1. Introduction
Many different stationary phases or supports have been used for sample preparation and chromatographic applications. In the field of HPLC, silica-based materials are prevailing; in FPLC, dextran- or agarose-based materials seem to be used most frequently. For affinity separations [
1,
2], crosslinked dextrans, agaroses [
3], celluloses, silica materials, acrylic, and epoxide polymers [
4], porous glass, and many others can be used, all with their pros and cons [
5,
6]. Also, cryogels, hydrogels, and hybrid materials have been suggested [
7]. The emergence of monolithic materials was considered a major step for more advanced stationary phases [
8,
9,
10,
11,
12]. Their usefulness for affinity separations was acknowledged, and the main solid supports were discussed in a review [
13]. Although some commercial products are available [
14], they could not gain a large market share yet. Some of their drawbacks seem to be their difficult manufacturing and the occurrence of radial pore gradients. Their low back-pressure was acknowledged as an advantage. However, their overall performance was not superior to conventional materials, particularly compared to modern UHPLC materials with small particles or core-shell structures. For affinity separations, soft materials in the form of beads are most common, which require quite low flow rates and very limited pressure. This also requires relatively large bead diameters, which causes peak broadening and hence slow kinetics. Also, most materials are not suitable for high-resolution separations and are not compatible with high-pressure systems, such as HPLC or UHPLC systems. Therefore, we aimed to develop a stationary phase mainly for affinity applications [
15,
16], which would overcome these limitations. First, we tried to use porous glass [
17] for this purpose, which turned out to be an excellent material for affinity separations. However, the problematic commercial availability and high cost motivated us to look for alternatives. Among other materials, we tested sintered borosilicate glass (
Figure 1), which is a proven material for filtration (fritted glass) and other purposes for many decades. These products are based on cheap borosilicate glass powder and are produced commercially in high volumes. Hence, the price of such materials is quite low.
Another advantage is the nearly complete lack of spatial inhomogeneity due to the bulk preparation of the semi-finished sintered plates, from which the monolithic borosilicate cylinders are drilled with a diamond tool. Here, we show the preparation of affinity columns in a step-by-step protocol, their respective holders, and their coating with biochemical ligands, such as protein A and the application for the analytical determination of IgG and the preparative isolation of IgG from human plasma (see
Supplementary Material). The same columns were used both in low-pressure (FPLC) and high-pressure (HPLC) systems. Reproducibility and other characteristic parameters were determined. High-speed separations down to 60 s could be shown, and 100 regeneration cycles have been performed without significant activity losses. Extrapolated from these data, the system might be useful for several thousand separation cycles. These columns can be used for semipreparative purposes, such as the fast and automated purification of some milligrams of an antibody, or for analytical purposes to quantify IgG precisely in a sample or lab-scale product.
2. Materials and Methods
2.1. Biochemicals
LyoPlas N-w (lyophilized human plasma) was obtained from Deutsches Rotes Kreuz (Essen, Germany). Recombinant protein A was obtained from ProSpec (Staphylococcal Protein-A 41 kDa, pro-774). Adalimumab (Humira obtained from Evidentic, Berlin, Germany, 100 mg/mL); Albumin from Bovine Serum (BSA, Sigma-Aldrich, Taufkichen, Germany, A7906-100G); Bevacizumab (Avastin obtained from Evidentic, 25 mg/mL); Serva Triple Color Protein standard III (Serva, Heidelberg, Germany, 39258.01). Buffers: PBS (10× powder, from AppliChem, Darmstadt, Germany, A0965,9010); binding buffer: Na2HPO4 · 2 H2O (8.7 mM), NaH2PO4 · 2 H2O (3.3 mM), pH 7.4; elution buffer: NaH2PO4 · 2 H2O (6.0 mM), H3PO4 (6.0 mM), pH 2.3; 4x loading buffer for SDS-PAGE (non-reducing): glycerol (40%), Tris-Base (1 M, pH 6.8) (25%), sodium dodecyl sulfate SDS (8%), bromophenol blue (0.02%); SDS running buffer (10×): tris-base (250 mM), glycine (1.92 M), SDS (1%); Tris-HCl buffer (Tris-base, 100 mM, pH 6.0 with HCl, Promega, Mannheim, Germany H5131). Solvents: Ethanol (absolute for HPLC, LabsoluteBerlin, Germany, 2222).
2.2. Other Reagents
EDTA disodium salt 2-hydrate (AppliChem, 131669.1209); formaldehyde (Sigma, 252549-25ML); (3-glycidoxypropyl)methyldimethoxysilane (Sigma-Aldrich, 539252, CAS 65799-47-5); (3-glycidyloxypropyl)trimethoxysilane (Sigma-Aldrich, 440167, CAS 2530-83-8); hydrochloric acid (ChemSolute, Berlin, Germany, 857,1011); Mucasol® (Brand, Wertheim, Germany, 230091); potassium hydroxide (AppliChem, A1575,1000); silver nitrate (Carl Roth, Karlsruhe, Germany, 9370.4); sodium carbonate (AppliChem, AP141648.1211); sodium chloride (Acros Organics, Geel, Belgium, 446212500); sodium cyanoborohydride (Sigma-Aldrich, 156159); sodium hydroxide (Sigma-Aldrich, 30620-1KG-R); sodium periodate (Sigma-Aldrich, S1878, CAS 7790-28-5); sodium thiosulfate (Roth, HN25.1); sulfuric acid (AppliChem, A0655,1000GL); Tween 20 (Serva, 37470.01). The water used was purified by an Milli-Q Reference A+ Ultra-Pure Water System from Merck KGaA (Darmstadt, Germany), with a resistivity of 18.2 MΩ·cm. Columns: For comparison, a column HiTrap rProtein A FF, 1 mL (Cytiva, Freiburg, Germany, 29-0485-81) was used.
2.3. Preparation of the Monolithic Raw Column
The sintered borosilicate cylinders were obtained from ROBU Glasfilter-Geräte GmbH (Hattert, Germany). They are based on glass filters of the porosity classes Por. 5 (P 1.6, Ultrafine, pore size 1–1.6 µm) or Por. 4 (P 16, Medium, pore size 10–16 µm) made of borosilicate glass 3.3. These classes are defined in ISO 4793-80 (International Standard) and ASTM EI28-99 (American Standard). The monolithic cylinders are prepared by custom order with the designation VitraPOR 5 or 4, diameter 8 mm, length 15 mm, front surfaces finely sawn. These raw cylinders of around 0.75 mL (
Figure 2) are glued into short tubes made of titanium grade 2 with outer dimensions of 15.2 mm × 12.0 mm and a wall thickness of 1.0 mm, respectively (
Figure 3). The tubes were cleaned and roughened with sodium hydroxide solution. A silicone glue with a suitable viscosity and chemical stability is needed, such as silicon F liquid, obtainable from WEICON (Münster, Germany, 13200310). The metal tube and the monolith are fixed concentrically on an adhesive tape. The interspace is filled with liquid silicone glue gently not to loosen the fixed monolith. To avoid that the second front face is contaminated with silicone, the second front should be protected with a round patch, cut from adhesive tape, which can be removed after the polymerization of the silicone. A column holder was prepared by additive manufacturing from acrylonitrile-butadiene-styrene-polymer (ABS) with a Zortrax M200 system (Olsztyn, Poland). The detailed description of the construction and manufacturing of the column and the column holder is shown in the
Supplementary Material supported by a picture story.
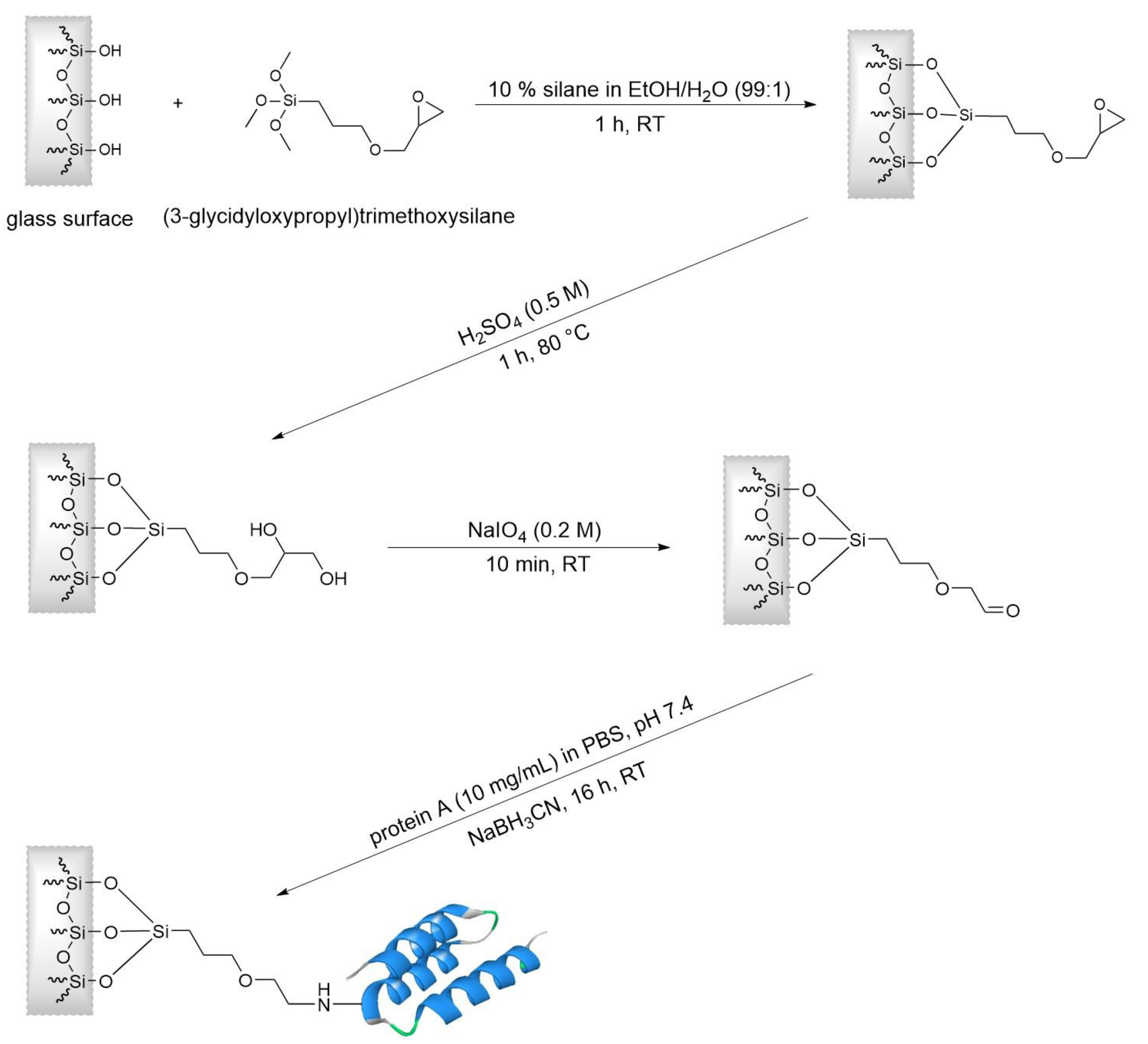
2.4. Optimized Coating Protocol of the Borosilicate Surface with Protein A
The assembled column system was connected to a syringe pump (Standard Infusion Only Pump 11 Elite from Harvard Apparatus, Holliston, MA, USA), and several reagents and washing solutions were pumped through the column. If not indicated otherwise, a flow rate of 1 mL/min was used, and the reactions took place at room temperature.
In order to clean the glass surface, 10 mL of KOH (20%) was pumped through the column. Afterward, the column was washed with 10 mL of PBS to obtain a neutral pH before the column was equilibrated with 10 mL of EtOH/H
2O (99:1). As the first reaction step (
Figure 4), 10 mL of (3-glycidyloxypropyl) trimethoxysilane (10% in EtOH/H
2O 99:1) was pumped through the column for 10 min. The column was sealed on both sides and incubated for 1 h. After a washing step with 10 mL of EtOH/H
2O (99:1), the column was flushed with 3 mL of water and 10 mL of H
2SO
4 (0.5 M) and sealed on both sides. The column was placed in a drying oven (UNB 200, Memmert, Schwabach, Germany) to incubate for 1 h at 80 °C. In this step, the epoxide groups were hydrolyzed to form a diol surface. After flushing the column with 10 mL of PBS to remove the acid and to achieve a neutral pH, the column was flushed with 10 mL of sodium periodate (0.2 M of NaIO
4). Subsequently, the column was washed with 10 mL of PBS, and the remaining PBS was pushed out with nitrogen. Later, 3 mL of a protein A solution (10 mg/mL in PBS) was run through the column. The column was sealed on both sides and incubated overnight. On the next morning, 10 mL of a blocking and reduction solution (10% of BSA + 10 mM of Tris + 150 mM of NaCl + 50 mM of NaBH
3CN, pH adjusted to 7.4 with HCl) was flushed through the column with a flow rate of 0.166 mL/min for 1 h. Subsequently, the column was washed with 10 mL of PBS-T (0.05% of Tween 20). Finally, the column was flushed with 10 mL of H
2O, 10 mL of EtOH (20% in H
2O) was stored in the fridge until use.
2.5. Analytical IgG Determination on an HPLC System
The HPLC experiments were performed on a bio-inert Infinity 1260 HPLC system from Agilent (Santa Clara, CA, USA). The HPLC experiments were performed on a bio-inert Infinity 1260 HPLC system from Agilent, and absorption was monitored at 280 nm. The maximum flow rate of 5 mL/min was used throughout. Lyophilized human plasma was reconstituted in water (100 mg/mL) and diluted in binding buffer (pH 7.4). The obtained solution was micro-filtered (0.22 µm). The column was connected to the system and then equilibrated with water, elution buffer, and binding buffer. A method was programmed and used for all future runs: The method injects 10–100 µL of the human plasma (diluted in binding buffer), then flushes the column with 2.5 mL of binding buffer to remove non-binding components before eluting IgG with 5.0 mL of elution buffer (pH 2.3) and eventually equilibrating the column back to pH 7.4 with 5.0 mL of binding buffer, and absorption was monitored at 280 nm. The maximum flow rate of 5 mL/min was used throughout. Lyophilized human plasma was reconstituted in water (100 mg/mL) and diluted in binding buffer (pH 7.4). The obtained solution was micro-filtered (0.22 µm). The column was connected to the system and then equilibrated with water, elution buffer, and binding buffer. A method was programmed and used for all future runs: The method injects 10–100 µL of the human plasma (diluted in binding buffer), then flushes the column with 2.5 mL of binding buffer to remove non-binding components before eluting IgG with 5.0 mL of elution buffer (pH 2.3) and eventually equilibrating the column back to pH 7.4 with 5.0 mL of binding buffer.
2.6. Preparative IgG Isolation on an FPLC System
The FPLC experiments were performed on an ÄKTA pure 25 L from GE Healthcare Life Sciences (Freiburg, Germany), and absorption was monitored at 280 nm. Flow rates between 5–25 mL/min were used, depending on the experiments performed. Lyophilized human plasma was reconstituted in water (100 mg/mL) and diluted in binding buffer (pH 7.4). The obtained solution was micro-filtered (0.22 µm) and then filled into the super loop (up to 10 mL) of the system.
The column was connected to the system and then equilibrated with water, elution buffer, and binding buffer. In general, all methods injected between 0.5–5 mL of the human plasma (typically diluted 1:10) at flow rates between 5–25 mL/min. In a typical method, the column was first equilibrated with 2.5 mL of binding buffer, and then the sample was injected. After a washing step with 10.0 mL of binding buffer to remove loosely bound components, IgG was eluted with 3.0 mL of elution buffer (pH 2.3). Finally, the column was re-equilibrated with 8.0 mL binding buffer back to a pH of 7.4.
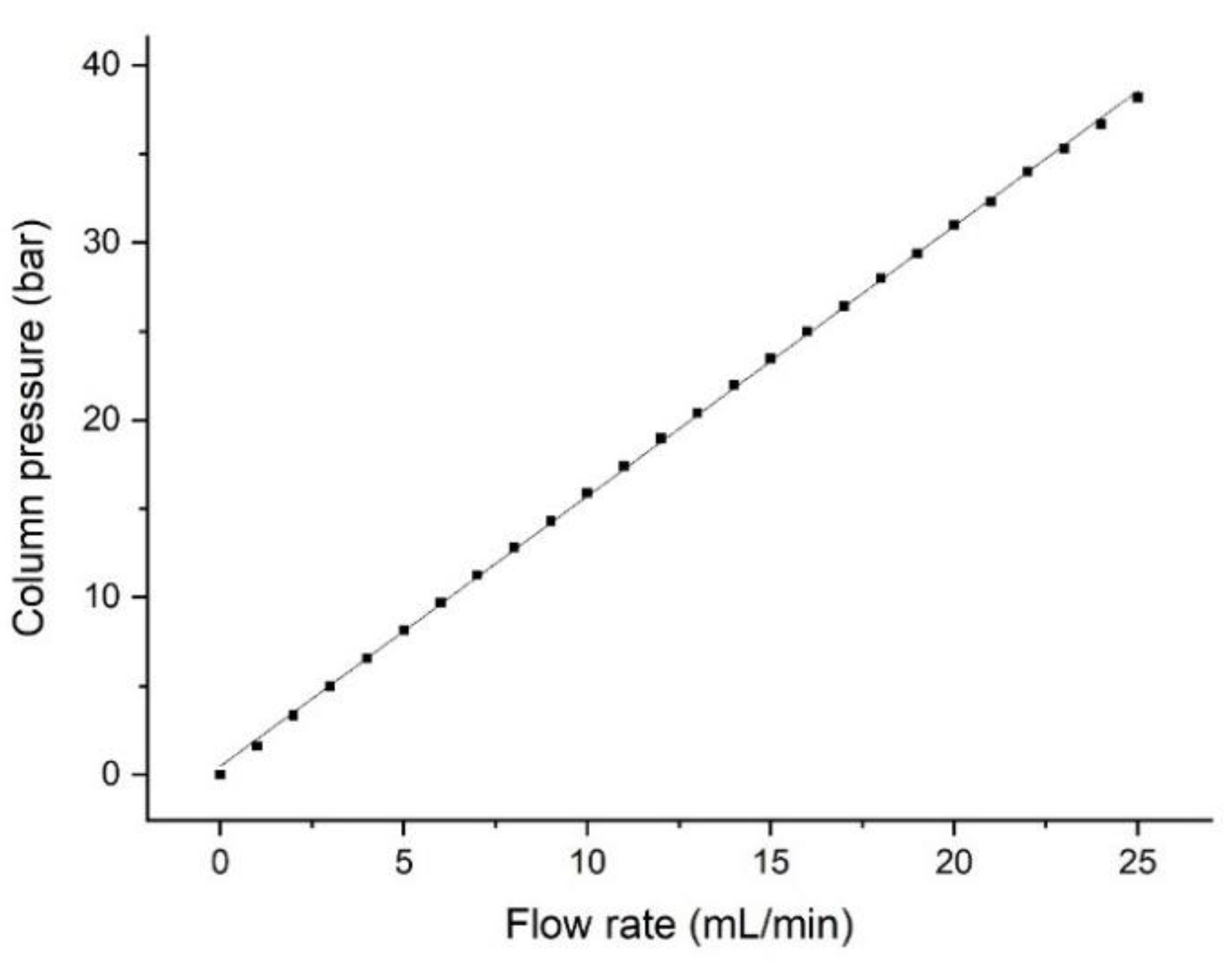
2.7. Pressure Characterization of the System
In order to determine the maximum pressure limits, the column was connected directly to the FPLC pump to avoid any back-pressure from the system itself. A solution of 20% ethanol was flushed through the column with 1 mL/min, and the flow rate was then increased by 1 mL/min every 12 s until the maximum system flow rate of 25 mL/min was reached.
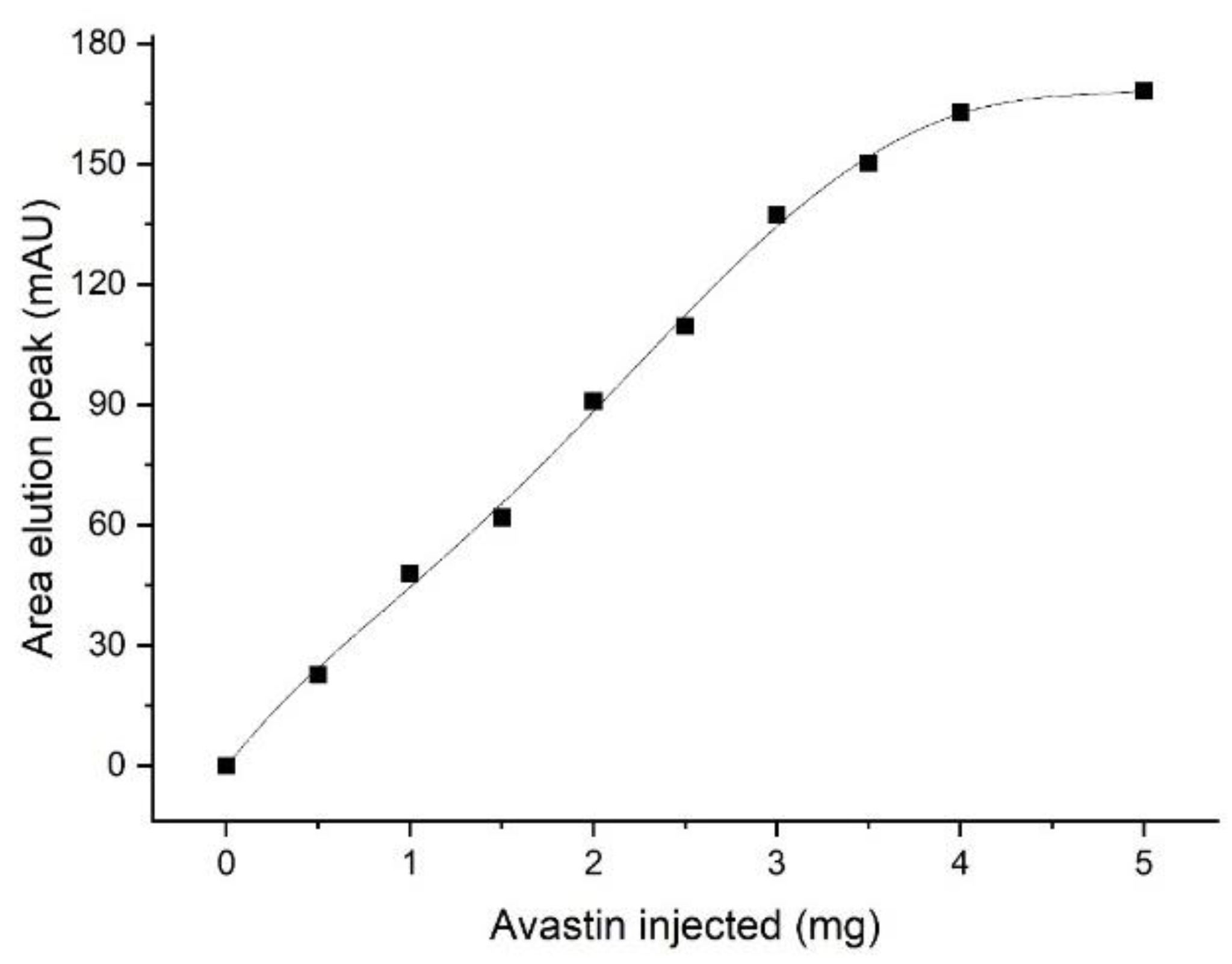
2.8. Determination of the Monolith Capacity (IgG on Protein A)
This experiment was performed on the FPLC system. From a stock solution of the therapeutic antibody Avastin (25 mg/mL), several dilutions were prepared (0/2/4/6/8/10 mg/mL). For each run, 500 µL was injected with a flow rate of 5 mL/min, and the peak areas of the resulting elution peaks were compared (UV, 280 nm).
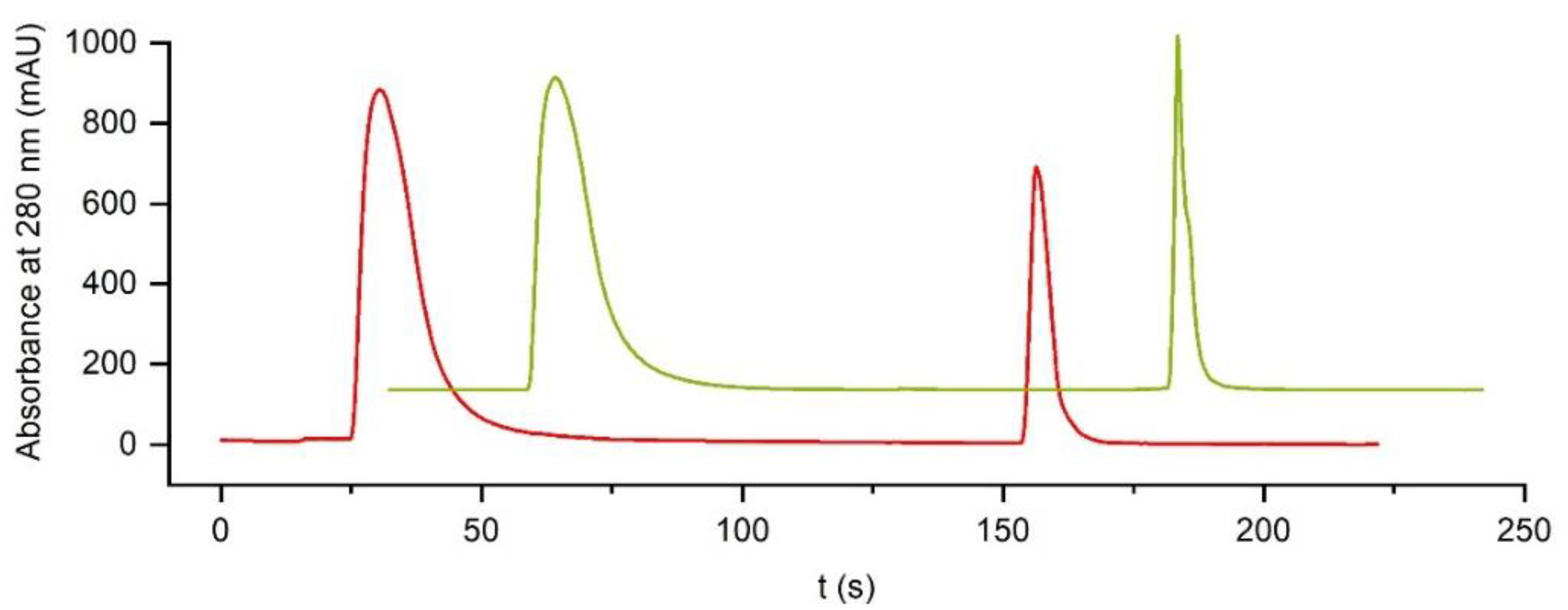
2.9. Reversal of Flow Direction (Counterflow)
This experiment was also performed on the FPLC system at a flow rate of 5 mL/min. 1 mL of human plasma (diluted 1:20 in binding buffer) was injected, and the bound antibodies were eluted in the normal flow direction (up-flow) with elution buffer (pH 2.3). Eventually, the same run was performed, but with a reversed elution direction (counterflow).
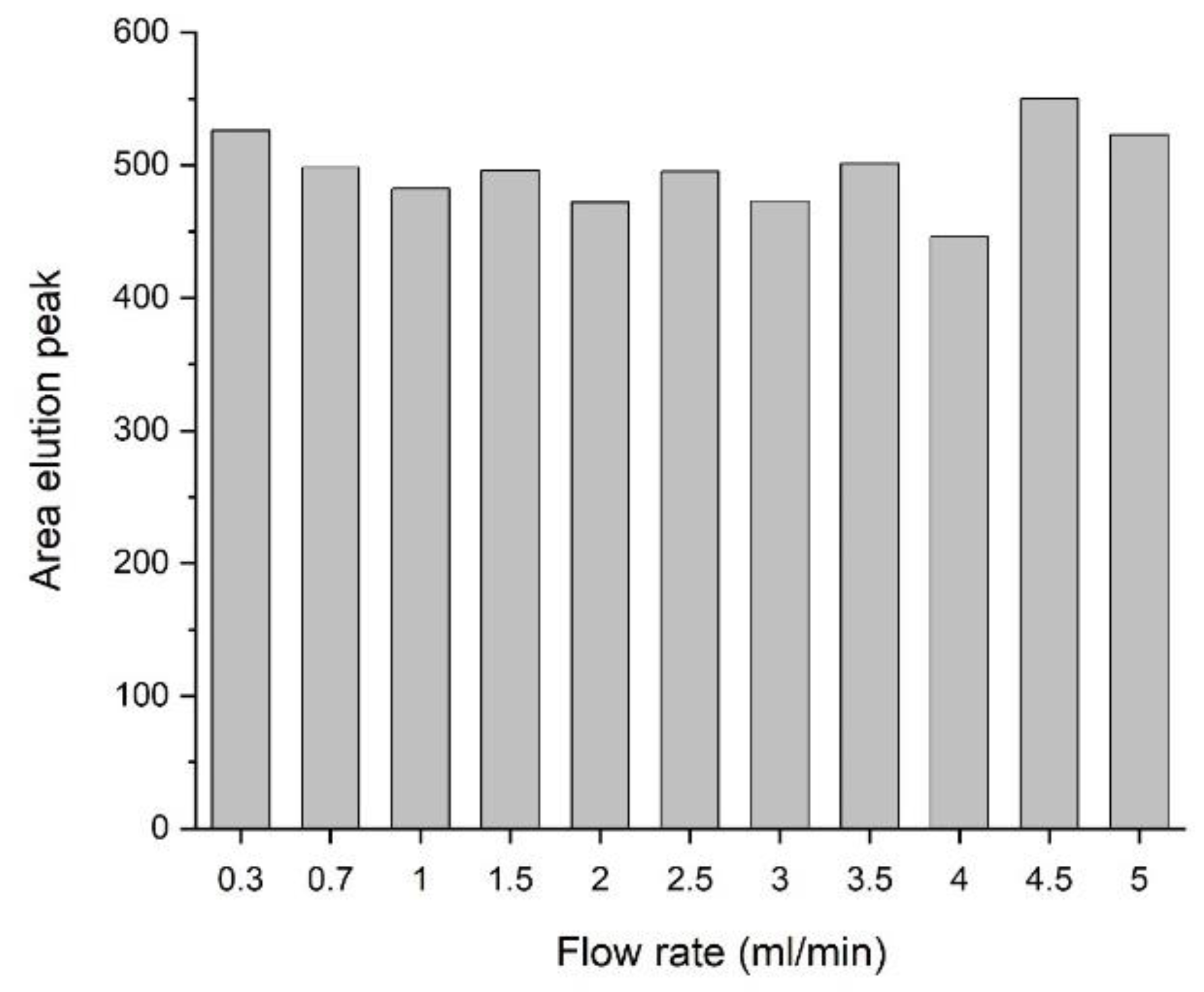
2.10. Examination of the Dynamic Capacity
This experiment was performed on the HPLC system. Human plasma was diluted 1:2 in binding buffer, and 10 µL were injected. The flow rates of each run were varied between 0.33 and 5 mL/min. The flow-rate corrected areas of each elution peak were compared. This experiment used only a fraction of the total column binding capacity.
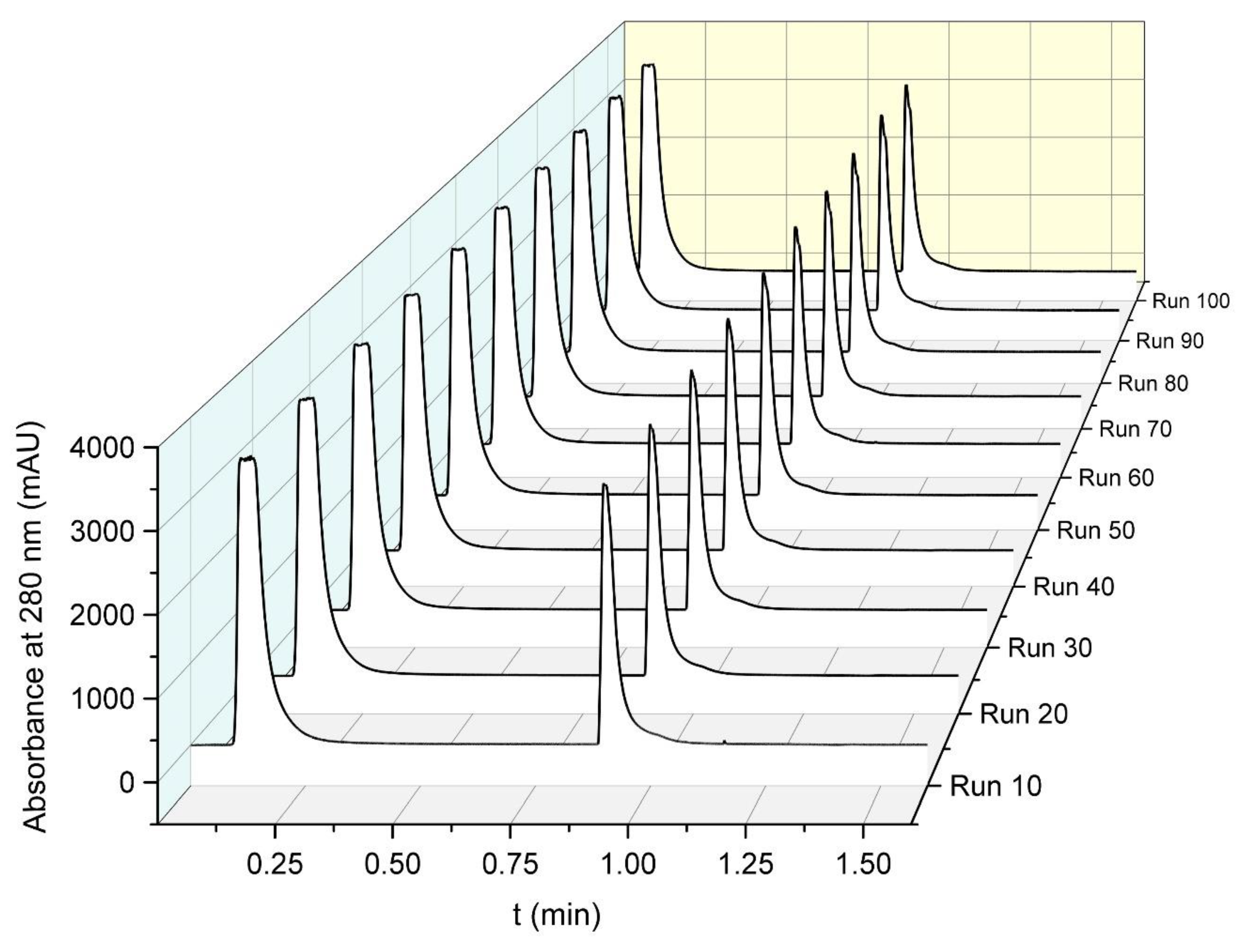
2.11. Repeatability Test (100 Cycles)
This experiment was performed on the HPLC system. Human plasma was diluted 1:10 in binding buffer. A method was programmed, which injected 80 µL of plasma solution at 5 mL/min. This method was run 100 times successively without human intervention.
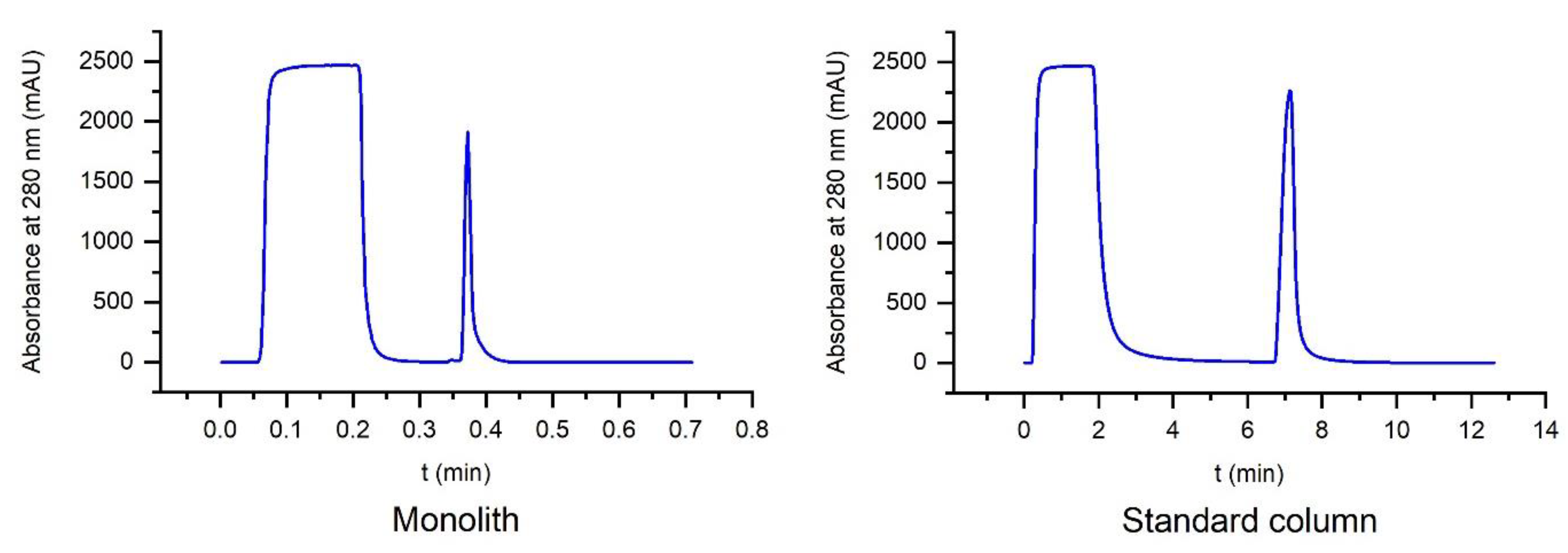
2.12. Productivity Test (Amount of Purified IgG per Hour)
This experiment was performed on the FPLC system. It was determined how much IgG from human plasma could be collected within one hour with each column at their respective maximum flow rates. Each column was connected to the FPLC, and the standard protocol was performed. For the monolith, a flow rate of 25 mL/min was chosen, which is also the maximum flow rate of the system (run duration of 0.7 min). The compared column from GE was limited to a flow rate of 4 mL/min (run duration 13 min). The IgG yield of each run was calculated by comparing the area of the elution peaks with a calibration line from calibration experiments and extrapolated up to one hour for each column.
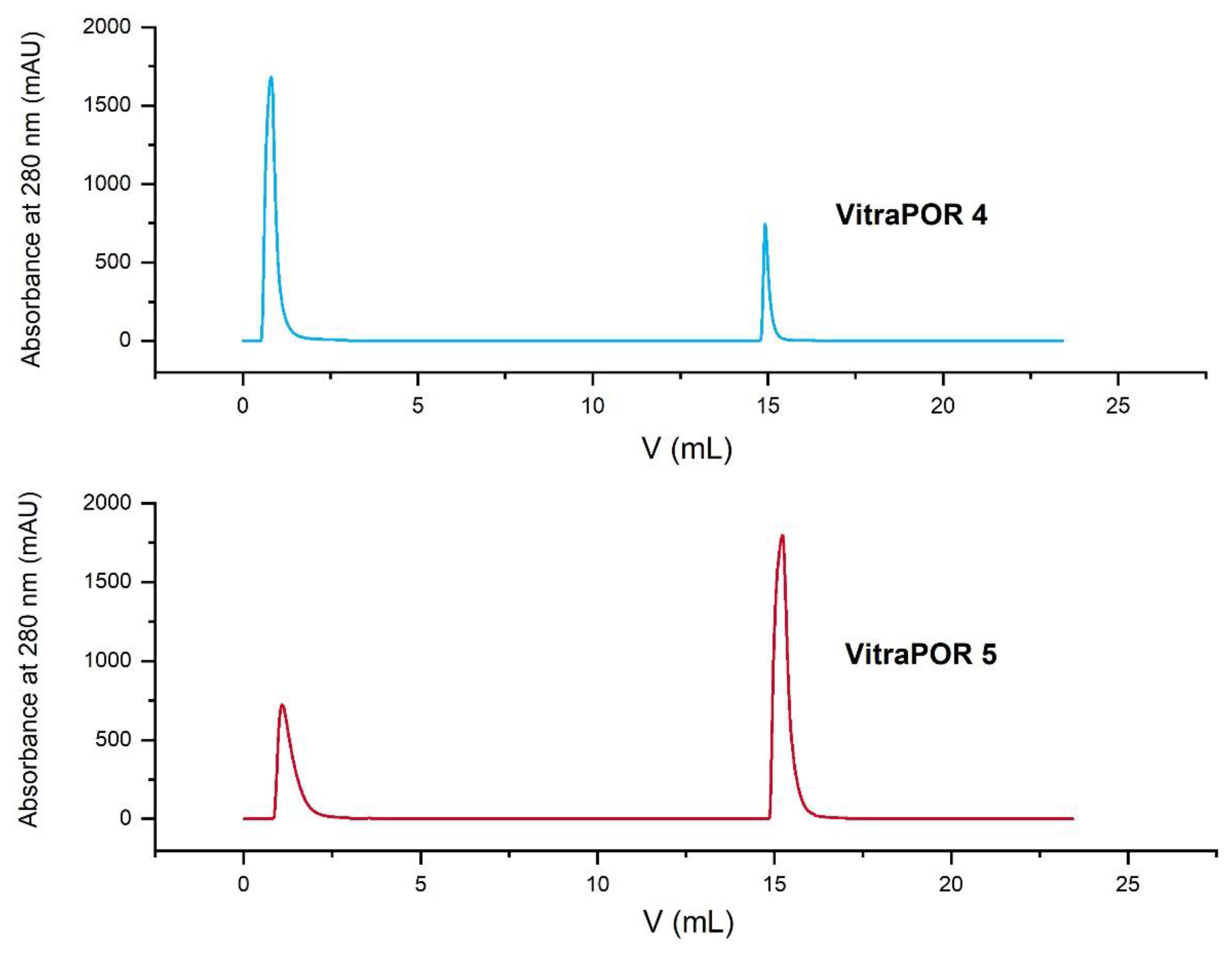
2.13. Comparison of the Capacity of VitraPOR 4 and VitraPOR 5 Columns
This experiment was performed on the FPLC system. As a sample, 500 µL Adalimumab (Humira®, from Evidentic) with a concentration of 10 mg/mL was injected with a flow rate of 5 mL/min on a VitraPOR 4 (pore size 10–16 µm) and a VitraPOR 5 (pore size 1–1.6 µm) column. The IgG yield of each run was calculated by comparing the area of the elution peaks with a calibration line from calibration experiments.
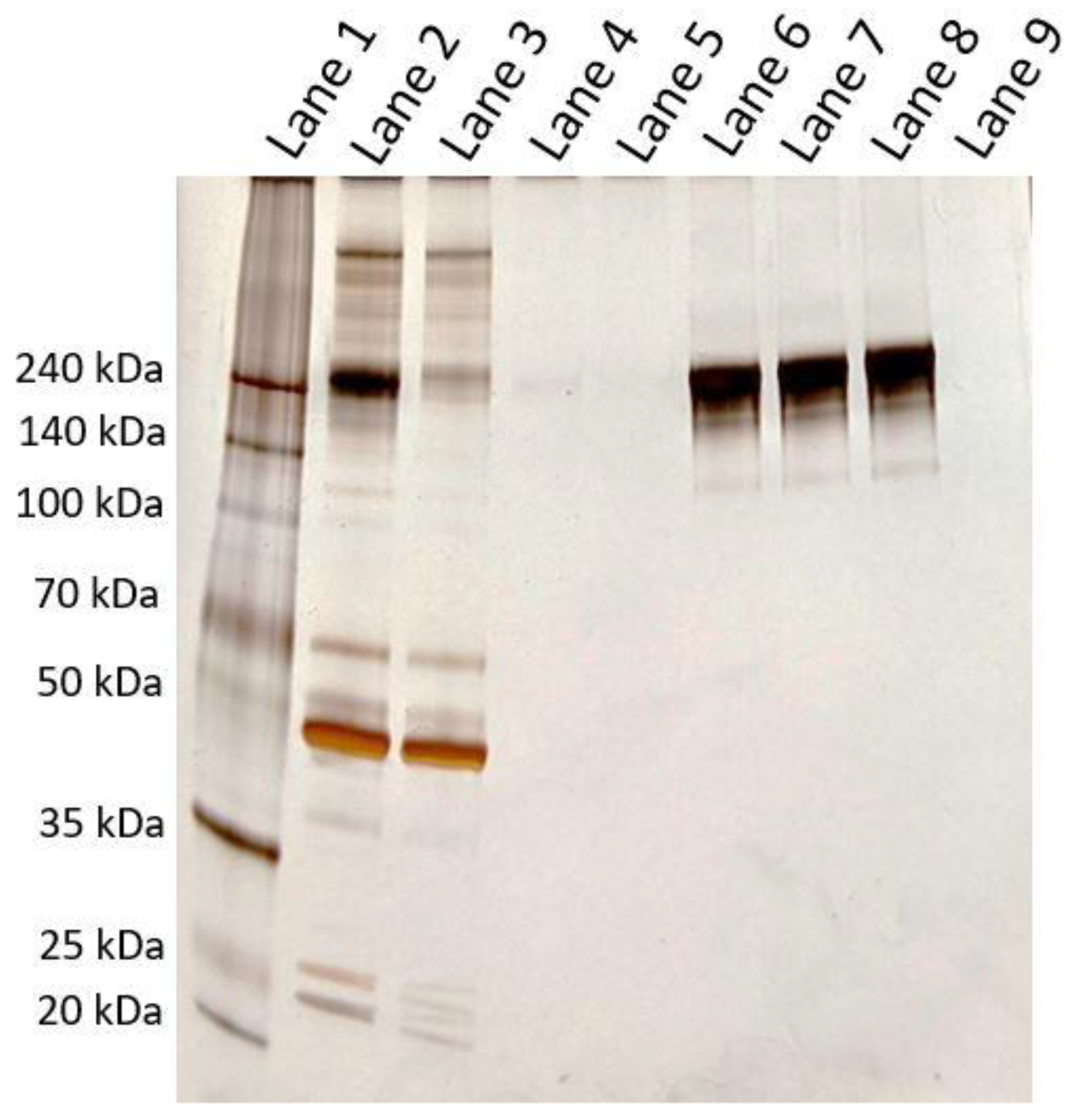
2.14. Determination of IgG Purity
The chromatography was performed on the FPLC system and the following SDS-PAGE on a Hoefer SE260 with gels from Serva (SERVAGel™ TG PRiME™ 4–12%, 43273.01). Human plasma was diluted 1:10 in binding buffer, and 2 mL were injected on the FPLC with a run flow rate of 10 mL/min. The binding and elution peaks were fractionated, and the latter neutralized with 1 µL of NaOH (1 M) per 100 µL eluate. Of each fraction, 100 µL were used for the SDS-PAGE characterization. The rest of the obtained elution fraction was reinjected and run with the same method. Again, both peaks were collected, some aliquots were stored, and the elution fraction was injected again. Eventually, six samples were obtained: Three binding and three elution fractions. The three elution fractions were each diluted to a total volume of 2 mL with binding buffer. The three elution fractions and the first binding fraction were examined on a NanoPhotometer NP 80 (Implen, Munich, Germany) to estimate their IgG concentration, using its integrated method for human IgG. In total, 2 µL of the sample were used for the absorbance measurement at 280 nm.
For SDS-PAGE analysis, human plasma was diluted 1:100 in binding buffer. The flow-through fraction was diluted 1:10 in binding buffer. The three elution fractions were adjusted to the same IgG concentration based on NanoPhotometer determinations. Of all samples, 18 µL were taken and each supplemented with 6 µL of 4x loading buffer (non-reducing), mixed, and heated to 95 °C in a ThermoMixer C (Eppendorf, Hamburg, Germany, 5382000015) for 10 min. The samples were mixed again, and subsequently, 10 µL of each sample was loaded onto the gel, which was run with 400 mL of SDS running buffer in a fridge at 4 °C. Run times: 15 min at 70 V + 60 min at 180 V + 10 min at 200 V. The following silver staining protocol was applied to visualize protein bands: The gel was washed twice in water for 5 min each. Afterward, the gel was incubated in sodium thiosulfate Na2S2O3 (1.3 mM) for 1 min and then washed with water for 10 s. The gel was then transferred into a staining container containing freshly prepared AgNO3 (5.9 mM) and incubated for 30 min. The gel was washed twice with water for 10 s each and then incubated for 10 s with sodium carbonate Na2CO3 (236 mM). In the next step, the gel was incubated in a solution of Na2CO3 (236 mM) and formaldehyde (2.5 mM) for 10 min. After the completed staining, the gel was washed twice with water and then incubated in Na2EDTA (50 mM) for 20 min. Finally, the gel was washed twice with water, and a photograph of the final gel was taken.
2.15. Calibration Curve of IgG
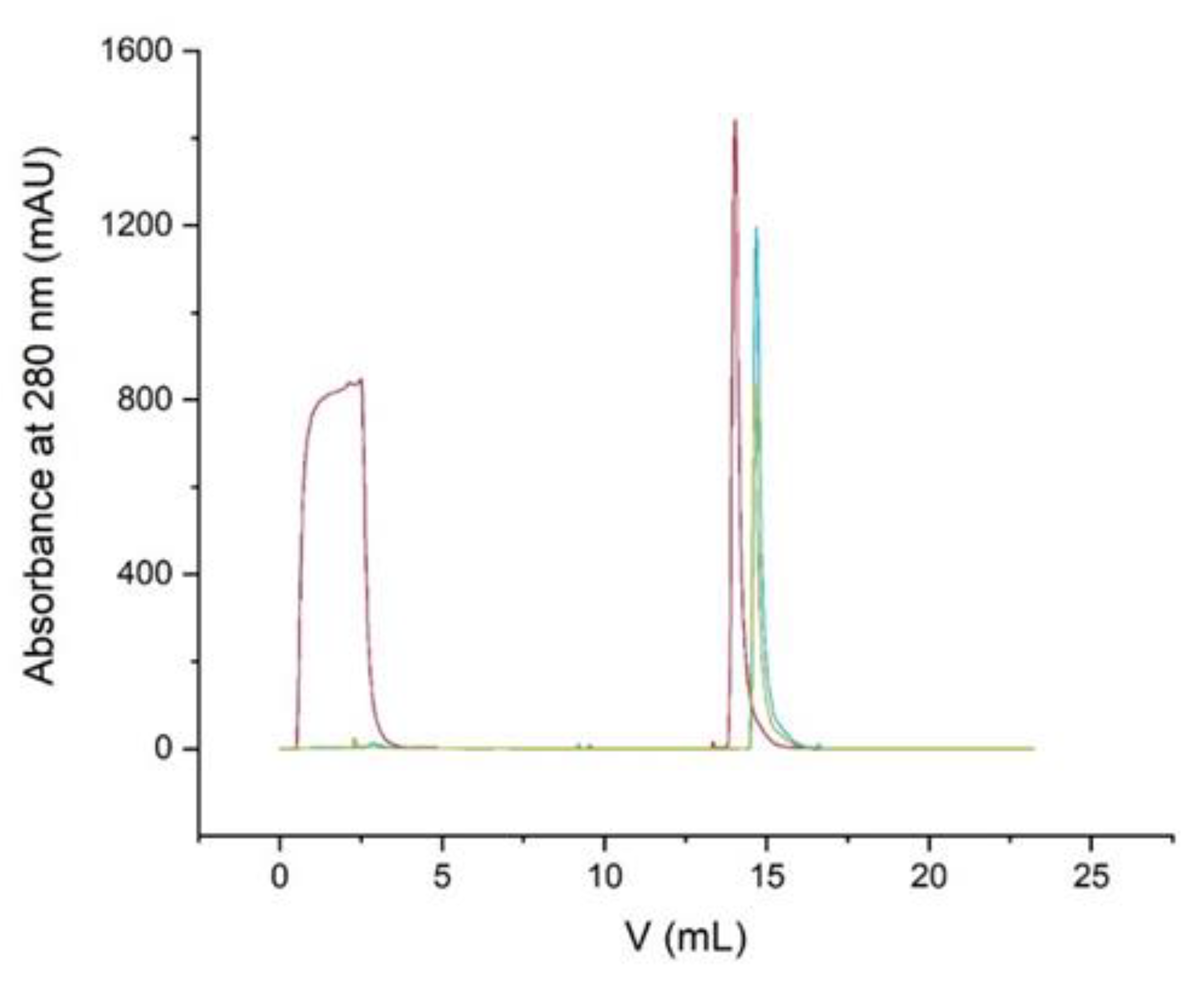
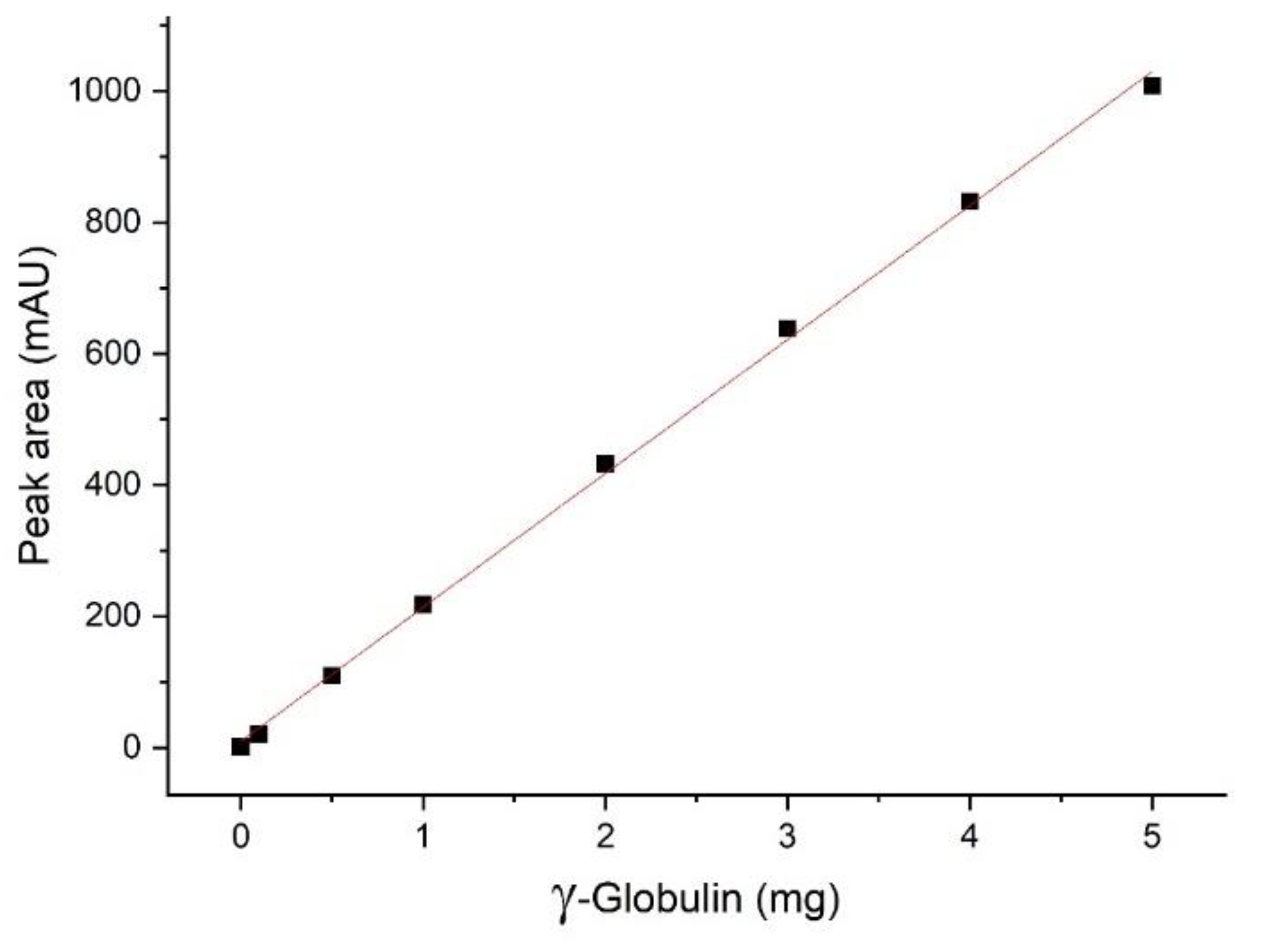
The experiment was performed on the FPLC without a column connected. On each run, 500 µL of different concentrations of bovine γ-globulin (0/0.2/1.0/2/4/6/8/10/20 mg/mL) was injected at a flow rate of 5 mL/min. The obtained peak areas were compared, and a calibration line with a linear regression equation was prepared.
2.16. Determination of the Limit of Detection (LOD) for Human IgG
This experiment was performed on the HPLC system. A series of Avastin solutions (0/0.5/1/1.5 µg/mL) were prepared from the stock solution, and 100 µL of each solution was injected with a flow rate of 5 mL/min. Each sample was injected 10 times for statistical evaluation.
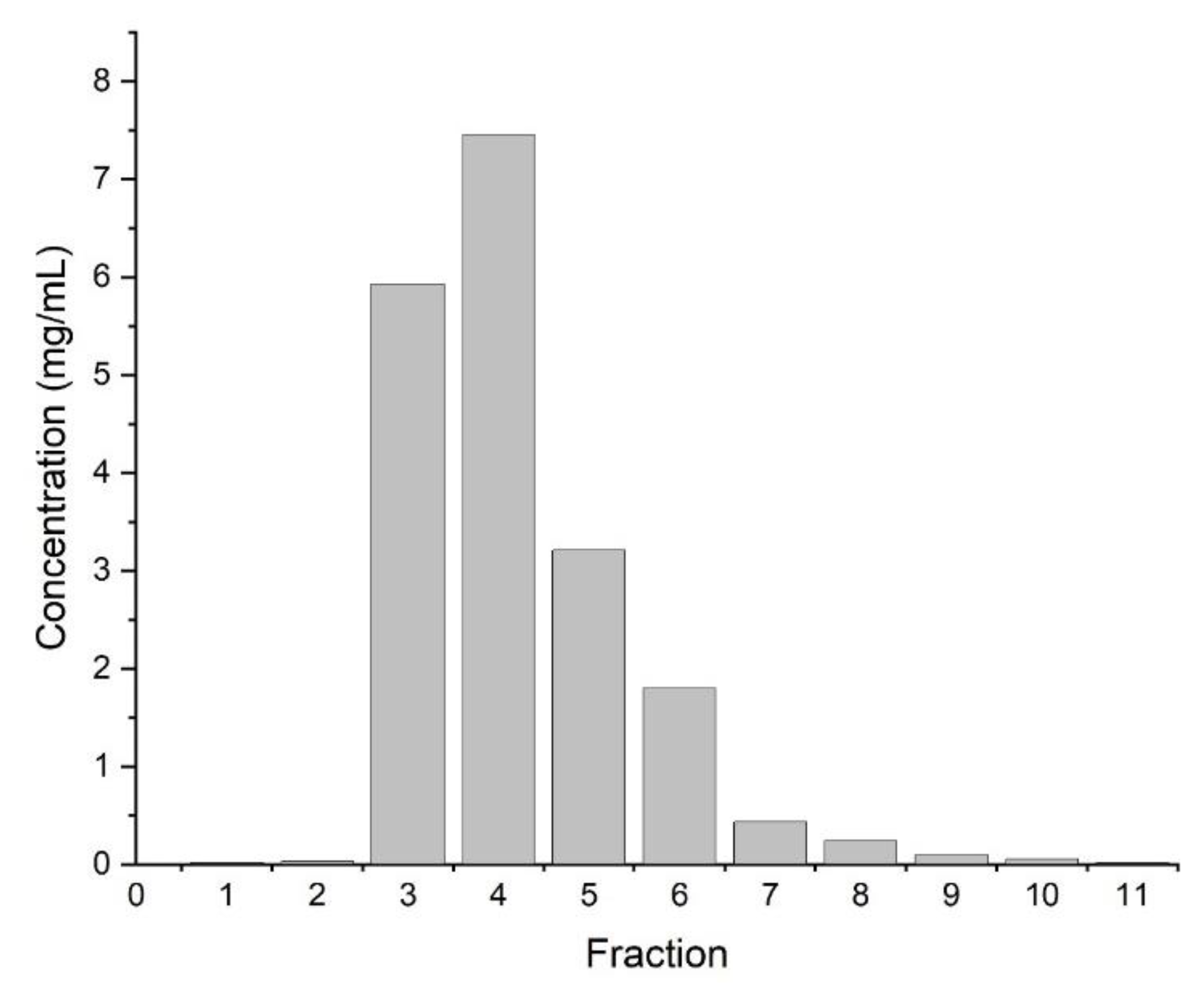
2.17. The Maximum Concentration of IgG in the Eluate
This experiment was performed on the FPLC system. Human plasma was diluted 1:5 in binding buffer, and 4 mL was injected onto the column with a flow rate of 5 mL/min. The elution peak was fractionated in 150 µL fractions and neutralized with 1 µL of NaOH (1 M) per 1 mL eluate. A calibration solution of γ-globulin from bovine in binding buffer (0/0.1/0.2/0.3/0.4/0.5 mg/mL) was diluted from a stock solution. The calibration samples and each FPLC fraction were examined on a UV/VIS-photometer at 280 nm (Evolution 220 from Thermo Fisher, Darmstadt, Germany). A calibration line was created to determine the concentration of each fraction.