Enantioselective Study on the Biodegradation of Verapamil and Cytalopram by Chiral Capillary Electrophoresis
Abstract
1. Introduction
2. Materials and Methods
2.1. Instrumentation
2.2. Chemicals and Solutions
2.3. EKC-CFT Methods
2.4. Biodegradability Assays
3. Results and Discussion
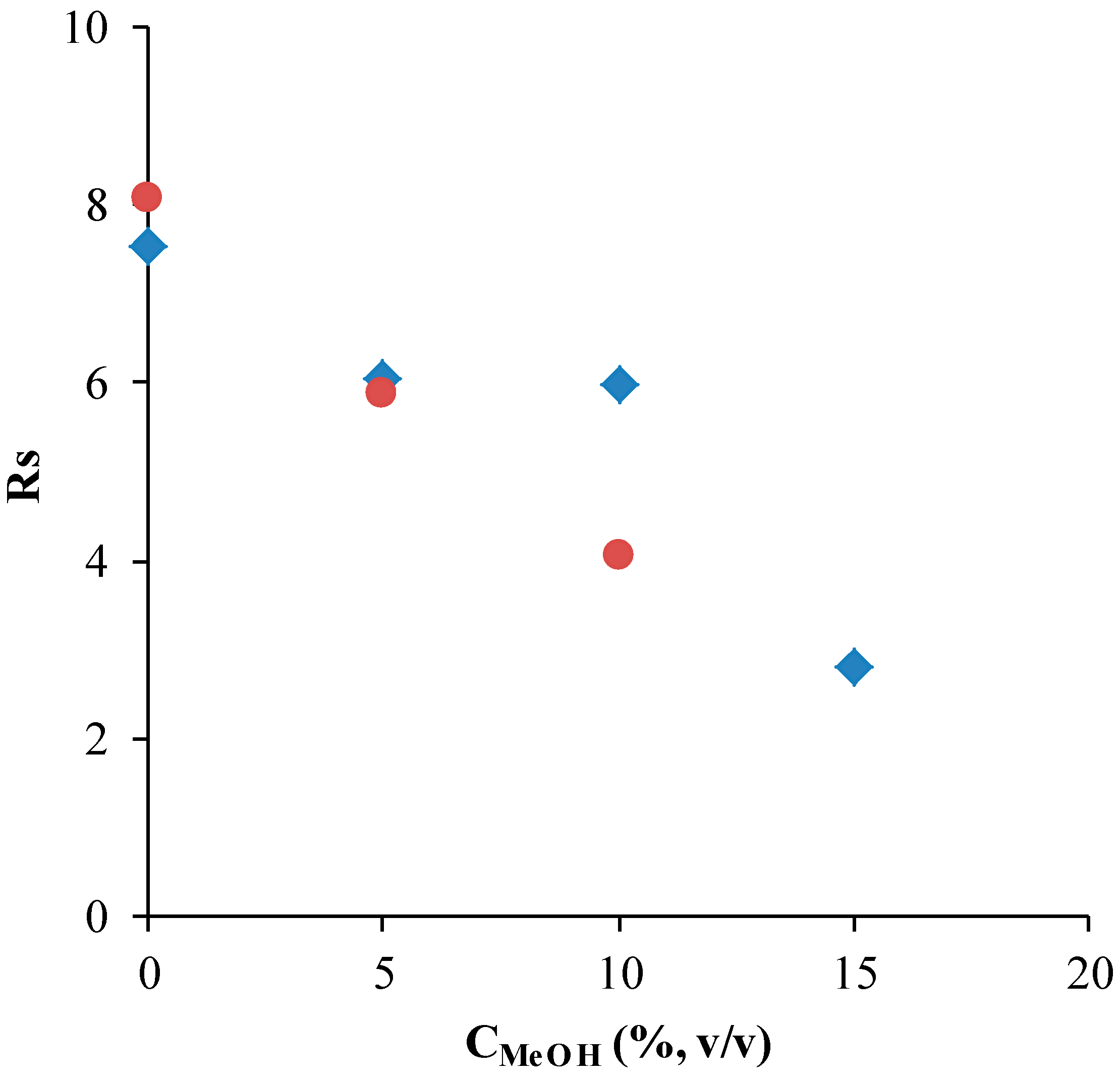
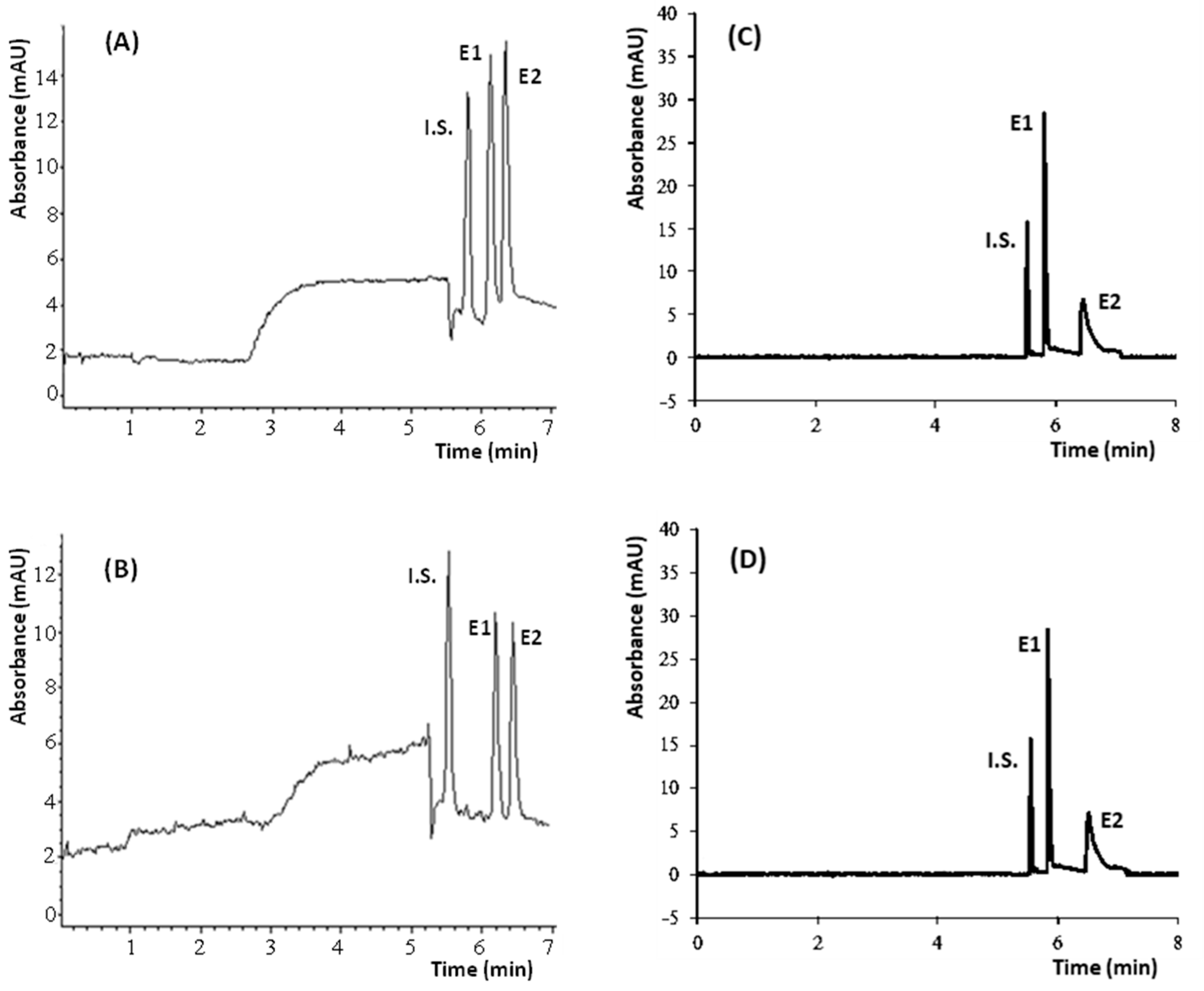
3.1. Chiral Electrophoretic Analysis
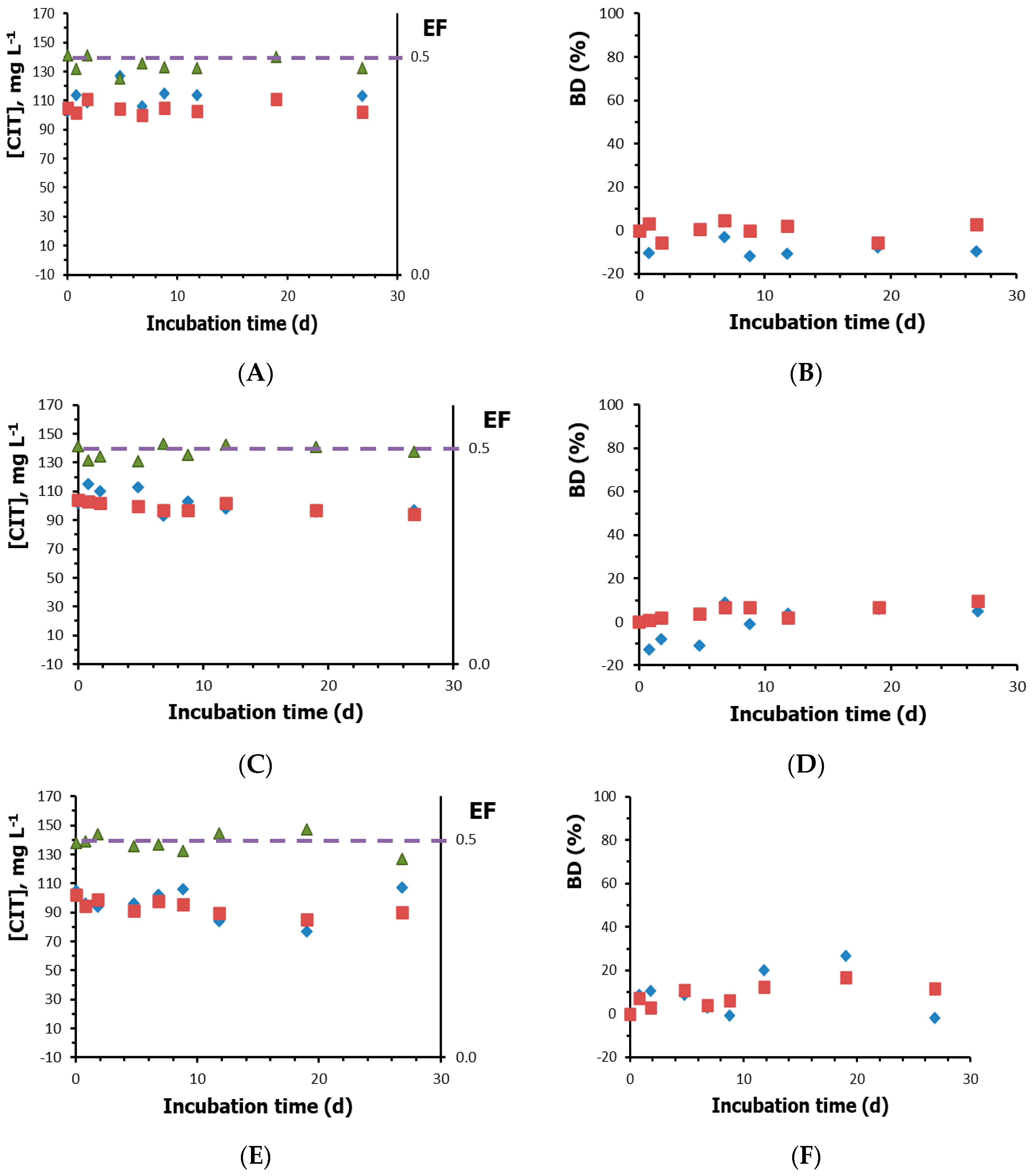
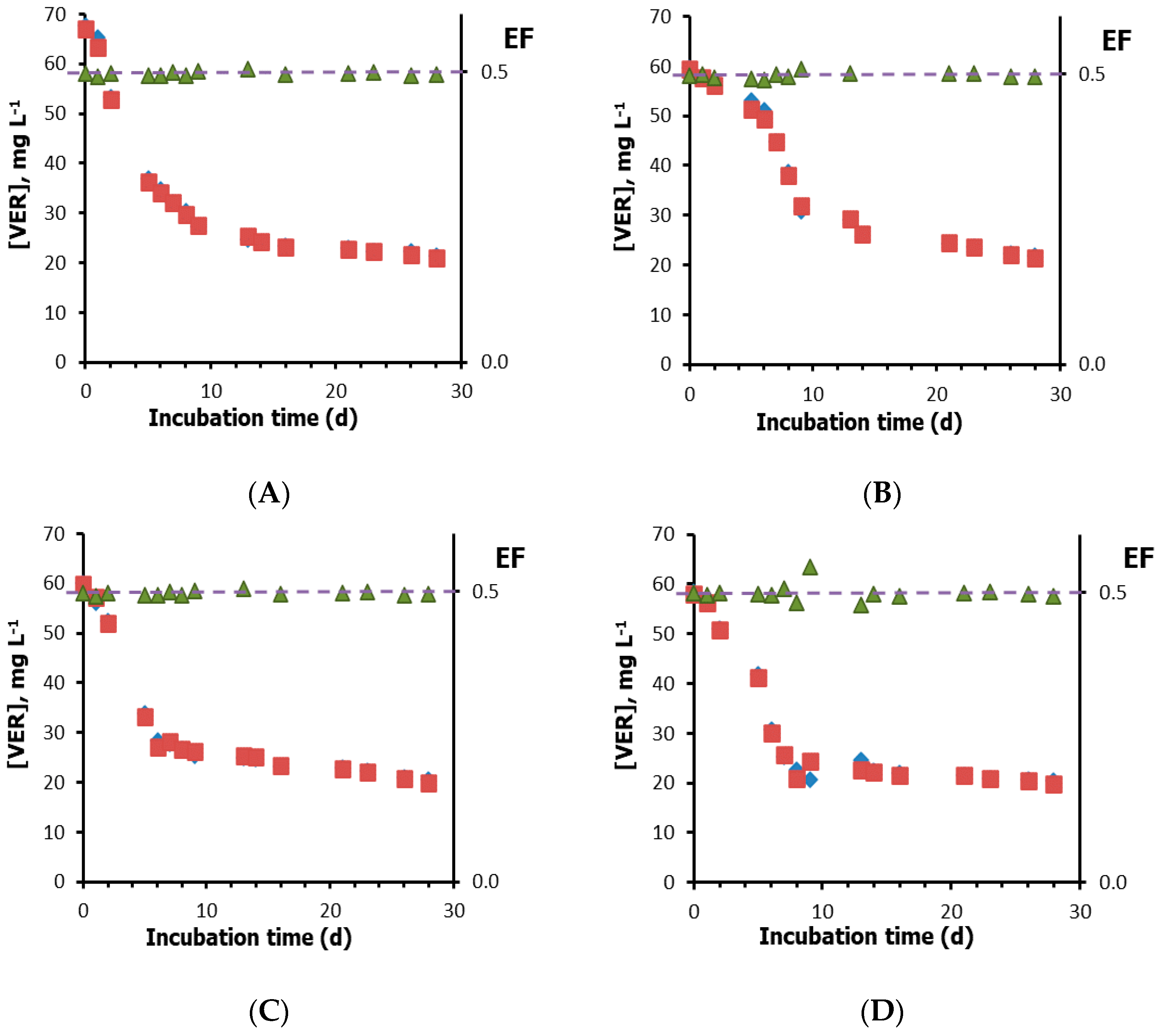
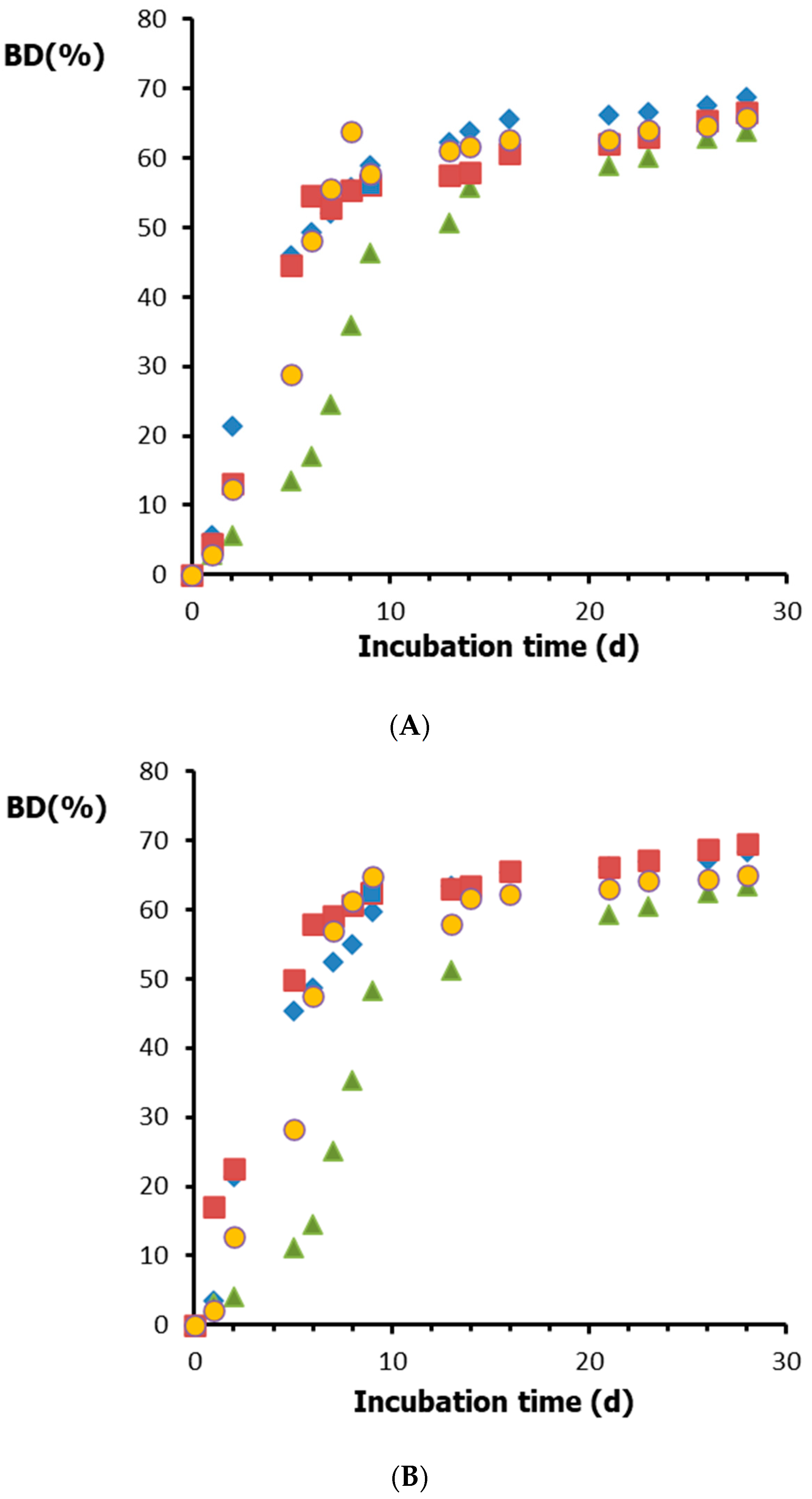
3.2. Biodegradation Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hernando, M.D.; Mezcua, M.; Fernández-Alba, A.R.; Barceló, D. Environmental risk assessment of pharmaceutical residues in wastewater effluents, surface waters and sediments. Talanta 2006, 69, 370–376. [Google Scholar] [CrossRef]
- Hernando, M.D.; Fernández-Alba, A.R.; Tauler, R.; Barceló, D. Toxicity assays applied to wastewater treatment. Talanta 2005, 65, 358–366. [Google Scholar] [CrossRef]
- Kümmerer, K. The presence of pharmaceuticals in the environment due to human use- present knowledge and future changes. J. Environ. Manag. 2009, 90, 2354–2366. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.R.; Santos, L.H.M.L.M.; Maia, A.S.; Delerue-Matos, C.; Castro, P.M.L.; Tiritan, M.E. Enantiomeric fraction evaluation of pharmaceuticals in environmental matrices by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2014, 1363, 226–235. [Google Scholar] [CrossRef]
- Escuder-Gilabert, L.; Martín-Biosca, Y.; Pérez-Baeza, M.; Sagrado, S.; Medina-Hernández, M.J. Direct chromatographic study of the enantioselective biodegradation of ibuprofen and ketoprofen by an activated sludge. J. Chromatogr. A 2018, 1568, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Roig, P.; Kasprzyk-Hordem, B.; Blasco, C.; Picó, Y. Stereoisomeric profiling of drugs of abuse and pharmaceuticals in wastewaters of Valencia (Spain). Sci. Total Environ. 2014, 494–495, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Kasprzyk-Hordem, B.; Baker, D.R. Enantiomeric profiling of chiral drugs in wastewater and receiving waters. Environ. Sci. Technol. 2012, 46, 1681–1691. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.E.; Bagnall, J.; Kasprzyk-Hordem, B. Enantioselective degradation of amphetamine-like environmental micropollutants (amphetamine, methamphetamine, MDMA and MDA) in urban water. Environ. Pollut. 2016, 215, 154–163. [Google Scholar] [CrossRef]
- Ribeiro, A.R.; Maia, A.S.; Moreira, I.S.; Afonso, C.M.; Castro, P.M.L.; Tiritan, M.E. Enantioselective quantification of fluoxetine and norfluoxetine by HPLC in wastewater effluents. Chemosphere 2014, 95, 589–596. [Google Scholar] [CrossRef]
- Ready Biodegradability Test. OECD Guideline for testing of chemicals. OECD 1992, 71, 1–9. [Google Scholar]
- Evans, S.; Bagnall, J.; Kasprzyk-Hordern, B. Enantiomeric profiling of a chemically diverse mixture of chiral pharmaceuticals in urban water. Environ. Pollut. 2017, 230, 368–377. [Google Scholar] [CrossRef]
- Bagnall, J.; Malia, L.; Lubben, A.; Kasprzyk-Hordem, B. Stereoselective biodegradation of amphetamine and methamphetamine in river microcosms. Water Res. 2013, 47, 5708–5718. [Google Scholar] [CrossRef]
- Ribeiro, A.R.; Afonso, C.M.; Castro, P.M.L.; Tiritan, M.E. Enantioselective HPLC analysis and biodegradation of atenolol, metoprolol and fluoxetine. Environ. Chem. Lett. 2013, 11, 83–90. [Google Scholar] [CrossRef]
- Ribeiro, A.R.; Afonso, C.M.; Castro, P.M.L.; Tiritan, M.E. Enantioselective biodegradation of pharmaceuticals, alprenolol and propranolol, by an activated sludge inoculum. Ecotoxicol. Environ. Saf. 2013, 87, 108–114. [Google Scholar] [CrossRef]
- Li, Z.; Gomez, E.; Fenet, H.; Chiron, S. Chiral signature of venlafaxine as a marker of biological attenuation processes. Chemosphere 2013, 90, 1933–1938. [Google Scholar] [CrossRef]
- Moreira, I.S.; Ribeiro, A.R.; Afonso, C.M.; Castro, P.M.L.; Tiritan, M.E. Enantioselective biodegradation of fluoxetine by the bacterial strain Labrys portucalensis F11. Chemosphere 2014, 111, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Escuder-Gilabert, L.; Martín-Biosca, Y.; Pérez-Baeza, M.; Sagrado, S.; Medina-Hernández, M.J. Trimeprazine is enantioselectively degraded by an activated sludge in ready biodegradability test conditions. Water Res. 2018, 141, 57–64. [Google Scholar] [CrossRef]
- Klein, C.; Schneider, R.J.; Meyer, M.T.; Aga, D.S. Enantiomeric separation of metolachlor and its metabolites using LC–MS and CZE. Chemosphere 2006, 62, 1591–1599. [Google Scholar] [CrossRef] [PubMed]
- Chu, B.L.; Guo, B.Y.; Peng, Z.; Wang, Z.; Guo, G.; Lin, J.M. Studies on degradation of imazalil enantiomers in soil using capillary electrophoresis. J. Sep. Sci. 2007, 30, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Chankvetadze, B.; Blaschke, G. Enantioseparations in capillary electromigration techniques: Recent developments and future trends. J. Chromatogr. A 2001, 906, 309–363. [Google Scholar] [CrossRef]
- Cserháti, T. New applications of cyclodextrins in electrically driven chromatographic systems: A review. Biomed. Chromatogr. 2008, 22, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Fanali, S.; Chankvetadze, B. Some thoughts about enantioseparations in capillary electrophoresis. Electrophoresis 2019, 40, 2420–2437. [Google Scholar] [CrossRef] [PubMed]
- Bernardo-Bermejo, S.; Sanchez-Lopez, E.; Castro-Puyana, M.; Marina, M.L. Chiral capillary electrophoresis. TrAC Trends Anal. Chem. 2020, 124, 115807. [Google Scholar] [CrossRef]
- Amini, A.; Paulsen-Sörman, U.; Westerlund, D. Principle and applications of the partial filling technique in capillary electrophoresis. Chromatographia 1999, 50, 497–506. [Google Scholar] [CrossRef]
- Asensi-Bernardi, L.; Martín-Biosca, Y.; Sagrado, S.; Medina-Hernández, M.J. Characterizing the interaction between enantiomers of eight psychoactive drugs and highly sulfated-β-cyclodextrin by counter-current capillary electrophoresis. Biomed. Chromatogr. 2014, 28, 120–126. [Google Scholar] [CrossRef]
- Ruzicka, M.; Koval, D.; Vavra, J.; Reyes-Gutierrez, P.E.; Teply, F.; Kasicka, V. Interactions of helquats with chiral acidic aromatic analytes investigated by partial-filling affinity capillary electrophoresis. J. Chromatogr. A 2016, 1467, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Asensi-Bernardi, L.; Martín-Biosca, Y.; Escuder-Gilabert, L.; Sagrado, S.; Medina-Hernández, M.J. Modeling the chiral resolution ability of highly sulfated β-cyclodextrin for basic compounds in electrokinetic chromatography. J. Chromatogr. A 2013, 1308, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Escuder-Gilabert, L.; Martín-Biosca, Y.; Medina-Hernández, M.J.; Sagrado, S. Enantioresolution in electrokinetic chromatography-complete filling technique using sulfated gamma-cyclodextrin. Software-free topological anticipation. J. Chromatogr. A 2016, 1467, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Baumann, P.; Zullino, D.F.; Eap, C.B. Enantiomers’ potential in psychopharmacology—A critical analysis with special emphasis on the antidepressant escitalopram. Eur. Neuropsychopharmacol. 2002, 2, 433–444. [Google Scholar] [CrossRef]
- Budău, M.; Hancu, G.; Muntean, D.L.; Papp, L.A.; Cârje, A.G.; Garaj, V. Enantioseparation of citalopram enantiomers by capillary electrophoresis: Method development through experimental design and computational modeling. Chirality 2020, 32, 1119–1128. [Google Scholar] [CrossRef]
- Hamidi, S.; Jouyban, A. Capillary electrophoresis with UV detection, on-line stacking and off-line dispersive liquid-liquid microextraction for determination of verapamil enantiomers in plasma. Anal. Methods 2015, 7, 5820. [Google Scholar] [CrossRef]
- Trautwein, C.; Kümmerer, K.; Metzger, J.W. Aerobic biodegradability of the calcium antagonist verapamil and identification of a microbial dead-end transformation product studies by LC-MS/MS. Chemosphere 2008, 72, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Mechelke, J.; Rust, D.; Jaeger, A.; Hollender, J. Enantiomeric Fractionation during Biotransformation of Chiral Pharmaceuticals in Recirculating Water-Sediment Test Flumes. Environ. Sci. Technol. 2020, 54, 7291–7301. [Google Scholar] [CrossRef] [PubMed]
- Valimaña-Traverso, J.; Morante-Zarcero, S.; Pérez-Quintanilla, D.; García, M.A.; Sierra, I.; Marina, M.L. Periodic mesoporous organosilica materials as sorbents for solid-phase extraction of drugs prior to simultaneous enantiomeric separation by capillary electrophoresis. J. Chromatogr. A 2018, 1566, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Matthijs, N.; Hemelryck, S.V.; Maftouh, M.; Massart, D.L.; Heyden, Y.V. Electrophoretic separation strategy for chiral pharmaceuticals using highly-sulfated and neutral cyclodextrins based dual selector systems. Anal. Chim. Acta 2004, 525, 247–263. [Google Scholar] [CrossRef]
- Andersen, S.; Halvorsen, T.G.; Pedersen-Bjergaard, S.; Rasmussen, K.E.; Tanum, L.; Refsum, H. Stereospecific determination of citalopram and desmethylcitalopram by capillary electrophoresis and liquid-phase microextraction. J. Pharm. Biomed. Anal. 2003, 33, 263–273. [Google Scholar] [CrossRef]
- Mandrioli, R.; Fanali, S.; Pucci, V.; Raggi, M.A. Enantiomeric separation of citalopram and its metabolites by capillary electrophoresis. Electrophoresis 2003, 24, 2608–2616. [Google Scholar] [CrossRef]
- Deng, X.; De Wolf, J.; Vervoort, R.; Pamperin, D.; Adams, E.; Schepdael, A.V. Development and validation of a capillary electrophoresis method for the determination of escitalopram and sensitive quantification of its enantiomeric impurity in formulations. Electrophoresis 2012, 33, 1648–1651. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, S.; Breitbach, Z.S.; Petersen, H.; Ellegaard, P.; Armstrong, D.W. Enantioseparation of citalopram analogues with sulfated beta-cyclodextrin by capillary electrophoresis. Electrophoresis 2016, 37, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Shuler, M.L.; Kargi, F. (Eds.) Bioprocess Engineering: Basic Concepts, 2nd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2002. [Google Scholar]
| Compound | Structure | pKa | logP |
|---|---|---|---|
| Verapamil | 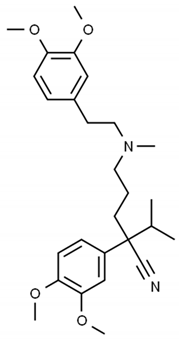 | 8.9 | 3.79 |
| Citalopram | 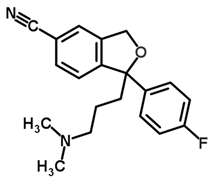 | 9.78 | 2.51 |
| Acetanilide |  | 0.5 | 1.16 |
| Ranitidine |  | 7.8 | 1.23 |
| Compound Interval b, mg/L−1 | E1 | E2 | |||||
|---|---|---|---|---|---|---|---|
| Working Session | b1 ± ts | b0 ± ts | R2 | b1 ± ts | b0 ± ts | R2 | |
| Verapamil 10–200 | 1 | 0.023 ± 0.003 | 0.0 ± 0.2 | 0.993 | 0.023 ± 0.004 | 0.0 ± 0.3 | 0.996 |
| 2 | 0.024 ± 0.004 | 0.0 ± 0.3 | 0.9998 | 0.024 ± 0.002 | −0.01 ± 0.12 | 0.998 | |
| 3 | 0.026 ± 0.002 | −0.09 ± 0.11 | 0.999 | 0.026 ± 0.002 | −0.08 ± 0.10 | 0.999 | |
| 4 | 0.026 ± 0.003 | −0.1 ± 0.2 | 0.996 | 0.026 ± 0.003 | −0.1 ± 0.2 | 0.996 | |
| 5 | 0.021 ± 0.005 | 0.2 ± 0.4 | 0.98 | 0.023 ± 0.003 | 0.18 ± 0.18 | 0.99 | |
| 6 | 0.020 ± 0.002 | −0.01 ± 0.09 | 0.993 | 0.020 ± 0.002 | 0.01 ± 0.09 | 0.994 | |
| 7 | 0.017 ± 0.002 | −0.01 ± 0.08 | 0.993 | 0.017 ± 0.002 | −0.02 ± 0.08 | 0.994 | |
| Citalopram 5–300 | - | 0.029 ± 0.007 | −0.1 ± 0.7 | 0.991 | 0.016 ± 0.003 | 0.3 ± 0.3 | 0.997 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-Biosca, Y.; Escuder-Gilabert, L.; Sagrado, S.; Medina-Hernández, M.J. Enantioselective Study on the Biodegradation of Verapamil and Cytalopram by Chiral Capillary Electrophoresis. Separations 2021, 8, 29. https://doi.org/10.3390/separations8030029
Martín-Biosca Y, Escuder-Gilabert L, Sagrado S, Medina-Hernández MJ. Enantioselective Study on the Biodegradation of Verapamil and Cytalopram by Chiral Capillary Electrophoresis. Separations. 2021; 8(3):29. https://doi.org/10.3390/separations8030029
Chicago/Turabian StyleMartín-Biosca, Yolanda, Laura Escuder-Gilabert, Salvador Sagrado, and María José Medina-Hernández. 2021. "Enantioselective Study on the Biodegradation of Verapamil and Cytalopram by Chiral Capillary Electrophoresis" Separations 8, no. 3: 29. https://doi.org/10.3390/separations8030029
APA StyleMartín-Biosca, Y., Escuder-Gilabert, L., Sagrado, S., & Medina-Hernández, M. J. (2021). Enantioselective Study on the Biodegradation of Verapamil and Cytalopram by Chiral Capillary Electrophoresis. Separations, 8(3), 29. https://doi.org/10.3390/separations8030029





