Determination of Fosetyl-Aluminum in Wheat Flour with Extract-Dilute-Shoot Procedure and Hydrophilic Interaction Liquid Chromatography Tandem Mass Spectrometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Preparation of Calibration Solutions
2.3. Instruments
2.4. Optimization of Extract-Dilute-Shoot Parameters
2.5. Sample Pretreatment
2.6. Hydrophilic Interaction Liquid Chromatography Tandem Mass Spectrometry
2.7. Method Validation
2.8. Real Sample Analysis
3. Results and Discussion
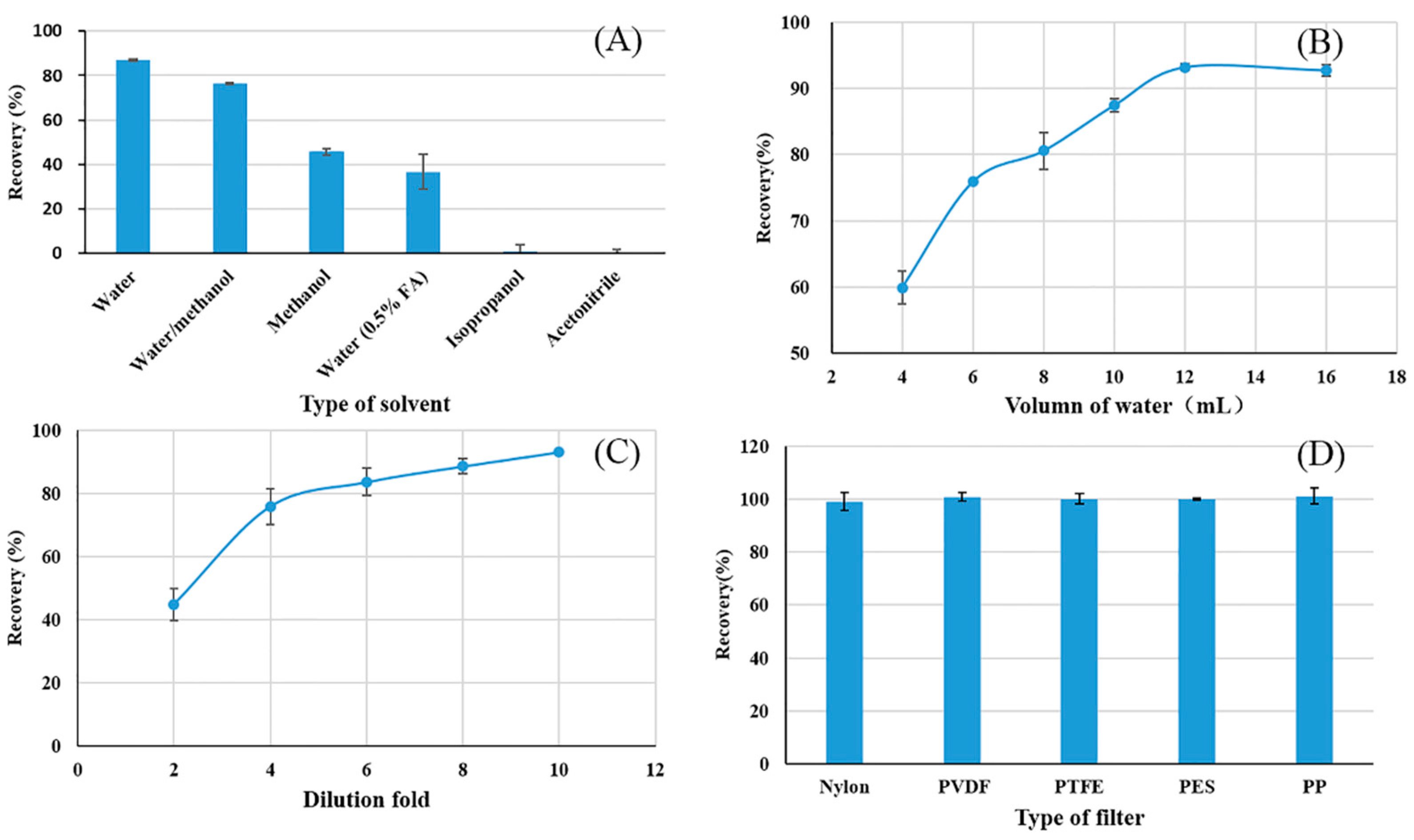
3.1. Extract-Dilute-Shoot Optimization
3.1.1. Type of Extraction Solvent
3.1.2. Volume of Extraction Solvent
3.1.3. Effect of Dilution Fold
3.1.4. Type of Filter
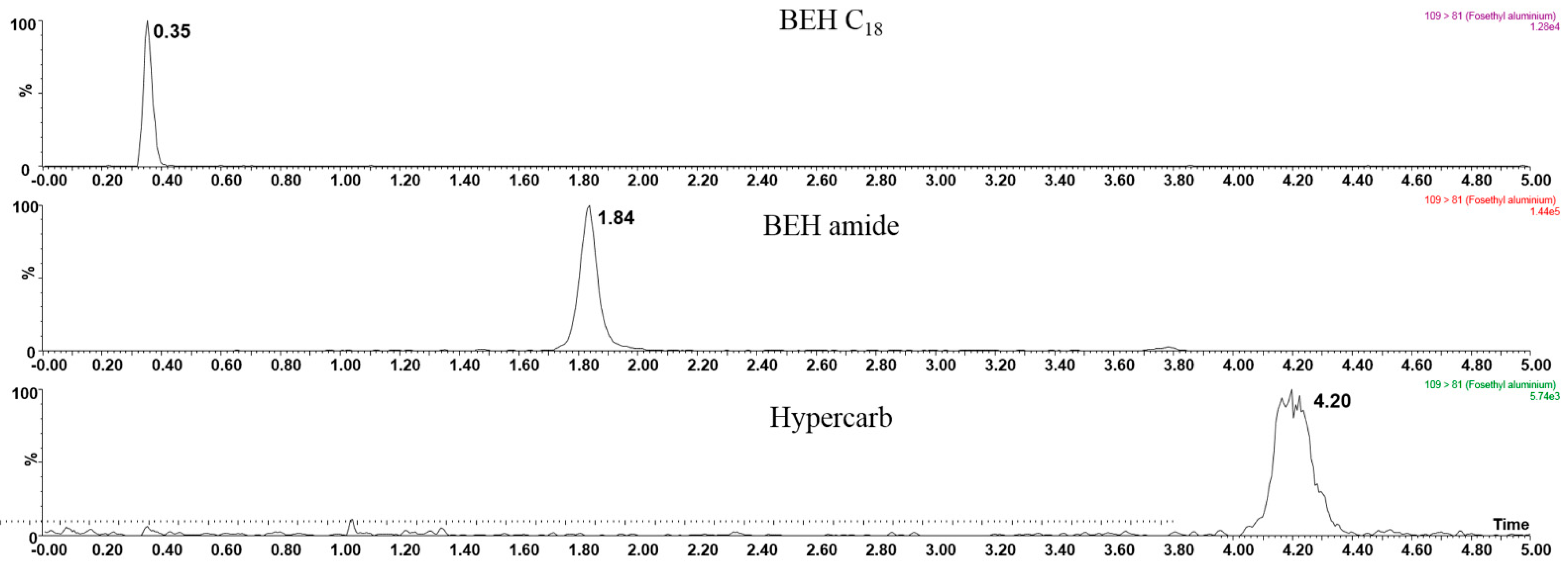
3.2. Hydrophilic Interaction Liquid Chromatography Tandem Mass Spectrometry
3.3. Method Validation
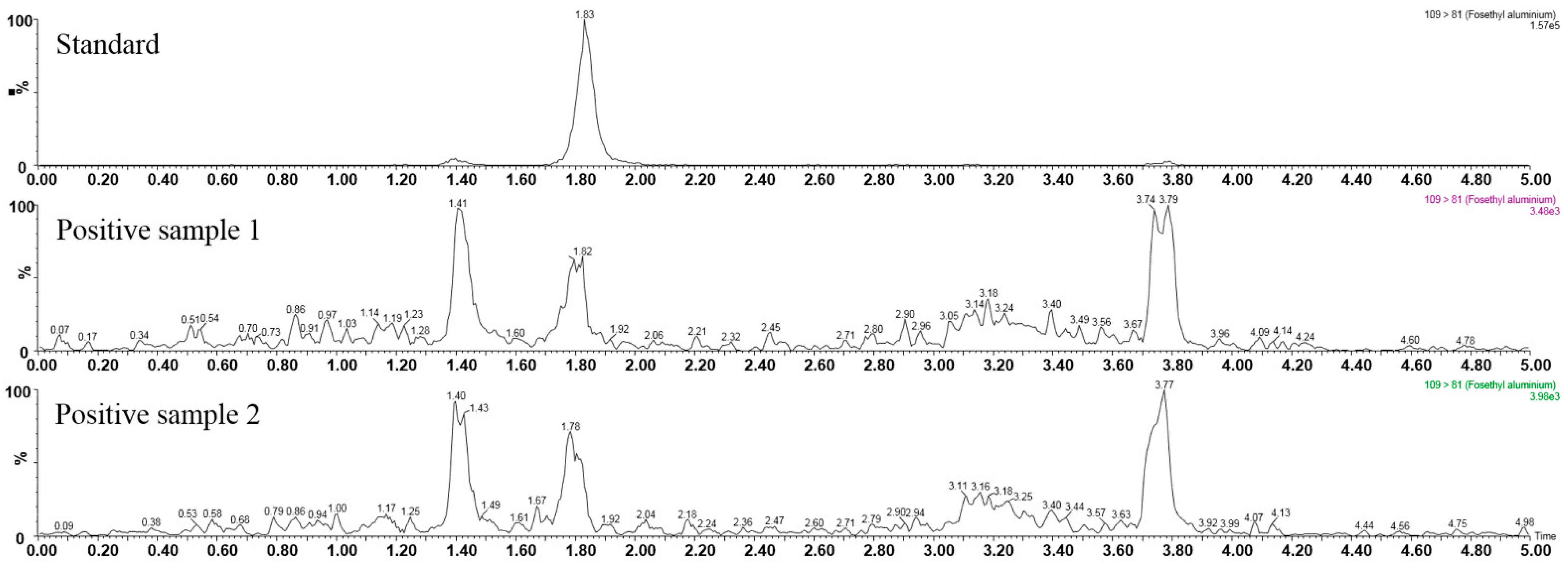
3.4. Real Sample Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Chamkasem, N. Determination of Glyphosate, Maleic Hydrazide, Fosetyl Aluminum, and Ethephon in Grapes by Liquid Chromatography/Tandem Mass Spectrometry. J. Agric. Food. Chem. 2017, 65, 7535–7541. [Google Scholar] [CrossRef] [PubMed]
- Pesticide Residues in Food; Joint FAO/WHO Meeting on Pesticide Residues: Geneva, Switzerland, 2017; Available online: http://www.fao.org/fileadmin/templates/agphome/documents/Pests_Pesticides/JMPR/Report2017/web_2017_JMPR_Report_Final.pdf (accessed on 20 September 2021).
- European Food Safety Authority. Modification of the existing maximum residue level for fosetyl in blackberry, celeriac and Florence fennel. EFSA J. 2015, 13, 4327. [Google Scholar] [CrossRef] [Green Version]
- NHC; MoA; SAMR of China. National Food Safety Standard—Maximum Residue Limits for Pesticides in Food; China Agriculture Press: Beijing, China, 2020.
- Aluminum Tris (O-ethylphosphonate); Tolerances for Residues. Available online: https://www.ecfr.gov/cgi-bin/text-idx?SID=c30d88a8176fe20021d927e53e9422c7&mc=true&node=se40.26.180_1415&rgn=div8 (accessed on 20 September 2021).
- Buiarelli, F.; Di Filippo, P.; Riccardi, C.; Pomata, D.; Marsiglia, R.; Console, C.; Puri, D. Hydrophilic Interaction Liquid Chromatography-Tandem Mass Spectrometry Analysis of Fosetyl-Aluminum in Airborne Particulate Matter. J. Anal. Methods Chem. 2018, 2018, 8792085. [Google Scholar] [CrossRef] [PubMed]
- Hernández, F.; Sancho, J.V.; Pozo, Ó.J.; Villaplana, C.; Ibáñez, M.; Grimalt, S. Rapid Determination of Fosetyl-Aluminum Residues in Lettuce by Liquid Chromatography/Electrospray Tandem Mass Spectrometry. J. AOAC Int. 2003, 86, 832–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Wang, H.; Li, X.; Zeng, X.; Du, Z.; Cao, J.; Jiang, W. Metal–organic framework for the extraction and detection of pesticides from food commodities. Compr. Rev. Food Sci. F. 2021, 20, 1009–1035. [Google Scholar] [CrossRef]
- Manzano-Sanchez, L.; Martinez-Martinez, J.A.; Dominguez, I.; Martinez Vidal, J.L.; Frenich, A.G.; Romero-Gonzalez, R. Development and Application of a Novel Pluri-Residue Method to Determine Polar Pesticides in Fruits and Vegetables through Liquid Chromatography High Resolution Mass Spectrometry. Foods 2020, 9, 553. [Google Scholar] [CrossRef]
- Marinho Pereira da Silva, H.C.; Galindo Bedor, D.C.; Cunha, A.N.; dos Santos Rodrigues, H.O.; Telles, D.L.; Pessoa Araujo, A.C.; de Santana, D.P. Ethephon and fosetyl residues in fruits from São Francisco Valley, Brazil. Food Addit. Contam. B 2019, 13, 16–24. [Google Scholar] [CrossRef]
- Nortes-Mendez, R.; Robles-Molina, J.; Lopez-Blanco, R.; Vass, A.; Molina-Diaz, A.; Garcia-Reyes, J.F. Determination of polar pesticides in olive oil and olives by hydrophilic interaction liquid chromatography coupled to tandem mass spectrometry and high resolution mass spectrometry. Talanta 2016, 158, 222–228. [Google Scholar] [CrossRef]
- López, S.H.; Scholten, J.; Kiedrowska, B.; de Kok, A. Method validation and application of a selective multiresidue analysis of highly polar pesticides in food matrices using hydrophilic interaction liquid chromatography and mass spectrometry. J. Chromatogr. A 2019, 1594, 93–104. [Google Scholar] [CrossRef]
- Savini, S.; Bandini, M.; Sannino, A. An Improved, Rapid, and Sensitive Ultra-High-Performance Liquid Chromatography-High-Resolution Orbitrap Mass Spectrometry Analysis for the Determination of Highly Polar Pesticides and Contaminants in Processed Fruits and Vegetables. J. Agric. Food. Chem. 2019, 67, 2716–2722. [Google Scholar] [CrossRef] [PubMed]
- Dias, J.; López, S.H.; Mol, H.; de Kok, A. Influence of different hydrophilic interaction liquid chromatography stationary phases on method performance for the determination of highly polar anionic pesticides in complex feed matrices. J. Sep. Sci. 2021, 44, 2165–2176. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yang, F.-Q.; Ge, L.; Hu, Y.-J.; Xia, Z.-N. Recent applications of hydrophilic interaction liquid chromatography in pharmaceutical analysis. J. Sep. Sci. 2017, 40, 49–80. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, S.M.; Makarem, S.; Alexovič, M.; Tabani, H. Simultaneous separation and quantification of acidic and basic dye specimens via a dual gel electro-membrane extraction from real environmental samples. J. Iran. Chem. Soc. 2021, 18, 2091–2099. [Google Scholar] [CrossRef]
- Li, X.; Li, H.; Ma, W.; Guo, Z.; Li, X.; Li, X.; Zhang, Q. Determination of patulin in apple juice by single-drop liquid-liquid-liquid microextraction coupled with liquid chromatography-mass spectrometry. Food Chem. 2018, 257, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ma, W.; Li, H.; Zhang, Q.; Ma, Z. Determination of residual fipronil and its metabolites in food samples: A review. Trends Food Sci. Technol. 2020, 97, 185–195. [Google Scholar] [CrossRef]
- Gao, S.; Wu, G.; Li, X.; Chen, J.; Wu, Y.; Wang, J.; Zhang, Z. Determination of Triazine Herbicides in Environmental Water Samples by Acetonitrile Inorganic Salt Aqueous Two-Phase Microextraction System. J. Anal. Test. 2018, 2, 322–331. [Google Scholar] [CrossRef]
- Rajski, L.; Diaz Galiano, F.J.; Cutillas, V.; Fernandez-Alba, A.R. Coupling Ion Chromatography to Q-Orbitrap for the Fast and Robust Analysis of Anionic Pesticides in Fruits and Vegetables. J. AOAC Int. 2018, 101, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Anastassiades, M.; Kolberg, D.I.; Wachtler, E.E.A.-K.; Benkenstein, A.; Zechmann, S.; Mack, D.; Wildgrube, C.; Barth, A.; Sigalov, I.; Görlich, S.; et al. Quick Method for the Analysis of Numerous Highly Polar Pesticides in Food Involving Extraction with Acidified Methanol and LC-MS/MS Measurement I. Food of Plant Origin (QuPPe-PO-Method), 11th ed.; EU Reference Laboratory for Pesticides: Stuttgart, Germany, 2020; Available online: https://www.eurl-pesticides.eu/userfiles/file/EurlSRM/meth_QuPPe_PO_V11(1).pdf (accessed on 20 September 2021).
- Greer, B.; Chevallier, O.; Quinn, B.; Botana, L.M.; Elliott, C.T. Redefining dilute and shoot: The evolution of the technique and its application in the analysis of foods and biological matrices by liquid chromatography mass spectrometry. Trends Anal. Chem. 2021, 141, 116284. [Google Scholar] [CrossRef]
- da Silva, L.P.; Madureira, F.; de Azevedo Vargas, E.; Faria, A.F.; Augusti, R. Development and validation of a multianalyte method for quantification of mycotoxins and pesticides in rice using a simple dilute and shoot procedure and UHPLC-MS/MS. Food Chem. 2019, 270, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Gebrehiwot, W.H.; Erkmen, C.; Uslu, B. A novel HPLC-DAD method with dilute-and-shoot sample preparation technique for the determination of buprofezin, dinobuton and chlorothalonil in food, environmental and biological samples. Int. J. Environ. Anal. Chem. 2020, 4, 1–15. [Google Scholar] [CrossRef]
- Petrarca, M.H.; Meinhart, A.D.; Godoy, H.T. Dilute-and-Shoot Liquid Chromatography Approach for Simple and High-throughput Analysis of 5-Hydroxymethylfurfural in Fruit-based Baby Foods. Food Anal. Methods 2020, 13, 942–951. [Google Scholar] [CrossRef]
- Tölgyesi, Á.; Sharma, V.K. Determination of acrylamide in gingerbread and other food samples by HILIC-MS/MS: A dilute-and-shoot method. J. Chromatogr. B 2020, 1136, 121933. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Xiao, W.; Wan, Y.; Li, Z.; Luo, D.; Yang, H. Dispersive solid-phase extraction using microporous metal-organic framework UiO-66: Improving the matrix compounds removal for assaying pesticide residues in organic and conventional vegetables. Food Chem. 2021, 345, 128807. [Google Scholar] [CrossRef]
- Alves, R.D.; Romero-González, R.; López-Ruiz, R.; Jiménez-Medina, M.L.; Frenich, A.G. Fast determination of four polar contaminants in soy nutraceutical products by liquid chromatography coupled to tandem mass spectrometry. Anal. Bioanal. Chem. 2016, 408, 8089–8098. [Google Scholar] [CrossRef] [PubMed]
- Mol, H.G.J.; van Dam, R.C.J. Rapid detection of pesticides not amenable to multi-residue methods by flow injection–tandem mass spectrometry. Anal. Bioanal. Chem. 2014, 406, 6817–6825. [Google Scholar] [CrossRef]
- Flour, Whole Wheat, Unenriched. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/790085/nutrients (accessed on 10 September 2021).
- Li, X.; Li, H.; Ma, W.; Guo, Z.; Li, X.; Song, S.; Tang, H.; Li, X.; Zhang, Q. Development of precise GC-EI-MS method to determine the residual fipronil and its metabolites in chicken egg. Food Chem. 2019, 281, 85–90. [Google Scholar] [CrossRef]
- Kozlik, P.; Molnarova, K.; Jecmen, T.; Krizek, T.; Goldman, R. Glycan-specific precipitation of glycopeptides in high organic content sample solvents used in HILIC. J. Chromatogr. B 2020, 1150, 122196. [Google Scholar] [CrossRef]
- Doulia, D.S.; Anagnos, E.K.; Liapis, K.S.; Klimentzos, D.A. Removal of pesticides from white and red wines by microfiltration. J. Hazard. Mater. 2016, 317, 135–146. [Google Scholar] [CrossRef]
- Chamkasem, N.; Harmon, T. Direct determination of glyphosate, glufosinate, and AMPA in soybean and corn by liquid chromatography/tandem mass spectrometry. Anal. Bioanal. Chem. 2016, 408, 4995–5004. [Google Scholar] [CrossRef]
| Linear Range (ng/g) | Linearity (r2) | LOD (ng/g) | ME | RSDs% (n = 5) | Recovery | ||
|---|---|---|---|---|---|---|---|
| 10 ng/g | 50 ng/g | 100 ng/g | |||||
| 10–2000 | 0.9999 | 5 | 88.33% | 6.2 | 95.6% | 105.2% | 104.6% |
| Matrix | Sample Preparation | ME (%) | LOD (ng/g) | LOQ (ng/g) | Linear Range (ng/g) | Linearity | Ref. |
|---|---|---|---|---|---|---|---|
| Grape | SPE | 13 | 9 | 29 | 10–1000 1 | 0.995 | [1] |
| Lettuce | Water extraction, 5-fold dilution | / | 50 | 200 | 5–10,001 | 0.9993 | [7] |
| Tomato | QuPPe method | 137 | / | 25 | 25–1000 | 0.999 | [9] |
| Mango | QuPPe method | 70 | / | 50 | 50–2000 | 0.99 | [10] |
| Olive oil | QuPPe method | 80 | / | 10 | 10–1000 | 0.9932 | [11] |
| Soy beans | Methanol extraction, 12.5-fold dilution | 96 | / | 20 | 10–10,000 | 0.9969 | [12] |
| Wheat flour | Water extraction, 6-fold dilution | 88.33 | 5 | 10 | 10–2000 | 0.9999 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Wang, S.; Guo, Z.; Li, X.; Zhang, Q.; Li, H. Determination of Fosetyl-Aluminum in Wheat Flour with Extract-Dilute-Shoot Procedure and Hydrophilic Interaction Liquid Chromatography Tandem Mass Spectrometry. Separations 2021, 8, 197. https://doi.org/10.3390/separations8110197
Li X, Wang S, Guo Z, Li X, Zhang Q, Li H. Determination of Fosetyl-Aluminum in Wheat Flour with Extract-Dilute-Shoot Procedure and Hydrophilic Interaction Liquid Chromatography Tandem Mass Spectrometry. Separations. 2021; 8(11):197. https://doi.org/10.3390/separations8110197
Chicago/Turabian StyleLi, Xianjiang, Sheng Wang, Zhen Guo, Xiuqin Li, Qinghe Zhang, and Hongmei Li. 2021. "Determination of Fosetyl-Aluminum in Wheat Flour with Extract-Dilute-Shoot Procedure and Hydrophilic Interaction Liquid Chromatography Tandem Mass Spectrometry" Separations 8, no. 11: 197. https://doi.org/10.3390/separations8110197
APA StyleLi, X., Wang, S., Guo, Z., Li, X., Zhang, Q., & Li, H. (2021). Determination of Fosetyl-Aluminum in Wheat Flour with Extract-Dilute-Shoot Procedure and Hydrophilic Interaction Liquid Chromatography Tandem Mass Spectrometry. Separations, 8(11), 197. https://doi.org/10.3390/separations8110197








