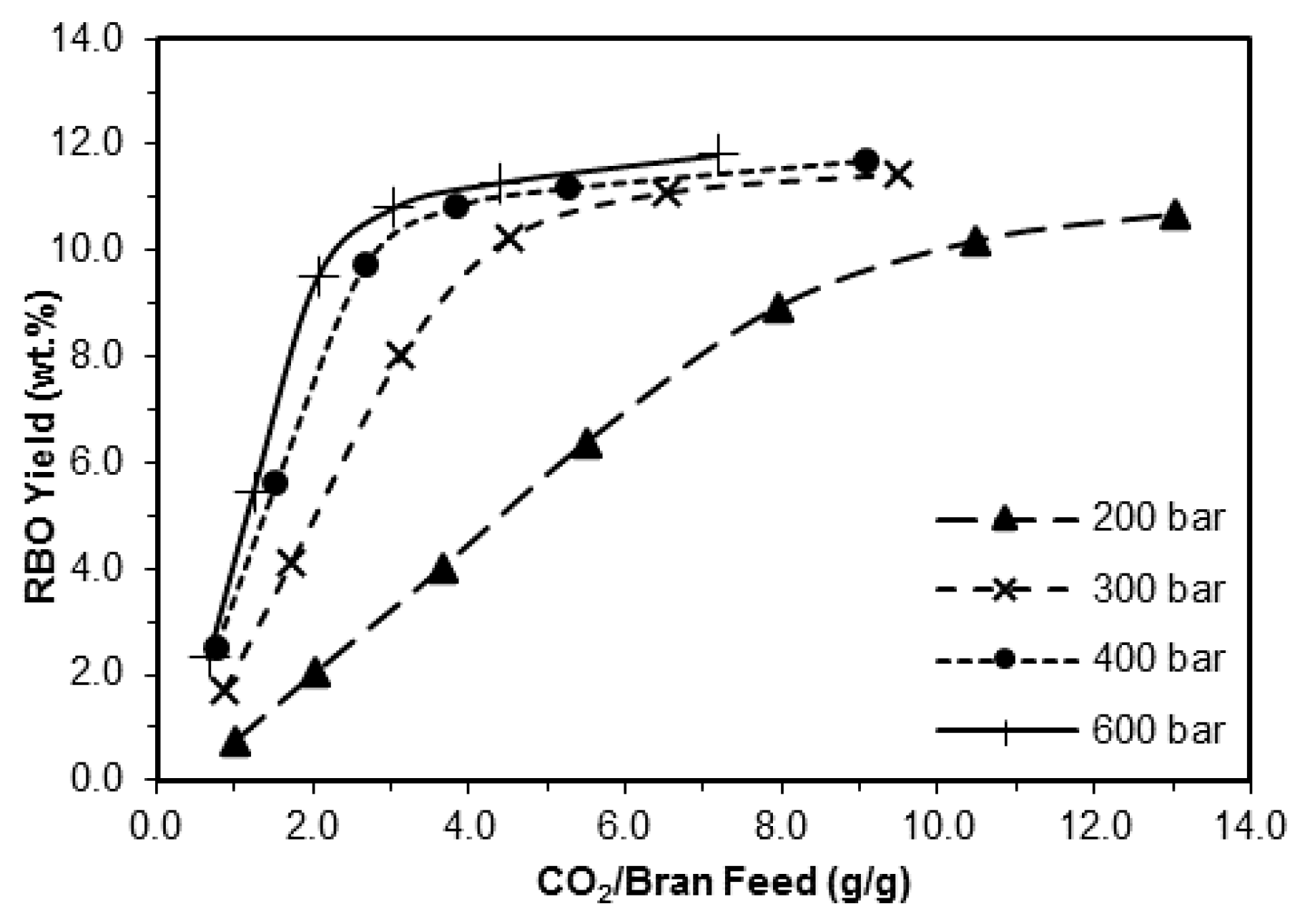
3.1.3. Influence of the Pressure
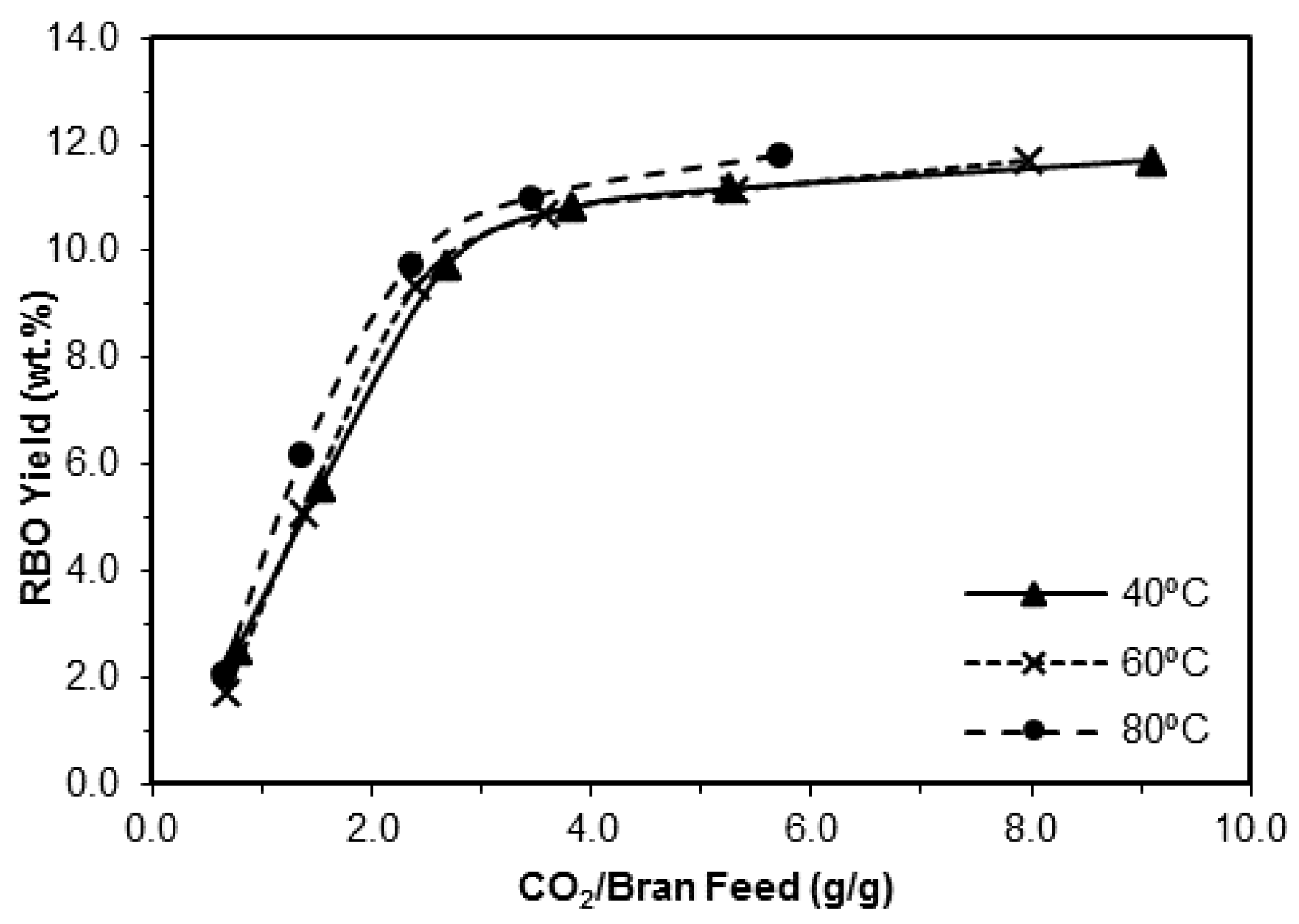
3.1.4. Influence of the Temperature
3.2. SFE and Antioxidant Activity of Bran Oil Varieties
3.2.1. Extraction Yields
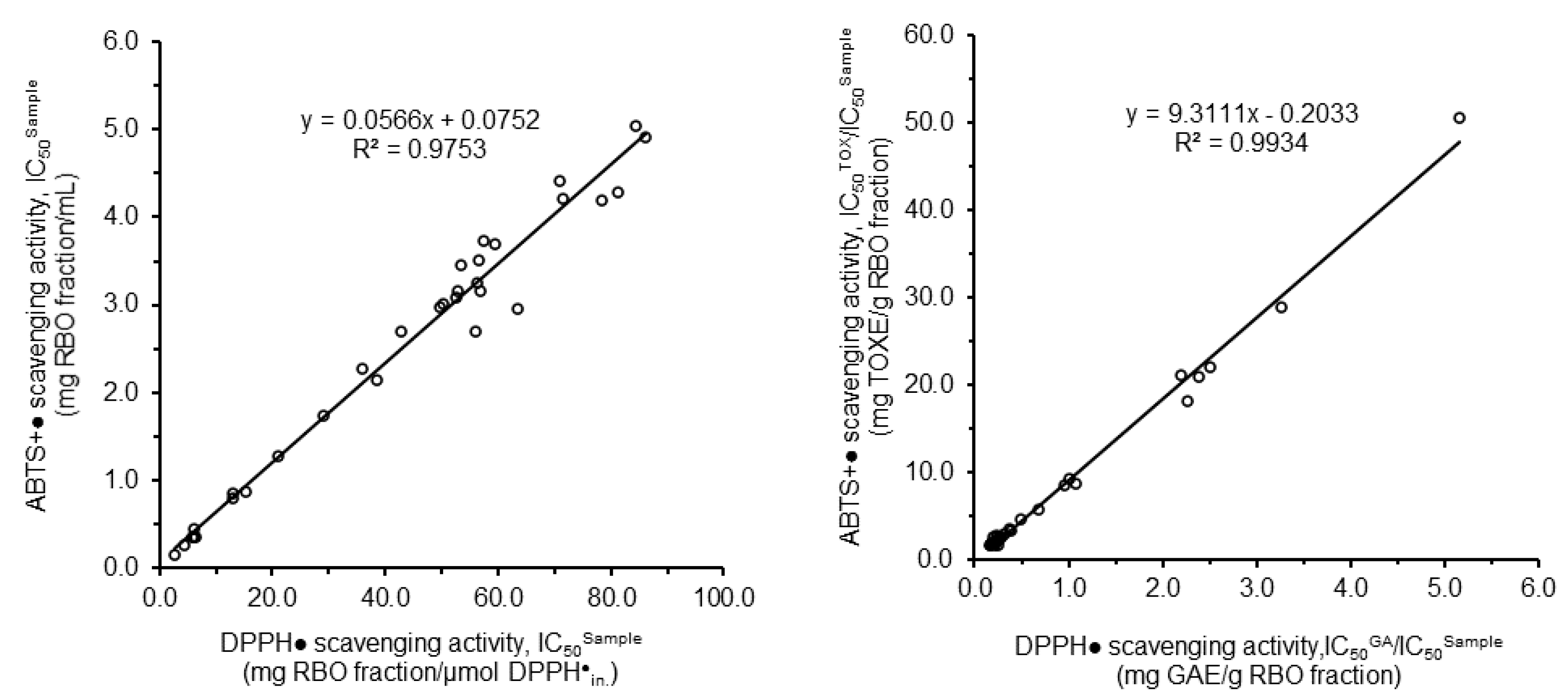
3.2.2. Antioxidant Activities of Rice Bran Oil Fractions
3.3. Fatty Acid Composition and Oxidation Stability Index Determination
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations, FAOSTAT Data, Crops. Available online: http://www.fao.org/faostat/en/#data/QC/visualize (accessed on 18 March 2021).
- Beloshapka, A.; Buff, P.; Fahey, G.; Swanson, K. Compositional analysis of whole grains, processed grains, grain co-products, and other carbohydrate sources with applicability to pet animal nutrition. Foods 2016, 5, 23. [Google Scholar] [CrossRef]
- Casas, G.A.; Overholt, M.F.; Dilger, A.C.; Boler, D.D.; Stein, H.H. Effects of full fat rice bran and defatted rice bran on growth performance and carcass characteristics of growing-finishing pigs. J. Anim. Sci. 2018, 96, 2293–2309. [Google Scholar] [CrossRef]
- Saleh, A.S.M.; Wang, P.; Wang, N.; Yang, L.; Xiao, Z. Brown rice versus white rice: Nutritional quality, potential health benefits, development of food products, and preservation technologies. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1070–1096. [Google Scholar] [CrossRef] [Green Version]
- Goufo, P.; Trindade, H. Rice antioxidants: Phenolic acids, flavonoids, anthocyanins, proanthocyanidins, tocopherols, tocotrienols, γ-oryzanol, and phytic acid. Food Sci. Nutr. 2014, 2, 75–104. [Google Scholar] [CrossRef]
- Sharif, M.K.; Butt, M.S.; Anjum, F.M.; Khan, S.H. Rice bran: A novel functional ingredient. Crit. Rev. Food Sci. Nutr. 2014, 54, 807–816. [Google Scholar] [CrossRef]
- Nagendra Prasad, M.N.; Sanjaya, K.R.; Sravya Khatokar, M.N.; Vismaya, M.N.; Najunda Swamy, S. Health benefits of rice bran—A review. J. Nutr. Food Sci. 2011, 1, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Amarasinghe, B.M.W.P.K.; Kumarasiri, M.P.M.; Gangodavilage, N.C. Effect of method of stabilization on aqueous extraction of rice bran oil. Food Bioprod. Process. 2009, 87, 108–114. [Google Scholar] [CrossRef]
- Khan, S.H.; Butt, M.S.; Sharif, M.K.; Sameen, A.; Mumtaz, S.; Sultan, M.T. Functional properties of protein isolates extracted from stabilized rice bran by microwave, dry heat, and parboiling. J. Agric. Food Chem. 2011, 59, 2416–2420. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Q.; Strappe, P.; Zhou, Z.K.; Blanchard, C. Impact on the nutritional attributes of rice bran following various stabilization procedures. Crit. Rev. Food Sci. Nutr. 2019, 59, 2458–2466. [Google Scholar] [CrossRef] [PubMed]
- Dorni, C.; Sharma, P.; Saikia, G.; Longvah, T. Fatty acid profile of edible oils and fats consumed in India. Food Chem. 2018, 238, 9–15. [Google Scholar] [CrossRef]
- Imsanguan, P.; Roaysubtawee, A.; Borirak, R.; Pongamphai, S.; Douglas, S.; Douglas, P.L. Extraction of α-tocopherol and γ-oryzanol from rice bran. LWT Food Sci. Technol. 2008, 41, 1417–1424. [Google Scholar] [CrossRef]
- Massarolo, K.C.; Ribeiro, A.C.; Furlong, E.B.; de Souza Soares, L.A. Effect of particle size of rice bran on gamma-oryzanol content and compounds. J. Cereal Sci. 2017, 75, 54–60. [Google Scholar] [CrossRef]
- Capellini, M.C.; Giacomini, V.; Cuevas, M.S.; Rodrigues, C.E.C. Rice bran oil extraction using alcoholic solvents: Physicochemical characterization of oil and protein fraction functionality. Ind. Crops Prod. 2017, 104, 133–143. [Google Scholar] [CrossRef]
- Bessa, L.C.B.A.; Ferreira, M.C.; Rodrigues, C.E.C.; Batista, E.A.C.; Meirelles, A.J.A. Simulation and process design of continuous countercurrent ethanolic extraction of rice bran oil. J. Food Eng. 2017, 202, 99–113. [Google Scholar] [CrossRef]
- Kamimura, J.A.A.M.; Aracava, K.K.; Rodrigues, C.E.C. Experimental data and modeling of rice bran oil extraction kinetics using ethanol as solvent. Sep. Sci. Technol. 2017, 52, 1921–1928. [Google Scholar] [CrossRef]
- Daud, N.S.M.; Zaidel, D.N.A.; Lai, K.S.; Khairuddin, N.; Jusoh, Y.M.M.; Muhamad, I.I. Crude oil yield and properties of rice bran oil from different varieties as affected by extraction conditions using soxhterm method. Arab. J. Sci. Eng. 2018, 43, 6237–6244. [Google Scholar] [CrossRef]
- Pandey, R.; Shrivastava, S.L. Comparative evaluation of rice bran oil obtained with two-step microwave assisted extraction and conventional solvent extraction. J. Food Eng. 2018, 218, 106–114. [Google Scholar] [CrossRef]
- Zigoneanu, I.G.; Williams, L.; Xu, Z.; Sabliov, C.M. Determination of antioxidant components in rice bran oil extracted by microwave-assisted method. Bioresour. Technol. 2008, 99, 4910–4918. [Google Scholar] [CrossRef]
- Kumar, P.; Yadav, D.; Kumar, P.; Panesar, P.S.; Bunkar, D.S.; Mishra, D.; Chopra, H.K. Comparative study on conventional, ultrasonication and microwave assisted extraction of γ-oryzanol from rice bran. J. Food Sci. Technol. 2016, 53, 2047–2053. [Google Scholar] [CrossRef] [Green Version]
- Terigar, B.G.; Balasubramanian, S.; Sabliov, C.M.; Lima, M.; Boldor, D. Soybean and rice bran oil extraction in a continuous microwave system: From laboratory- to pilot-scale. J. Food Eng. 2011, 104, 208–217. [Google Scholar] [CrossRef]
- Phan, V.M.; Junyusen, T.; Liplap, P.; Junyusen, P. Effects of ultrasonication and thermal cooking pretreatments on the extractability and quality of cold press extracted rice bran oil. J. Food Process Eng. 2019, 42, 1–8. [Google Scholar] [CrossRef]
- Pengkumsri, N.; Chaiyasut, C.; Sivamaruthi, B.S.; Saenjum, C.; Sirilun, S.; Peerajan, S.; Suwannalert, P.; Sirisattha, S.; Chaiyasut, K.; Kesika, P.; et al. The influence of extraction methods on composition and antioxidant properties of rice bran oil. Food Sci. Technol. 2015, 35, 493–501. [Google Scholar] [CrossRef] [Green Version]
- Mingyai, S.; Kettawan, A.; Srikaeo, K.; Singanusong, R. Physicochemical and antioxidant properties of rice bran oils produced from colored rice using different extraction methods. J. Oleo Sci. 2017, 66, 565–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mingyai, S.; Srikaeo, K.; Kettawan, A.; Singanusong, R.; Nakagawa, K.; Kimura, F.; Ito, J. Effects of extraction methods on phytochemicals of rice bran oils produced from colored rice. J. Oleo Sci. 2018, 67, 135–142. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Liu, R.; Shi, L.; Zhang, Z.; Zhang, T.; Lu, M.; Chang, M.; Jin, Q.; Wang, X. Effect of refining process on physicochemical parameters, chemical compositions and in vitro antioxidant activities of rice bran oil. LWT 2019, 109, 26–32. [Google Scholar] [CrossRef]
- Wongwaiwech, D.; Weerawatanakorn, M.; Tharatha, S.; Ho, C.T. Comparative study on amount of nutraceuticals in by-products from solvent and cold pressing methods of rice bran oil processing. J. Food Drug Anal. 2019, 27, 71–82. [Google Scholar] [CrossRef] [Green Version]
- Sairam, S.; Gopala Krishna, A.G.; Urooj, A. Physico-chemical characteristics of defatted rice bran and its utilization in a bakery product. J. Food Sci. Technol. 2011, 48, 478–483. [Google Scholar] [CrossRef] [Green Version]
- Han, S.W.; Chee, K.M.; Cho, S.J. Nutritional quality of rice bran protein in comparison to animal and vegetable protein. Food Chem. 2015, 172, 766–769. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Hettiarachchy, N.S.; Horax, R.; Eswaranandam, S. Physicochemical properties and functionality of rice bran protein hydrolyzate prepared from heat-stabilized defatted rice bran with the aid of enzymes. J. Food Sci. 2003, 68, 152–157. [Google Scholar] [CrossRef]
- Tomita, K.; Machmudah, S.; Fukuzato, R.; Kanda, H.; Quitain, A.T.; Sasaki, M.; Goto, M. Extraction of rice bran oil by supercritical carbon dioxide and solubility consideration. Sep. Purif. Technol. 2014, 125, 319–325. [Google Scholar] [CrossRef]
- Manosroi, A.; Ruksiriwanich, W.; Abe, M.; Sakai, H.; Manosroi, W.; Manosroi, J. Biological activities of the rice bran extract and physical characteristics of its entrapment in niosomes by supercritical carbon dioxide fluid. J. Supercrit. Fluids 2010, 54, 137–144. [Google Scholar] [CrossRef]
- Soares, J.F.; Dal Prá, V.; De Souza, M.; Lunelli, F.C.; Abaide, E.; Da Silva, J.R.F.; Kuhn, R.C.; Martínez, J.; Mazutti, M.A. Extraction of rice bran oil using supercritical CO2 and compressed liquefied petroleum gas. J. Food Eng. 2016, 170, 58–63. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.-H.; Chen, C.-R.; Wu, J.-J.; Wang, L.-Y.; Chang, C.-M.J.; Ho, W.-J. Designing supercritical carbon dioxide extraction of rice bran oil that contain oryzanols using response surface methodology. J. Sep. Sci. 2008, 31, 1399–1407. [Google Scholar] [CrossRef] [PubMed]
- Sparks, D.; Hernandez, R.; Zappi, M.; Blackwell, D.; Fleming, T. Extraction of rice brain oil using supercritical carbon dioxide and propane. J. Am. Oil Chem. Soc. 2006, 83, 885–891. [Google Scholar] [CrossRef]
- Balachandran, C.; Mayamol, P.N.; Thomas, S.; Sukumar, D.; Sundaresan, A.; Arumughan, C. An ecofriendly approach to process rice bran for high quality rice bran oil using supercritical carbon dioxide for nutraceutical applications. Bioresour. Technol. 2008, 99, 2905–2912. [Google Scholar] [CrossRef] [PubMed]
- Mariod, A.; Ismail, M.; Rahman, N.F.A.; Matthaus, B. Stability of rice bran oil extracted by SFE and soxhlet methods during accelerated shelf-life storage. Grasas y Aceites 2014, 65, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Coelho, J.P.; Bernotaityte, K.; Miraldes, M.A.; Mendonca, A.F.; Stateva, R.P. Solubility of ethanamide and 2-propenamide in supercritical carbon dioxide. Measurements and correlation. J. Chem. Eng. Data 2009, 54, 2546–2549. [Google Scholar] [CrossRef]
- Coelho, J.P.; Filipe, R.M.; Paula Robalo, M.; Boyadzhieva, S.; Cholakov, G.S.; Stateva, R.P. Supercritical CO2 extraction of spent coffee grounds. Influence of co-solvents and characterization of the extracts. J. Supercrit. Fluids 2020, 161, 1–13. [Google Scholar] [CrossRef]
- Coelho, J.; Veiga, J.; Karmali, A.; Nicolai, M.; Pinto Reis, C.; Nobre, B.; Palavra, A.; Reis, C.P.; Nobre, B.; Palavra, A. Supercritical CO2 extracts and volatile oil of basil (Ocimum basilicum L.) comparison with conventional methods. Separations 2018, 5, 21. [Google Scholar] [CrossRef] [Green Version]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Mechergui, K.; Coelho, J.A.; Serra, M.C.; Lamine, S.B.; Boukhchina, S.; Khouja, M.L. Essential oils of Origanum vulgare L. subsp. glandulosum (Desf.) Ietswaart from Tunisia: Chemical composition and antioxidant activity. J. Sci. Food Agric. 2010, 90, 1745–1749. [Google Scholar] [CrossRef] [PubMed]
- Chew, Y.L.; Lim, Y.Y.; Omar, M.; Khoo, K.S. Antioxidant activity of three edible seaweeds from two areas in South East Asia. LWT Food Sci. Technol. 2008, 41, 1067–1072. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved abts radical cation decolorization assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Knothe, G. Structure indices in FA chemistry. How relevant is the iodine value? J. Am. Oil Chem. Soc. 2002, 79, 847–854. [Google Scholar] [CrossRef]
- Kumar, M.; Sharma, M.P. Assessment of potential of oils for biodiesel production. Renew. Sustain. Energy Rev. 2015, 44, 814–823. [Google Scholar] [CrossRef]
- Knothe, G.; Dunn, R.O. Dependence of oil stability index of fatty compounds on their structure and concentration and presence of metals. J. Am. Oil Chem. Soc. 2003, 80, 1021–1026. [Google Scholar] [CrossRef]
- Yoon, S.W.; Pyo, Y.; Lee, J.; Lee, J.; Kim, B.H.; Kim, I. The concentrations of tocols and γ-oryzanol compounds in rice bran oil obtained by fractional extraction with supercritical carbon dioxide. J. Oleo Sci. 2014, 63, 47–53. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.R.; Wang, L.Y.; Wang, C.H.; Ho, W.J.; Chang, C.M.J. Supercritical carbon dioxide extraction of rice bran oil and column partition fractionation of γ-oryzanols. Sep. Purif. Technol. 2008, 61, 358–365. [Google Scholar] [CrossRef]
- Perretti, G.; Miniati, E.; Montanari, L.; Fantozzi, P. Improving the value of rice by-products by SFE. J. Supercrit. Fluids 2003, 26, 63–71. [Google Scholar] [CrossRef]
- Danielski, L.; Zetzl, C.; Hense, H.; Brunner, G. A process line for the production of raffinated rice oil from rice bran. J. Supercrit. Fluids 2005, 34, 133–141. [Google Scholar] [CrossRef]
- Trevisani Juchen, P.; Nolasco Araujo, M.; Hamerski, F.; Corazza, M.L.; Pedersen Voll, F.A. Extraction of parboiled rice bran oil with supercritical CO2 and ethanol as co-solvent: Kinetics and characterization. Ind. Crop. Prod. 2019, 139, 111506. [Google Scholar] [CrossRef]
- Lerma-García, M.J.; Herrero-Martínez, J.M.; Simó-Alfonso, E.F.; Mendonça, C.R.B.; Ramis-Ramos, G. Composition, industrial processing and applications of rice bran γ-oryzanol. Food Chem. 2009, 115, 389–404. [Google Scholar] [CrossRef]
- Castanho, A.; Lageiro, M.; Calhelha, R.C.; Ferreira, I.C.F.R.; Sokovic, M.; Cunha, L.M.; Brites, C. Exploiting the bioactive properties of γ-oryzanol from bran of different exotic rice varieties. Food Funct. 2019, 10, 2382–2389. [Google Scholar] [CrossRef] [PubMed]
- Janu, C.; Kumar, D.R.S.; Reshma, M.V.; Jayamurthy, P.; Sundaresan, A.; Nisha, P. Comparative study on the total phenolic content and radical scavenging activity of common edible vegetable oils. J. Food Biochem. 2014, 38, 38–49. [Google Scholar] [CrossRef]
- Xu, Z.; Godber, J.S. Comparison of supercritical fluid and solvent extraction methods in extracting γ-oryzanol from rice bran. J. Am. Oil Chem. Soc. 2000, 77, 547–551. [Google Scholar] [CrossRef]
- Jesus, S.P.; Grimaldi, R.; Hense, H. Recovery of γ-oryzanol from rice bran oil byproduct using supercritical fluid extraction. J. Supercrit. Fluids 2010, 55, 149–155. [Google Scholar] [CrossRef]
- Liu, H.M.; Wang, F.Y.; Li, H.Y.; De Wang, X.; Qin, G.Y. Subcritical butane and propane extraction of oil from rice bran. BioResources 2015, 10, 4652–4662. [Google Scholar] [CrossRef]
- Dwivedi, G.; Sharma, M.P. Potential and limitation of straight vegetable oils as engine fuel—An Indian perspective. Renew. Sustain. Energy Rev. 2014, 33, 316–322. [Google Scholar] [CrossRef]
- Sierra-Cantor, J.F.; Guerrero-Fajardo, C.A. Methods for improving the cold flow properties of biodiesel with high saturated fatty acids content: A review. Renew. Sustain. Energy Rev. 2017, 72, 774–790. [Google Scholar] [CrossRef]
- Giakoumis, E.G. Analysis of 22 vegetable oils’ physico-chemical properties and fatty acid composition on a statistical basis, and correlation with the degree of unsaturation. Renew. Energy 2018, 126, 403–419. [Google Scholar] [CrossRef]




| Plant Loading (g) | CO2 Flow Rate (L/min) | Temperature (°C) | Pressure (MPa) | Oil Yield (% wt.) |
|---|---|---|---|---|
| Influence of plant feed | ||||
| 10 | 1.0 | 40 | 40.0 | 11.83 ± 0.49 |
| 20 | 11.72 ± 0.49 | |||
| Influence of CO2 flow rate | ||||
| 20 | 0.5 | 40 | 40.0 | 11.42 ± 0.43 |
| 1.0 | 11.72 ± 0.48 | |||
| 1.5 | 11.14 ± 0.45 | |||
| Influence of temperature | ||||
| 20 | 1.0 | 40 | 40.0 | 11.72 ± 0.46 |
| 60 | 11.70 ± 0.50 | |||
| 80 | 11.79 ± 0.48 | |||
| Influence of pressure | ||||
| 20 | 1.0 | 40 | 20.0 | 10.68 ± 0.42 |
| 30.0 | 11.46 ± 0.44 | |||
| 40.0 | 11.72 ± 0.48 | |||
| 60.0 | 11.80 ± 0.47 | |||
| Rice Variety | RBO Yield wt.% | MeOH-RBO | Hex-RBO | |||
|---|---|---|---|---|---|---|
| TPC mg/kg | DPPH• mg/kg | ABTS− mg/kg | DPPH mg/kg | ABTS•+ mg/kg | ||
| J. Ariete | 11.72 ± 0.48 a | 5.71 ± 0.11a | 1.30 ± 0.06 a | 13.99 ± 0.20 a | 77.36 ± 2.39 a | 795.6 ± 6.6 a |
| J. Euro | 11.48 ± 0.46 ab | 3.24 ± 0.17 b | 0.84 ± 0.05 b | 9.41 ± 0.11 b | 59.83 ± 0.85 b | 484.2 ± 3.2 b |
| J. Opale | 13.00 ± 0.48 ac | 5.83 ± 0.19 ac | 1.18 ± 0.04 ac | 14.71 ± 0.10 c | 72.67 ± 1.11ac | 659.7 ± 5.3 c |
| I. Minima | 12.79 ± 0.51 abcd | 2.91 ± 0.18 b | 0.73 ± 0.05 b | 8.53 ± 0.15 d | 51.44 ±0.58 d | 442.7 ± 12.3 b |
| I. Ellebi | 14.46 ± 0.55 e | 7.48 ± 0.21 e | 1.77 ± 0.04 d | 18.89 ± 0.15 e | 54.30 ± 0.43 bd | 500.6 ± 9.8 bd |
| I. Sprint | 13.55 ± 0.56 cdef | 5.84 ± 0.18 acd | 1.47 ± 0.07 a | 15.26 ± 0.12 f | 74.41 ± 3.67 ac | 704.5 ± 20.4 c |
| I. Gladio | 13.11 ± 0.52 acdef | 5.10 ± 0.23 d | 1.14 ± 0.03 ac | 12.72 ± 0.19 g | 86.87 ± 4.03 e | 767.1 ± 46.8 a |
| I. Sirio | 15.60 ± 0.55 e | 8.90 ± 0.22 f | 1.77 ± 0.14 d | 21.34 ± 0.17 h | 62.51 ± 2.59 b | 538.8 ± 11.7 bd |
| p (ANOVA) | (p < 0.05) | (p < 0.01) | (p < 0.01) | (p < 0.01) | (p < 0.01) | (p < 0.01) |
| Fatty Acid (%) | J. Ariete | J. Euro | J. Opale | I. Minima | I. Ellebi | I. Sprint | I. Gladio | I. Sirio |
|---|---|---|---|---|---|---|---|---|
| C14:0 (Myristic) | 0.06 ± 0.01 | 0.12 ± 0.01 | 0.06 ± 0.01 | 0.07 ± 0.01 | 0.09 ± 0.01 | 0.11 ± 0.01 | 0.07 ± 0.01 | 0.09 ± 0.01 |
| C16:0 (Palmitic) | 15.39 ± 0.94 a | 14.63 ± 0.85 ab | 16.32 ± 0.95 abc | 13.37 ± 0.78 ab | 14.94 ± 0.83 abc | 14.16 ± 0.81 abc | 13.94 ± 0.81 ab | 13.76 ± 0.74 ab |
| C18:0 (Stearic) | 1.43 ± 0.08 a | 1.77 ± 0.10 b | 1.53 ± 0.09 abc | 2.01 ± 0.12 bd | 1.84 ± 0.10 bd | 2.11 ± 0.12 de | 1.93 ± 0.11 bde | 1.75 ± 0.09 bcd |
| C18:1 (Oleic) | 52.56 ± 3.15 ab | 47.47 ± 2.75 ab | 45.85 ± 2.60 ab | 49.63 ± 2.86 ab | 46.50 ± 2.55 ab | 49.85 ± 2.83 ab | 44.60 ± 2.54 b | 48.87 ± 2.61ab |
| C18:2 (Linoleic) | 29.90 ± 1.82 a | 35.32 ± 2.06 b | 35.50 ± 2.05 ab | 34.06 ± 1.99 ab | 35.83 ± 1.98 b | 32.86 ± 1.87 ab | 38.41 ± 2.22 b | 34.67 ± 1.87 ab |
| C20:0 (Arachidic) | 0.24 ± 0.01 | 0.33 ± 0.02 | 0.32 ± 0.02 | 0.39 ± 0.02 | 0.37 ± 0.02 | 0.45 ± 0.03 | 0.47 ± 0.03 | 0.39 ± 0.02 |
| C20:1 (Gadoleic) | 0.11 ± 0.01 | 0.18 ± 0.01 | 0.18 ± 0.01 | 0.18 ± 0.01 | 0.19 ± 0.01 | 0.18 ± 0.01 | 0.22 ± 0.01 | 0.19 ± 0.01 |
| C22:0 (Behenic) | 0.06 ± 0.01 | 0.06 ± 0.01 | 0.07 ± 0.01 | 0.07 ± 0.01 | 0.07 ± 0.01 | 0.08 ± 0.01 | 0.09 ± 0.01 | 0.04 ± 0.01 |
| C24:0 (Lignoceric) | 0.11 ± 0.01 | 0.11 ± 0.01 | 0.16 ± 0.01 | 0.17 ± 0.01 | 0.15 ± 0.01 | 0.17 ± 0.01 | 0.10 ± 0.01 | 0.16 ± 0.01 |
| J. Ariete | J. Euro | J. Opale | I.Minima | I. Ellebi | I. Sprint | I. Gladio | I. Sirio | |
|---|---|---|---|---|---|---|---|---|
| SFA | 17.29 ± 1.06 | 17.02 ± 1.00 | 18.46 ± 1.09 | 16.11 ± 0.95 | 17.48 ± 0.98 | 17.08 ± 0.99 | 16.63 ± 0.98 | 16.26 ± 0.88 |
| MUFA | 52.67 ± 3.16 | 47.95 ± 2.76 | 46.03 ± 2.61 | 49.81 ± 2.87 | 46.69 ± 2.56 | 50.03 ± 2.84 | 44.82 ± 2.55 | 49.06 ± 2.62 |
| DUFA | 29.9 ± 1.82 | 35.32 ± 2.06 | 35.5 ± 2.05 | 34.06 ± 1.99 | 35.83 ±1.98 | 32.86 ± 1.87 | 38.51 ± 2.22 | 34.67± 1.87 |
| UI | 1.125 ± 0.068 | 1.186 ± 0.069 | 1.170 ± 0.067 | 1.179 ± 0.069 | 1.184 ± 0.065 | 1.158 ± 0.066 | 1.218 ± 0.070 | 1.184 ± 0.064 |
| APE | 1.649 ± 0.099 | 1.662 ± 0.096 | 1.627 ± 0.093 | 1.674 ± 0.097 | 1.647 ± 0.091 | 1.654 ± 0.094 | 1.662 ± 0.095 | 1.671 ± 0.090 |
| BAPE | 0.299 ± 0.018 | 0.353 ± 0.021 | 0.355 ± 0.021 | 0.341 ± 0.020 | 0.358 ± 0.020 | 0.329 ± 0.019 | 0.385 ± 0.022 | 0.347 ± 0.019 |
| OX | 0.310 ± 0.019 | 0.363 ± 0.021 | 0.364 ± 0.021 | 0.351 ± 0.020 | 0.368 ± 0.020 | 0.339 ± 0.019 | 0.394 ± 0.023 | 0.356 ± 0.019 |
| OSI | 3.897 ± 0.001 | 3.894 ± 0.001 | 3.894 ± 0.001 | 3.895 ± 0.001 | 3.894 ± 0.001 | 3.895 ± 0.001 | 3.893 ± 0.001 | 3.894 ± 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinto, T.I.; Coelho, J.A.; Pires, B.I.; Neng, N.R.; Nogueira, J.M.; Bordado, J.C.; Sardinha, J.P. Supercritical Carbon Dioxide Extraction, Antioxidant Activity, and Fatty Acid Composition of Bran Oil from Rice Varieties Cultivated in Portugal. Separations 2021, 8, 115. https://doi.org/10.3390/separations8080115
Pinto TI, Coelho JA, Pires BI, Neng NR, Nogueira JM, Bordado JC, Sardinha JP. Supercritical Carbon Dioxide Extraction, Antioxidant Activity, and Fatty Acid Composition of Bran Oil from Rice Varieties Cultivated in Portugal. Separations. 2021; 8(8):115. https://doi.org/10.3390/separations8080115
Chicago/Turabian StylePinto, Tânia I., José A. Coelho, Bruna I. Pires, Nuno R. Neng, José M. Nogueira, João C. Bordado, and José P. Sardinha. 2021. "Supercritical Carbon Dioxide Extraction, Antioxidant Activity, and Fatty Acid Composition of Bran Oil from Rice Varieties Cultivated in Portugal" Separations 8, no. 8: 115. https://doi.org/10.3390/separations8080115
APA StylePinto, T. I., Coelho, J. A., Pires, B. I., Neng, N. R., Nogueira, J. M., Bordado, J. C., & Sardinha, J. P. (2021). Supercritical Carbon Dioxide Extraction, Antioxidant Activity, and Fatty Acid Composition of Bran Oil from Rice Varieties Cultivated in Portugal. Separations, 8(8), 115. https://doi.org/10.3390/separations8080115