Application of Concentrated Growth Factors Membrane for Human Umbilical Cord Wharton’s Jelly Mesenchymal Stem Cell Differentiation towards Keratinocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Concentrated Growth Factors Membrane
2.2. Culture of Human Umbilical Cord Wharton’s Jelly Mesenchymal Stem Cell Line and HaCaT Cell Line
2.3. In Vitro Culture of Human Umbilical Cord Wharton’s Jelly Mesenchymal Stem Cells or HaCaT Cells on CGF Membrane
2.4. In Vitro Culture of Human Umbilical Cord Wharton’s Jelly Mesenchymal Stem Cells on PPP Membrane
2.5. Histological Analysis
2.6. Immunohistochemical Analysis
2.7. Digital Slide Scanning Analysis
Statistical Evaluation
3. Results
 ) observed in an inverted microscope (Figure 1I), and a multi-layer epidermal-like tissue (
) observed in an inverted microscope (Figure 1I), and a multi-layer epidermal-like tissue ( ) formed on the surface of the concentrated growth factors membrane (Figure 1J). The thickness of the tissue ranged from 89.91 µm to 204.19 µm (mean ± SD = 144.36 µm ± 43.14 µm) (Figure 2A). We performed immunohistochemical staining on the sections of these multi-layer epidermal-like tissues and found that 79.8% ± 7.2% of the cells expressed the pancytokeratin marker (Figure 2B), 29.5% ± 9.4% of the cells expressed the P63 marker (Figure 2C), and 71.7% ± 3.9% of the cells expressed the vimentin marker (Figure 2D). In comparison, no labelled cells were found in the blank controls where a pancytokeratin, P63, or vimentin antibody was not added (Figure 2E). Under Masson trichome staining, thick dark-red fiber bands could be seen around the cell clusters in the tissue section. However, in other places where there was no cell clusters in the blood clot, pink was displayed (Figure 2F).
) formed on the surface of the concentrated growth factors membrane (Figure 1J). The thickness of the tissue ranged from 89.91 µm to 204.19 µm (mean ± SD = 144.36 µm ± 43.14 µm) (Figure 2A). We performed immunohistochemical staining on the sections of these multi-layer epidermal-like tissues and found that 79.8% ± 7.2% of the cells expressed the pancytokeratin marker (Figure 2B), 29.5% ± 9.4% of the cells expressed the P63 marker (Figure 2C), and 71.7% ± 3.9% of the cells expressed the vimentin marker (Figure 2D). In comparison, no labelled cells were found in the blank controls where a pancytokeratin, P63, or vimentin antibody was not added (Figure 2E). Under Masson trichome staining, thick dark-red fiber bands could be seen around the cell clusters in the tissue section. However, in other places where there was no cell clusters in the blood clot, pink was displayed (Figure 2F).4. Discussion
- (1)
- The 3D fibrin mesh in the concentrated growth factors membrane is suitable for the attachment and growth of mesenchymal stem cells;
- (2)
- The concentrated growth factors membrane contains various growth factors and cytokines [4,5,6]. Using the same Medifuge centrifugation system (Silfradent, S.R,L, Santa Sofia, Italy) as ours, the growth factors and proinflammatory cytokines contents in concentrated growth factors (CGF) had been reported in detail [6]. It was reported that growth factors (such as epidermal growth factors, fibroblast growth factors, insulin-like growth factors, and vascular endothelial growth factor), and cytokine (such as interleukin 1, 4, and 6) might play important roles in tissue regeneration and wound healing [24]. It was also reported that some cytokines could extensively affect mesenchymal stem cell function [25,26];
- (3)
- Therefore, it is proposed that the related growth factors and cytokines contained in CGF membrane play an important role in promoting mesenchymal stem cells differentiating toward keratinocytes;
- (4)
- It can maintain the roof surface of the concentrated growth factors membrane where mesenchymal stem cells attach and grow on the air–fluid surface. When cultured in vitro for 2 weeks, the physical environment change can be used to promote the proliferation and overlap of keratinocyte-like cells into a multi-layer epidermal-like tissue;
- (5)
- Under Masson trichome staining, the tissue section of the UMST–CGF membrane showed a small amount of thicker red fibers in the fibrin clot near the cell clusters (Figure 2F). It is speculated that mesenchymal stem cells and fibroblast are the same, and fibroblasts are in fact aged mesenchymal stem cells [27]. After attaching to the concentrated growth factors membrane, the mesenchymal stem cells may not only proliferate to form cell clusters, but also secrete extracellular matrix-like or collagen-like substances, which then fill on the original fibrin mesh to form a new dermis-like structure [28]. This change may stop the degradation of the CGF gel and remodel it to make it more suitable for mesenchymal stem cells to proliferate, differentiate towards keratinocytes and form an epidermal-like tissue. However, the quality and quantity of these substances released by mesenchymal stem cells are still subject to further research in the future;
- (6)
- In terms of application in regenerative medicine, compared with previous literature reports on the method of transforming mesenchymal stem cells into keratinocyte-like cells by culture, the CGF membrane is a fully autologous fibrin mesh, growth factors, and cytokines, rather than heterologous [23] or artificial [22]. Of course, even in the same healthy adult, the composition of the CGF membrane will be different under different physiological conditions. However, our own CGF membrane is unique and it can avoid the problem of external infection or rejection, while the materials (autologous venous blood) are easy to obtain, the production process is simple and the cost is low.
- (1)
- To understand the role of concentrated growth factors on different stem cells, such as bone marrow stem cells, adipose stem cells, or hair follicle stem cells;
- (2)
- To develop efficient qualitative and quantitative methods to measure various effective ingredients in concentrated growth factors [29], and further explore the effects of its various growth factors (such as epidermal growth factor, transforming growth factor β, fibroblast growth factor, vascular endothelial growth factors, platelet-derived growth factors, and insulin-like growth factor) and cytokines (such as interleukin 1, 2, 4, 6) on promoting the differentiation of mesenchymal stem cells towards keratinocytes. Whether the promotion of stem cell differentiation towards keratinocytes was a result of a single component or multiple components remains to be determined;
- (3)
- To develop a method that effectively preserves a large amount of concentrated growth factors membrane for large-scale use, such as large-scale trauma or burn;
- (4)
- Together with different types of autologous stem cells, we can perform in vitro tissue culture under different environments, providing different autologous tissue, or using the same type of autologous stem cells, but gradually changing to different in vitro tissue environments and gradually transforming the same type of autologous stem cells into tissues in which cell groups of different characteristics coexist.
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Borie, E.; Oliví, D.G.; Orsi, I.A.; Garlet, K. Platelet-rich fibrin application in dentistry: A litera-ture review. Int. J. Clin. Exp. Med. 2015, 8, 7922–7929. [Google Scholar]
- Bonazza, V.; Borsani, E.; Buffoli, B.; Castrezzati, S.; Rezzani, R.; Rodella, L.F. How the different material and shape of the blood collection tube influences the Concentrated Growth Factors production. Microsc. Res. Tech. 2016, 79, 1173–1178. [Google Scholar] [CrossRef]
- Pirpir, C.; Yilmaz, O.; Candirli, C.; Balaban, E. Evaluation of effectiveness of concentrated growth factor on osseointegration. Int. J. Implant. Dent. 2017, 3, 1–6. [Google Scholar] [CrossRef]
- Amable, P.R.; Carias, R.B.V.; Teixeira, M.V.T.; Pacheco Ítalo, D.C.; Amaral, R.J.F.C.D.; Granjeiro, J.M.; Borojevic, R. Platelet-rich plasma preparation for regenerative medicine: Optimization and quantification of cytokines and growth factors. Stem. Cell Res. Ther. 2013, 4, 67. [Google Scholar] [CrossRef]
- Yu, M.; Wang, X.; Liu, Y.; Qiao, J. Cytokine release kinetics of concentrated growth factors in different scaffolds. Clin. Oral Investig. 2018, 23, 1663–1671. [Google Scholar] [CrossRef]
- Masuki, H.; Okudera, T.; Watanebe, T.; Suzuki, M.; Nishiyama, K.; Okudera, H.; Nakata, K.; Uematsu, K.; Su, C.-Y.; Kawase, T. Growth factor and pro-inflammatory cytokine contents in platelet-rich plasma (PRP), plasma rich in growth factors (PRGF), advanced platelet-rich fibrin (A-PRF), and concentrated growth factors (CGF). Int. J. Implant. Dent. 2016, 2, 1–6. [Google Scholar] [CrossRef]
- Verma, R.; Negi, G.; Kandwal, A.; Chandra, H.; Gaur, D.S.; Harsh, M. Effect of autologous PRP on wound healing in dental regenerative surgeries and its correlation with PDGF levels. Asian J. Transfus. Sci. 2019, 13, 47–53. [Google Scholar] [CrossRef]
- Tukaram, K.; Shirsagar, J.; Rubine, S. Innovation in regeneration–Concentrated growth factor. Int. J. Appl. Dent. Sci. 2017, 3, 206–208. [Google Scholar]
- Zhang, M.; Park, G.; Zhou, B.; Luo, D. Applications and efficacy of platelet-rich plasma in dermatology: A clinical review. J. Cosmet. Dermatol. 2018, 17, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Kao, C. Use of concentrate growth factors gel or membrane in chronic wound healing: Description of 18 cases. Int. Wound J. 2019, 17, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Serra, R.; Buffone, G.; Dominijanni, A.; Molinari, V. Application of platelet-rich gel to enhance healing of trans metatarsal amputations in diabetic dysvascular patients. Int. Wound J. 2013, 10, 612–615. [Google Scholar] [CrossRef] [PubMed]
- Houdek, M.T.; Wyles, C.C.; Stalboerger, P.G.; Terzic, A.; Behfar, A.; Moran, S.L. Collagen and Fractionated Platelet-Rich Plasma Scaffold for Dermal Regeneration. Plast. Reconstr. Surg. 2016, 137, 1498–1506. [Google Scholar] [CrossRef] [PubMed]
- Barbon, S.; Stocco, E.; Macchi, V.; Contran, M.; Grandi, F.; Borean, A.; Parnigotto, P.P.; Porzionato, A.; De Caro, R. Platelet-Rich Fibrin Scaffolds for Cartilage and Tendon Regenerative Medicine: From Bench to Bedside. Int. J. Mol. Sci. 2019, 20, 1701. [Google Scholar] [CrossRef]
- Llames, S.; Del Rio, M.; Larcher, F.; García, E.; García, M.; Escámez, M.J.; Jorcano, J.L.; Holguín, P.; Meana, A. Human plasma as a dermal scaffold for the generation of a completely autologous bioengineered skin. Transplant 2004, 77, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.A.; Dare, E.V.; Hincke, M. Fibrin: A versatile scaffold for tissue engineering applications. Tissue Eng. Part B Rev. 2008, 14, 199–215. [Google Scholar] [CrossRef]
- Santos, J.F.D.; Borcari, N.R.; Araujo, M.D.S.; Numes, V.A. Mesenchymal stem cells differentiate into keratinocytes and express epidermal kallikrelins: Towards an in vitro model of human epidermis. J. Cell. Biochem. 2019, 120, 13141–13155. [Google Scholar]
- Martin-Piedra, M.A.; Alfonso-Rodriguez, C.A.; Zapater, A.; Durand-Herrera, D. Effective use of mesenchymal stem cells in human skin substitutes generated by tissue engineering. Eur. Cell Mater. 2019, 37, 233–249. [Google Scholar] [CrossRef]
- Garzón, I.; Alfonso-Rodríguez, C.; Martínez-Gómez, C.; Carriel, V.; Martin-Piedra, M.; Fernández-Valadés, R.; Sanchez-Quevedo, M.; Alaminos, M. Expression of epithelial markers by human umbilical cord stem cells. A topographical analysis. Placenta 2014, 35, 994–1000. [Google Scholar] [CrossRef]
- Azmi, S.M.; Salih, M.; Abdelrazeg, S.; Roslan, F.F.; Mohamed, R.; Jie, T.J.; Shaharuddin, B. Human umbilical cord-mesenchymal stem cells: A promising strategy for corneal epithelial regeneration. Regen. Med. 2020, 15, 1381–1397. [Google Scholar] [CrossRef] [PubMed]
- Maia, F.R.; Lourenço, A.H.; Granja, P.L.; Gonçalves, R.M.; Barrias, C.C.; Maia, R. Effect of Cell Density on Mesenchymal Stem Cells Aggregation in RGD-Alginate 3D Matrices under Osteoinductive Conditions. Macromol. Biosci. 2014, 14, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Fatima, Q.; Choudhry, N.; Choudhery, M.S. Umbilical Cord Tissue Derived Mesenchymal Stem Cells Can Differentiate into Skin Cells. Open Life Sci. 2018, 13, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Garzon, I.; Martin-Piedra, M.A.; Alfonso-Rodriguez, C.; Gonzalez-Andrades, M. Generation of Biomimetic Human Artificial Cornea Model Using Wharton’s Jelly Mesenchymal Stem Cells. Cornea 2014, 5517, 4073–4083. [Google Scholar] [CrossRef]
- Salah, R.A.; Mohamed, I.K.; EI-Badin, N. Development of decellularized amniotic membrane as a bioscaffold for bone marrow-derived mesenchymal stem cells: Ultrastructural study. J. Mol. Histol. 2018, 49, 289–301. [Google Scholar] [CrossRef]
- Verma, U.P.; Yadav Rk, D.; Gupta, A. Platelet-rich Fibrin: A Paradigm in Periodontal Therapy—A Systemic Review. J. Int. Soc. Prev. Community Dent. 2017, 7, 227–233. [Google Scholar]
- Selich, A.; Ha, T.C.H.; Morgan, M.; Falk, C.S. Cytokine Selection of MSC clones with Different Functionality. Stem. Cell Rep. 2019, 13, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, W.; Sakaguchi, K.; Kawai, T.; Wakayama, Y.; Osugi, M.; Hibi, H. A defined mix of cytokines mimics conditioned medium from cultures of bone marrow-derived mesenchymal stem cells and elicits bone regeneration. Cell Prolif. 2017, 50, e12333. [Google Scholar] [CrossRef]
- Soundararajam, M.; Kannan, S. Fibroblasts and mesenchymal stem cells: Two sides of the same coin? J. Cell Physiol. 2018, 233, 9099–9109. [Google Scholar] [CrossRef]
- Assis-Ribas, T.; Fernanda-Forri, M.; Winnischofer, S.M.B.; Sogayar, M.C. Extracellular matrix dynamics during mesenchymal stem cell differentiation. Dev. Biol. 2018, 437, 63–74. [Google Scholar] [CrossRef]
- Astori, G.; Arrati, E.; Bambi, F.; Benardi, M. Platelet lysate as a substitute for animal serum for the ex-vivo explanation of mesenchymal stem/stromal cells: Present and future. Stem. Cell Res. Ther. 2016, 7, 93. [Google Scholar] [CrossRef] [PubMed]
 ). (H): photograph of umbilical cord mesenchymal stem cells and CGF membrane cultured for 4 weeks (
). (H): photograph of umbilical cord mesenchymal stem cells and CGF membrane cultured for 4 weeks ( ). (I): at the end of the second culture stage, the cells proliferated on the roof of CGF membrane and appeared as cuboid shape cells (
). (I): at the end of the second culture stage, the cells proliferated on the roof of CGF membrane and appeared as cuboid shape cells ( ) observed in inverted microscope. (J): Multi-layered epidermal-like cells in the roof of a tissue section of umbilical cord mesenchymal stem cells and CGF membrane (
) observed in inverted microscope. (J): Multi-layered epidermal-like cells in the roof of a tissue section of umbilical cord mesenchymal stem cells and CGF membrane ( ) cultured for 4 weeks. Hematoxylin–eosin staining. (K): at the end of the second culture stage, multiple spindle-shaped cells distributed in the whole tissue sections (
) cultured for 4 weeks. Hematoxylin–eosin staining. (K): at the end of the second culture stage, multiple spindle-shaped cells distributed in the whole tissue sections ( ). Abbreviations: CGF—concentrated growth factors; H&E staining—Hematoxylin–eosin staining; PPP—platelet-poor plasma; and UMST—umbilical cord mesenchymal stem cells.
). Abbreviations: CGF—concentrated growth factors; H&E staining—Hematoxylin–eosin staining; PPP—platelet-poor plasma; and UMST—umbilical cord mesenchymal stem cells.
 ). (H): photograph of umbilical cord mesenchymal stem cells and CGF membrane cultured for 4 weeks (
). (H): photograph of umbilical cord mesenchymal stem cells and CGF membrane cultured for 4 weeks ( ). (I): at the end of the second culture stage, the cells proliferated on the roof of CGF membrane and appeared as cuboid shape cells (
). (I): at the end of the second culture stage, the cells proliferated on the roof of CGF membrane and appeared as cuboid shape cells ( ) observed in inverted microscope. (J): Multi-layered epidermal-like cells in the roof of a tissue section of umbilical cord mesenchymal stem cells and CGF membrane (
) observed in inverted microscope. (J): Multi-layered epidermal-like cells in the roof of a tissue section of umbilical cord mesenchymal stem cells and CGF membrane ( ) cultured for 4 weeks. Hematoxylin–eosin staining. (K): at the end of the second culture stage, multiple spindle-shaped cells distributed in the whole tissue sections (
) cultured for 4 weeks. Hematoxylin–eosin staining. (K): at the end of the second culture stage, multiple spindle-shaped cells distributed in the whole tissue sections ( ). Abbreviations: CGF—concentrated growth factors; H&E staining—Hematoxylin–eosin staining; PPP—platelet-poor plasma; and UMST—umbilical cord mesenchymal stem cells.
). Abbreviations: CGF—concentrated growth factors; H&E staining—Hematoxylin–eosin staining; PPP—platelet-poor plasma; and UMST—umbilical cord mesenchymal stem cells.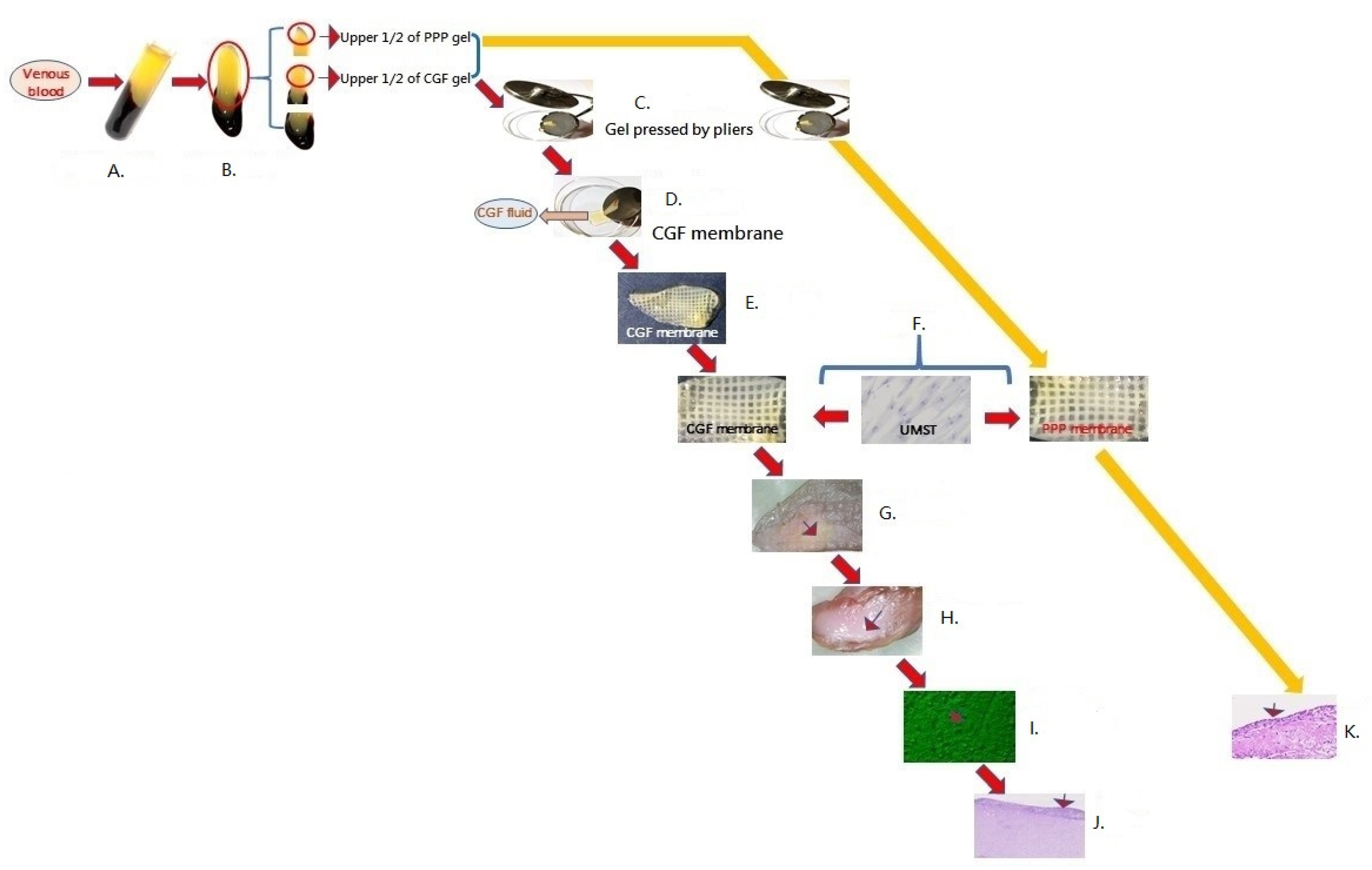
 ) formed on the surface of the concentrated growth factors membrane. The maximal thickness was 144.36 µm ± 43.14 µm (mean ± SD) (
) formed on the surface of the concentrated growth factors membrane. The maximal thickness was 144.36 µm ± 43.14 µm (mean ± SD) ( ). (B) The tissue sections of multi-layer epidermal-like tissue further stained for pancytokeratin. Most cells were positive for pancytokeratin marker (
). (B) The tissue sections of multi-layer epidermal-like tissue further stained for pancytokeratin. Most cells were positive for pancytokeratin marker ( ), and only a few cells were negative (
), and only a few cells were negative ( ). (C) The tissue sections of multi-layer epidermal-like tissue further stained for P63. Some cells were P63-positive (
). (C) The tissue sections of multi-layer epidermal-like tissue further stained for P63. Some cells were P63-positive ( ), while most cells were negative (
), while most cells were negative ( ). (D) The tissue sections of multi-layer epidermal-like tissue further stained for vimentin. A few cells were vimentin-positive (
). (D) The tissue sections of multi-layer epidermal-like tissue further stained for vimentin. A few cells were vimentin-positive ( ), while few cells were negative (
), while few cells were negative ( ). (E) The blank controls of the multi-layer epidermal-like tissue were used for immunohistochemical staining. We found that the cell were pancytokeratin-negative, P63-negative, and vimentin-negative (
). (E) The blank controls of the multi-layer epidermal-like tissue were used for immunohistochemical staining. We found that the cell were pancytokeratin-negative, P63-negative, and vimentin-negative ( ). (F) under Masson trichome stain, thick dark-red fiber bands could be seen around the cell clusters in the tissue section (
). (F) under Masson trichome stain, thick dark-red fiber bands could be seen around the cell clusters in the tissue section ( ), while other fibrin clots without cell clusters were pink (
), while other fibrin clots without cell clusters were pink ( ).
).
 ) formed on the surface of the concentrated growth factors membrane. The maximal thickness was 144.36 µm ± 43.14 µm (mean ± SD) (
) formed on the surface of the concentrated growth factors membrane. The maximal thickness was 144.36 µm ± 43.14 µm (mean ± SD) ( ). (B) The tissue sections of multi-layer epidermal-like tissue further stained for pancytokeratin. Most cells were positive for pancytokeratin marker (
). (B) The tissue sections of multi-layer epidermal-like tissue further stained for pancytokeratin. Most cells were positive for pancytokeratin marker ( ), and only a few cells were negative (
), and only a few cells were negative ( ). (C) The tissue sections of multi-layer epidermal-like tissue further stained for P63. Some cells were P63-positive (
). (C) The tissue sections of multi-layer epidermal-like tissue further stained for P63. Some cells were P63-positive ( ), while most cells were negative (
), while most cells were negative ( ). (D) The tissue sections of multi-layer epidermal-like tissue further stained for vimentin. A few cells were vimentin-positive (
). (D) The tissue sections of multi-layer epidermal-like tissue further stained for vimentin. A few cells were vimentin-positive ( ), while few cells were negative (
), while few cells were negative ( ). (E) The blank controls of the multi-layer epidermal-like tissue were used for immunohistochemical staining. We found that the cell were pancytokeratin-negative, P63-negative, and vimentin-negative (
). (E) The blank controls of the multi-layer epidermal-like tissue were used for immunohistochemical staining. We found that the cell were pancytokeratin-negative, P63-negative, and vimentin-negative ( ). (F) under Masson trichome stain, thick dark-red fiber bands could be seen around the cell clusters in the tissue section (
). (F) under Masson trichome stain, thick dark-red fiber bands could be seen around the cell clusters in the tissue section ( ), while other fibrin clots without cell clusters were pink (
), while other fibrin clots without cell clusters were pink ( ).
).
 ) formed on the surface of the concentrated growth factors membrane. The maximal thickness was 74.05 µm ± 13.44 µm (mean ± SD) (
) formed on the surface of the concentrated growth factors membrane. The maximal thickness was 74.05 µm ± 13.44 µm (mean ± SD) ( ). (B) The tissue sections of multi-layer tissue further stained for pancytokeratin. Most cells were positive for pancytokeratin marker (
). (B) The tissue sections of multi-layer tissue further stained for pancytokeratin. Most cells were positive for pancytokeratin marker ( ), and only a few cells were negative (
), and only a few cells were negative ( ). (C) The tissue sections of multi-layer tissue further stained for P63. A few cells were P63-positive (
). (C) The tissue sections of multi-layer tissue further stained for P63. A few cells were P63-positive ( ), while few cells were negative (
), while few cells were negative ( ). (D) The tissue sections of multi-layer tissue further stained for vimentin. A few cells were vimentin-positive (
). (D) The tissue sections of multi-layer tissue further stained for vimentin. A few cells were vimentin-positive ( ), while few cells were negative (
), while few cells were negative ( ). (E) The blank controls of the multi-layer epidermal-like tissue were used for immunohistochemical staining. We found that the cell were pancytokeratin-negative, P63-negative, and vimentin-negative (
). (E) The blank controls of the multi-layer epidermal-like tissue were used for immunohistochemical staining. We found that the cell were pancytokeratin-negative, P63-negative, and vimentin-negative ( ). (F) Under Masson trichome satin, thick blue fiber bands could be seen around the cell clusters in the tissue section (
). (F) Under Masson trichome satin, thick blue fiber bands could be seen around the cell clusters in the tissue section ( ), while other fibrin clots without cell clusters were red (
), while other fibrin clots without cell clusters were red ( ). The maximal thickness of the blue band in tissue sections of each sample ranged from 169.92 µm to 41.11 µm (mean ± SD = 96.48 µm ± 44.7 µm). Abbreviations: H&E staining—hematoxylin–eosin staining; and IHC—immunohistochemical staining.
). The maximal thickness of the blue band in tissue sections of each sample ranged from 169.92 µm to 41.11 µm (mean ± SD = 96.48 µm ± 44.7 µm). Abbreviations: H&E staining—hematoxylin–eosin staining; and IHC—immunohistochemical staining.
 ) formed on the surface of the concentrated growth factors membrane. The maximal thickness was 74.05 µm ± 13.44 µm (mean ± SD) (
) formed on the surface of the concentrated growth factors membrane. The maximal thickness was 74.05 µm ± 13.44 µm (mean ± SD) ( ). (B) The tissue sections of multi-layer tissue further stained for pancytokeratin. Most cells were positive for pancytokeratin marker (
). (B) The tissue sections of multi-layer tissue further stained for pancytokeratin. Most cells were positive for pancytokeratin marker ( ), and only a few cells were negative (
), and only a few cells were negative ( ). (C) The tissue sections of multi-layer tissue further stained for P63. A few cells were P63-positive (
). (C) The tissue sections of multi-layer tissue further stained for P63. A few cells were P63-positive ( ), while few cells were negative (
), while few cells were negative ( ). (D) The tissue sections of multi-layer tissue further stained for vimentin. A few cells were vimentin-positive (
). (D) The tissue sections of multi-layer tissue further stained for vimentin. A few cells were vimentin-positive ( ), while few cells were negative (
), while few cells were negative ( ). (E) The blank controls of the multi-layer epidermal-like tissue were used for immunohistochemical staining. We found that the cell were pancytokeratin-negative, P63-negative, and vimentin-negative (
). (E) The blank controls of the multi-layer epidermal-like tissue were used for immunohistochemical staining. We found that the cell were pancytokeratin-negative, P63-negative, and vimentin-negative ( ). (F) Under Masson trichome satin, thick blue fiber bands could be seen around the cell clusters in the tissue section (
). (F) Under Masson trichome satin, thick blue fiber bands could be seen around the cell clusters in the tissue section ( ), while other fibrin clots without cell clusters were red (
), while other fibrin clots without cell clusters were red ( ). The maximal thickness of the blue band in tissue sections of each sample ranged from 169.92 µm to 41.11 µm (mean ± SD = 96.48 µm ± 44.7 µm). Abbreviations: H&E staining—hematoxylin–eosin staining; and IHC—immunohistochemical staining.
). The maximal thickness of the blue band in tissue sections of each sample ranged from 169.92 µm to 41.11 µm (mean ± SD = 96.48 µm ± 44.7 µm). Abbreviations: H&E staining—hematoxylin–eosin staining; and IHC—immunohistochemical staining.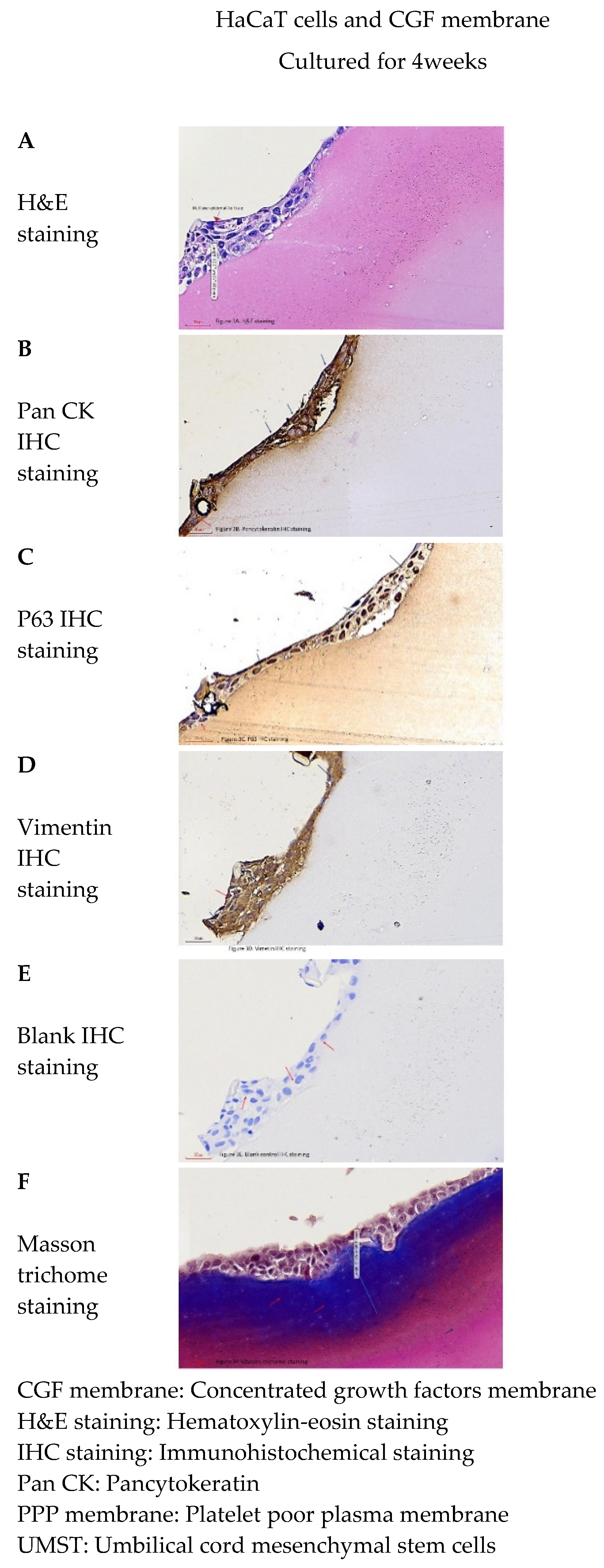
 ) were distributed in whole-tissue sections of platelet-poor plasma membrane. (B) The tissue sections further stained for pancytokeratin. Only few cells were positive for pancytokeratin marker (
) were distributed in whole-tissue sections of platelet-poor plasma membrane. (B) The tissue sections further stained for pancytokeratin. Only few cells were positive for pancytokeratin marker ( ), and most cells were negative (
), and most cells were negative ( ). (C) The tissue sections further stained for P63. Few cells were P63-positive (
). (C) The tissue sections further stained for P63. Few cells were P63-positive ( ), while most cells were negative(
), while most cells were negative( ). (D) The tissue sections further stained for vimentin. Most cells were vimentin-positive (
). (D) The tissue sections further stained for vimentin. Most cells were vimentin-positive ( ), while only few cells were negative (
), while only few cells were negative ( ). (E) The blank controls of the multi-layer epidermal-like tissue were used for immunohistochemical staining. We found that the cells were pancytokeratin-negative, P63-negative, and vimentin-negative (
). (E) The blank controls of the multi-layer epidermal-like tissue were used for immunohistochemical staining. We found that the cells were pancytokeratin-negative, P63-negative, and vimentin-negative ( ). (F) Under Masson trichome stain, multiple thin blue fiber bands could be seen around the cell clusters in the tissue section (
). (F) Under Masson trichome stain, multiple thin blue fiber bands could be seen around the cell clusters in the tissue section ( ). Abbreviations: H&E staining—hematoxylin–eosin staining; and IHC staining—immunohistochemical staining.
). Abbreviations: H&E staining—hematoxylin–eosin staining; and IHC staining—immunohistochemical staining.
 ) were distributed in whole-tissue sections of platelet-poor plasma membrane. (B) The tissue sections further stained for pancytokeratin. Only few cells were positive for pancytokeratin marker (
) were distributed in whole-tissue sections of platelet-poor plasma membrane. (B) The tissue sections further stained for pancytokeratin. Only few cells were positive for pancytokeratin marker ( ), and most cells were negative (
), and most cells were negative ( ). (C) The tissue sections further stained for P63. Few cells were P63-positive (
). (C) The tissue sections further stained for P63. Few cells were P63-positive ( ), while most cells were negative(
), while most cells were negative( ). (D) The tissue sections further stained for vimentin. Most cells were vimentin-positive (
). (D) The tissue sections further stained for vimentin. Most cells were vimentin-positive ( ), while only few cells were negative (
), while only few cells were negative ( ). (E) The blank controls of the multi-layer epidermal-like tissue were used for immunohistochemical staining. We found that the cells were pancytokeratin-negative, P63-negative, and vimentin-negative (
). (E) The blank controls of the multi-layer epidermal-like tissue were used for immunohistochemical staining. We found that the cells were pancytokeratin-negative, P63-negative, and vimentin-negative ( ). (F) Under Masson trichome stain, multiple thin blue fiber bands could be seen around the cell clusters in the tissue section (
). (F) Under Masson trichome stain, multiple thin blue fiber bands could be seen around the cell clusters in the tissue section ( ). Abbreviations: H&E staining—hematoxylin–eosin staining; and IHC staining—immunohistochemical staining.
). Abbreviations: H&E staining—hematoxylin–eosin staining; and IHC staining—immunohistochemical staining.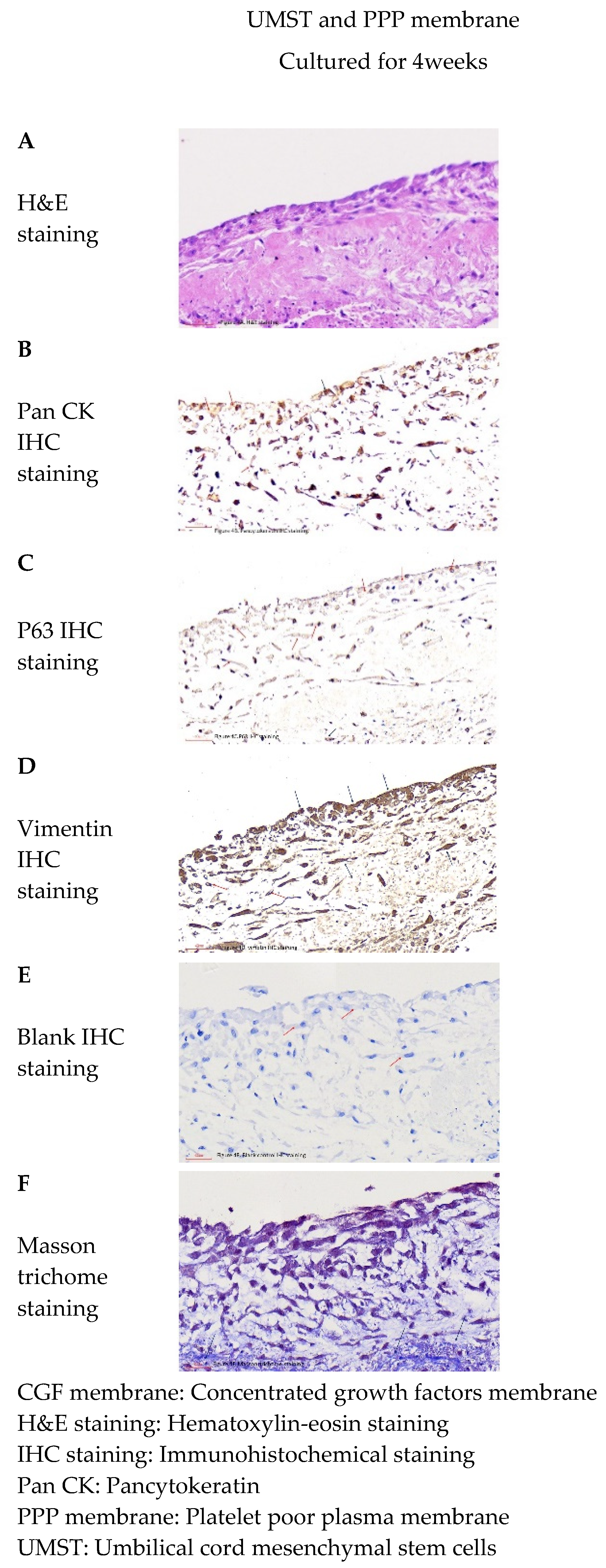
| Comparsion group 1 percentage of cells expressing marker (Data= Mean ± SD, N: Number of samples) | ||||
| cell marker | UMST + CGF | USMT + PPP | t value | p value |
| membrane group | membrane group | |||
| pancytokeratin | 79.8% ± 7.2%, N = 6 | 9.7% ± 2.4% | 12.3 | <0.05 |
| p-63 | 29.5% ± 9.4%, N = 6 | 7.45% ± 1.9% | 6.78 | <0.05 |
| vimentin | 71.7% ± 3.9%, N = 6 | 95.9% ± 3.7% | 6.4 | <0.05 |
| Comparsion group 2 percentage of cells expressing marker (Data= Mean ± SD, N: Number of samples) | ||||
| cell marker | UMST + CGF membrane group | HaCaT cells + CGF membrane group | t value | p value |
| pancytokeratin | 79.8% ± 7.2%, N = 6 | 88.1% ± 4.9%, N = 6 | 0.35 | >0.05 |
| p-63 | 29.5% ± 9.4%, N = 6 | 63.6% ± 11.4%, N = 6 | 1.19 | >0.05 |
| vimentin | 71.7% ± 3.9%, N = 6 | 79% ± 9.9%, N = 6 | 0.17 | >0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kao, C.-H. Application of Concentrated Growth Factors Membrane for Human Umbilical Cord Wharton’s Jelly Mesenchymal Stem Cell Differentiation towards Keratinocytes. Separations 2021, 8, 61. https://doi.org/10.3390/separations8050061
Kao C-H. Application of Concentrated Growth Factors Membrane for Human Umbilical Cord Wharton’s Jelly Mesenchymal Stem Cell Differentiation towards Keratinocytes. Separations. 2021; 8(5):61. https://doi.org/10.3390/separations8050061
Chicago/Turabian StyleKao, Chao-Hsing. 2021. "Application of Concentrated Growth Factors Membrane for Human Umbilical Cord Wharton’s Jelly Mesenchymal Stem Cell Differentiation towards Keratinocytes" Separations 8, no. 5: 61. https://doi.org/10.3390/separations8050061
APA StyleKao, C.-H. (2021). Application of Concentrated Growth Factors Membrane for Human Umbilical Cord Wharton’s Jelly Mesenchymal Stem Cell Differentiation towards Keratinocytes. Separations, 8(5), 61. https://doi.org/10.3390/separations8050061





