Extraction of Natural Pigments from Gardenia Jasminoides J.Ellis Fruit Pulp Using CO2-Expanded Liquids and Direct Sonication
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Conventional Solid–Liquid Extraction (SLE)
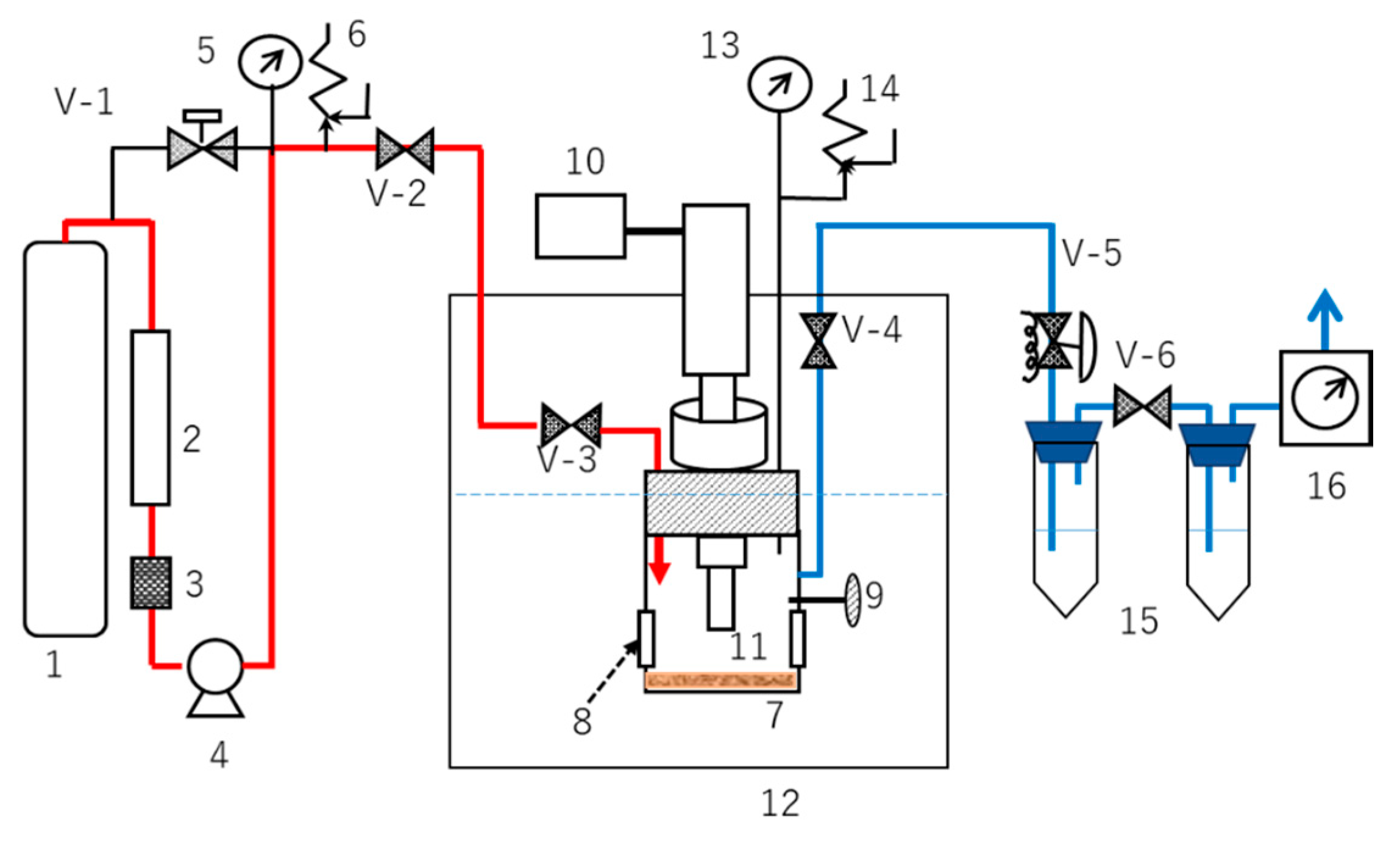
2.3. CO2-Expanded Liquids (CXLs) Extraction with or without Direct Sonication
2.4. Conventional Ultrasound-Assisted Extraction (UAE)
2.5. Analytical Procedures
2.5.1. High-Performance Liquid Chromatography (HPLC) Analysis
2.5.2. Analytical Method Validation
2.5.3. Standard Solutions and Calibration Curve
2.6. Statistical Analysis
3. Results and Discussion
3.1. HPLC-UV Profile
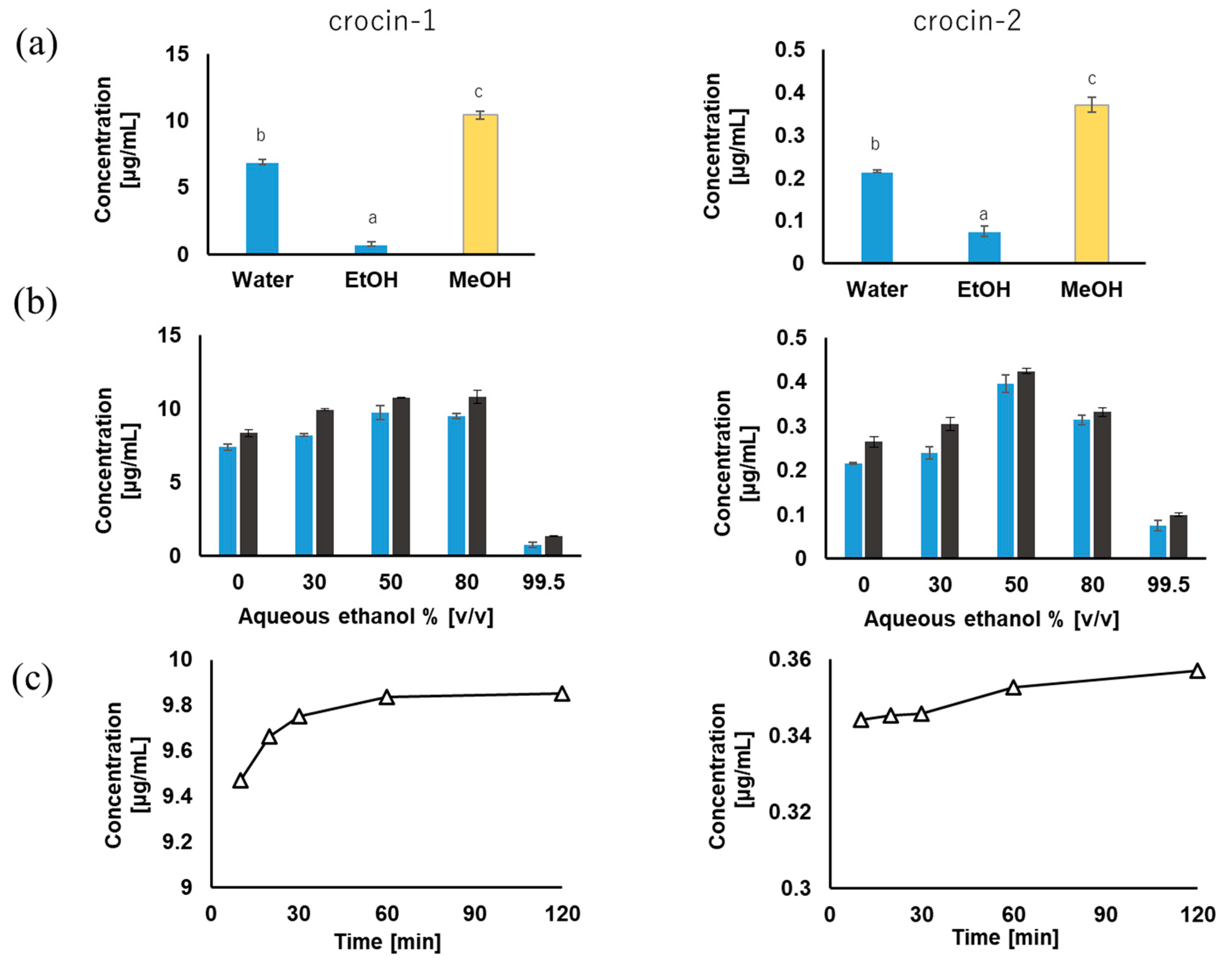
3.2. Conventional Solid–Liquid Extraction (SLE)
3.2.1. Effect of Solvents on Extraction Concentration
3.2.2. Effect of Ethanol Concentration on the Extraction
3.2.3. Effect of Extraction Time on the Concentration
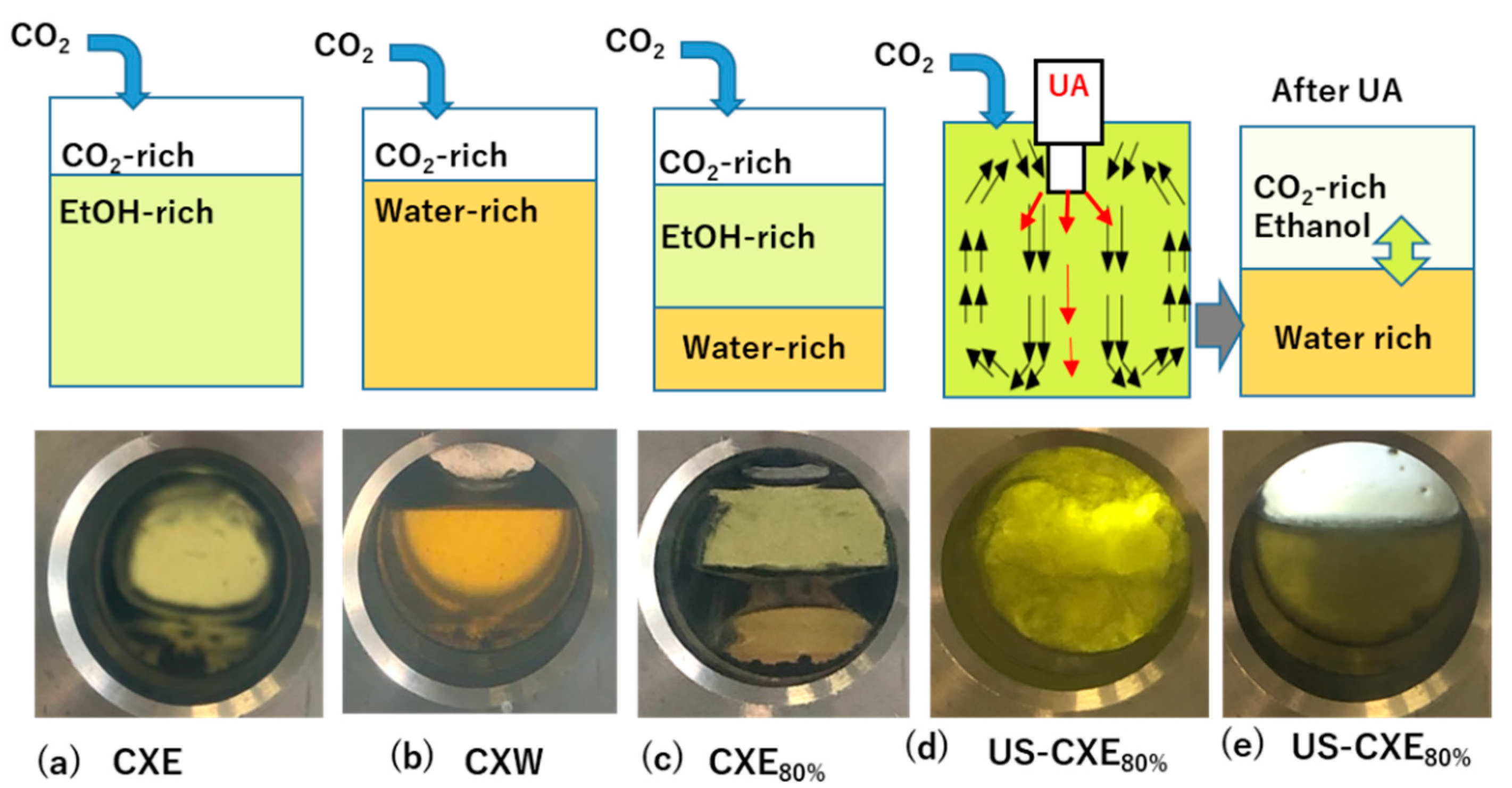
3.3. CO2-Expanded Liquid (CXL) Extraction System
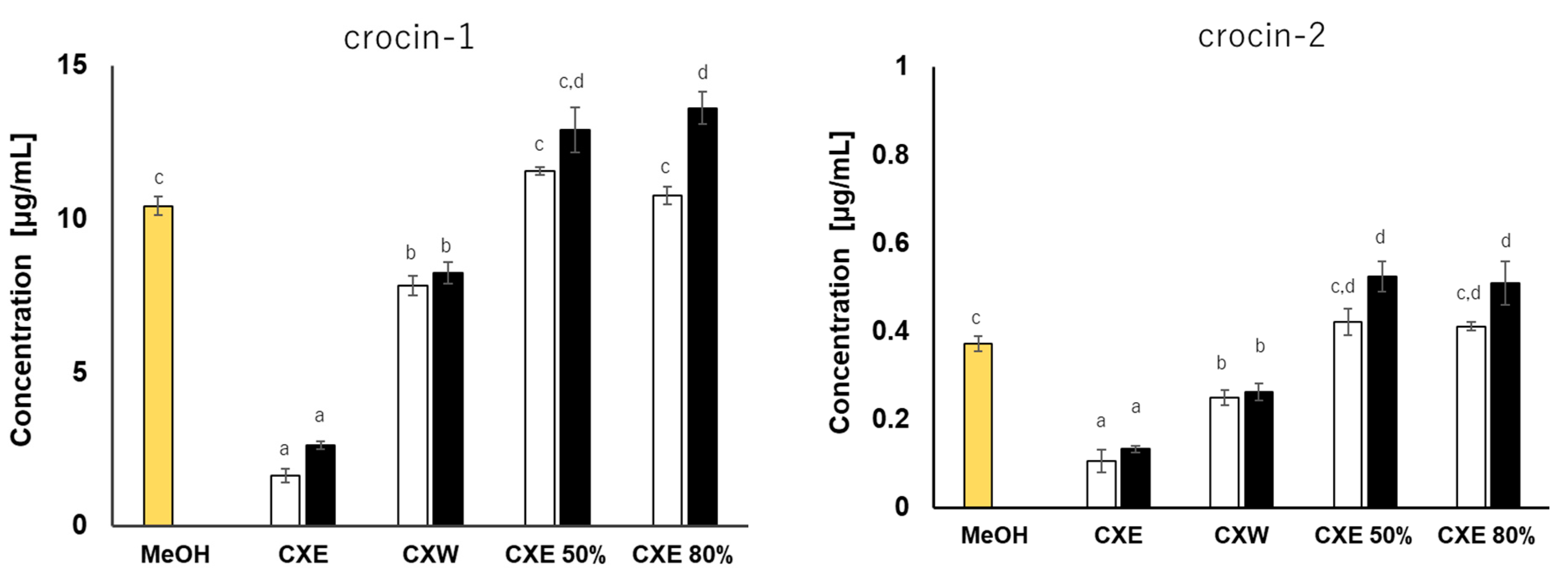
3.3.1. Effect of Modifiers on Extraction Concentration with or without Direct Sonication
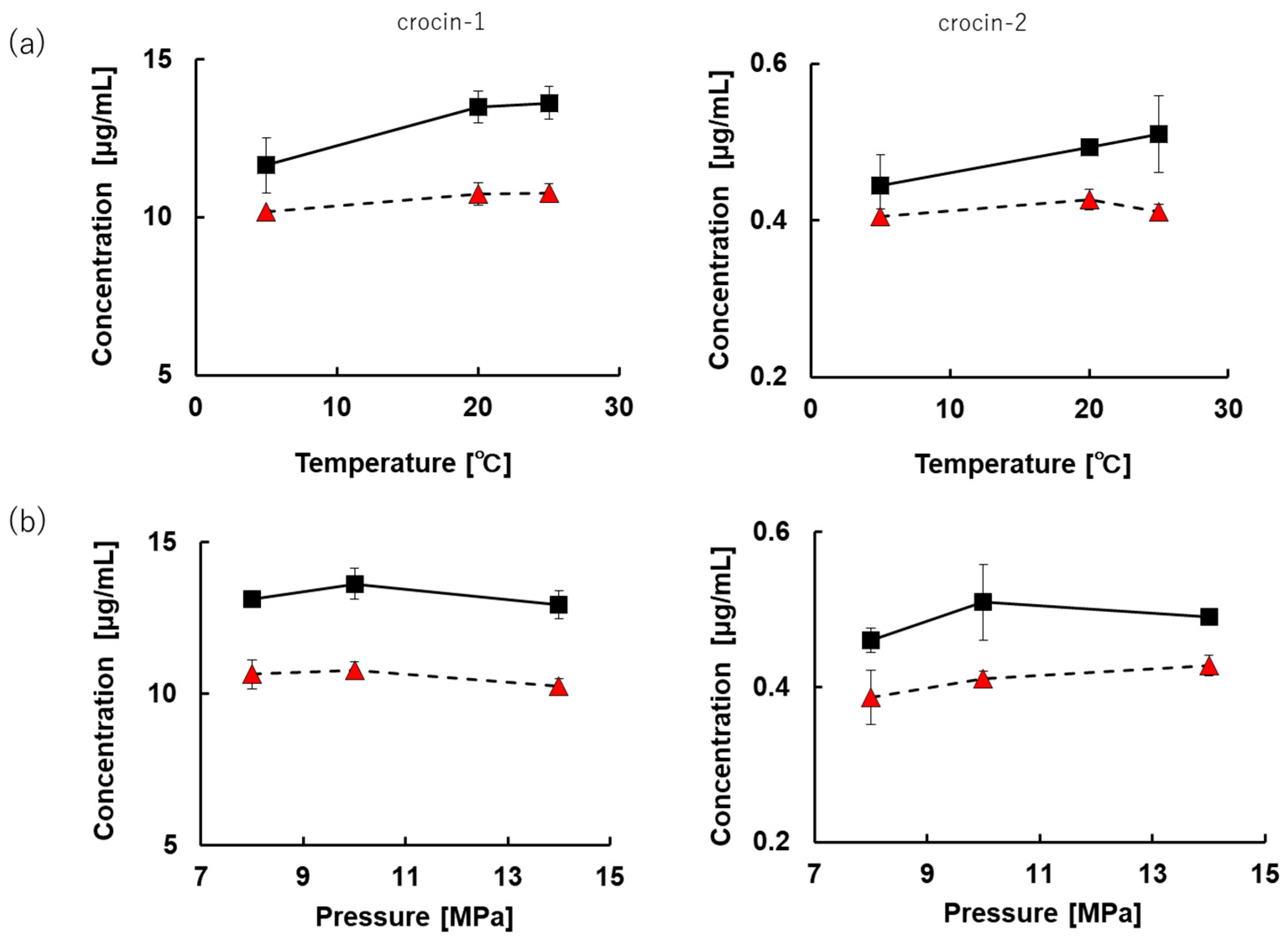
3.3.2. Effects of Temperature and Pressure on Extraction Concentration in CXE80% System with or without Direct Sonication
3.3.3. Effect of Sonication Time on Extraction Concentration in CXE80% System
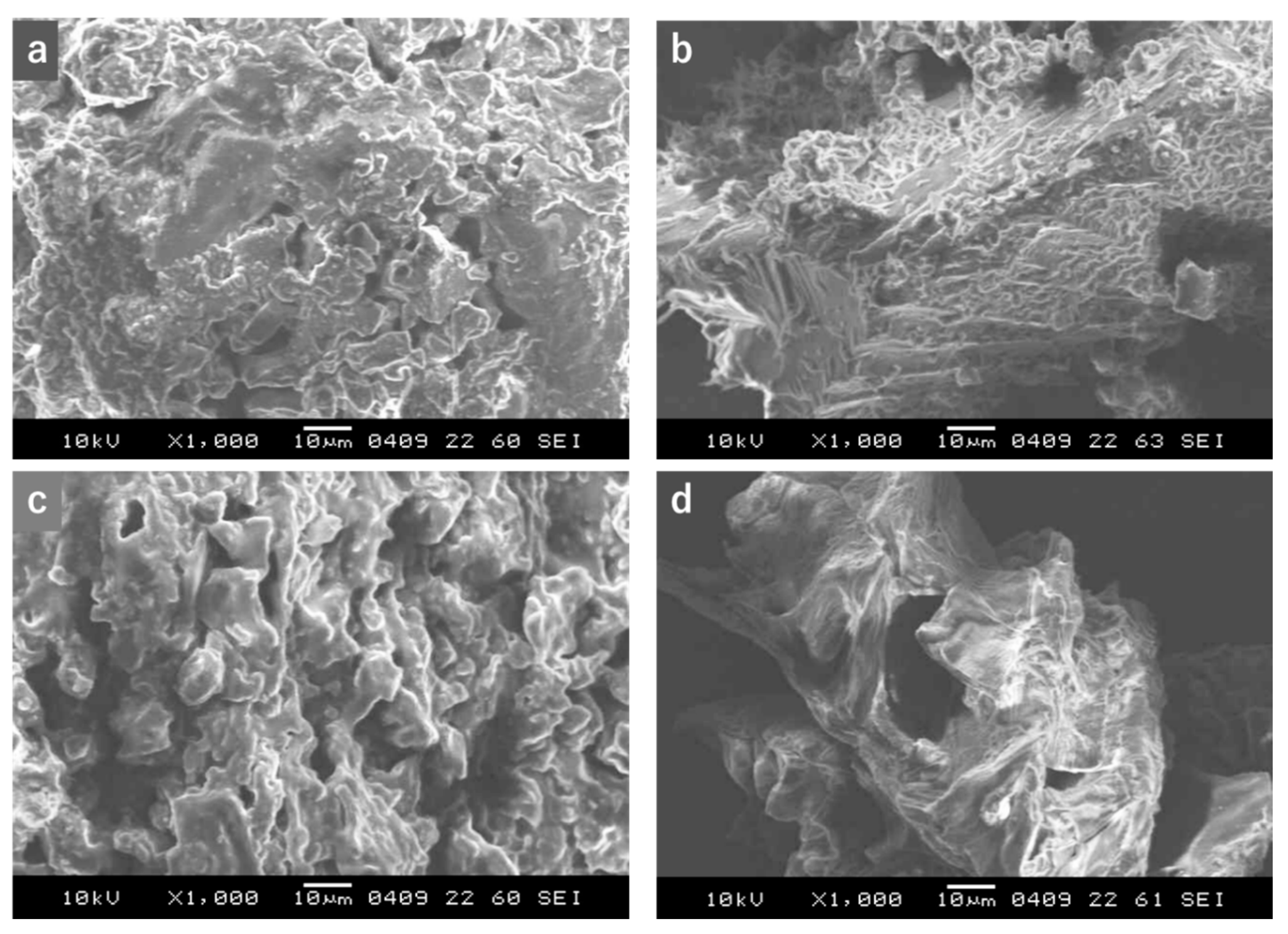
3.4. Proposed Mechanism of Extraction in CXL Extraction System with or without Direct Sonication
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hong, I.K.; Jeon, H.; Lee, S.B. Extraction of natural dye from Gardenia and chromaticity analysis according to chi parameter. J. Ind. Eng. Chem. 2015, 24, 326–332. [Google Scholar] [CrossRef]
- Yen, H.-W.; Yang, S.-C.; Chen, C.-H.; Jesisca; Chang, J.-S. Supercritical fluid extraction of valuable compounds from microalgal biomass. Bioresour. Technol. 2015, 184, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Sinha, K.; Saha, P.D.; Datta, S. Extraction of natural dye from petals of flame of forest (Butea monosperma) flower: Process optimization using response surface methodology (RSM). Dye. Pigment. 2012, 94, 212–216. [Google Scholar] [CrossRef]
- Ali, S.; Hussain, T.; Nawaz, R. Optimization of alkaline extraction of natural dye from Henna leaves and its dyeing on cotton by exhaust method. J. Clean. Prod. 2009, 17, 61–66. [Google Scholar] [CrossRef]
- Carmona, M.; Zalacain, A.; Sánchez, A.M.; Novella, J.L.; Alonso, G.L. Crocetin esters, picrocrocin and its related compounds present in Crocus sativus stigmas and Gardenia jasminoides fruits. Tentative identification of seven new compounds by LC-ESI-MS. J. Agric. Food Chem. 2006, 54, 973–979. [Google Scholar] [CrossRef]
- Yamada, S.; Oshima, H.; Saito, I.; Hayakawa, J. Adoption of crocetin as an indicator compound for detection of gardenia yellow in food products (Analysis of natural coloring matters in food V). J. Food Hyg. Soc. Jpn. 1996, 37, 372–377. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Wang, J.; Li, X.; Du, Z. A new method research for determination of natural pigment crocin yellow in foods by solid-phase extraction ultrahigh pressure liquid chromatography. J. Chromatogr. A 2011, 1218, 1423–1428. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, H.; Tian, X.; Zhao, C.; Cai, L.; Liu, Y.; Jia, L.; Yin, H.-X.; Chen, C. Antioxidant potential of crocins and ethanol extracts of Gardenia jasminoides ELLIS and Crocus sativus L.: A relationship investigation between antioxidant activity and crocin contents. Food Chem. 2008, 109, 484–492. [Google Scholar] [CrossRef]
- Nam, K.N.; Park, Y.-M.; Jung, H.-J.; Lee, J.Y.; Min, B.D.; Park, S.-U.; Jung, W.-S.; Cho, K.-H.; Park, J.-H.; Kang, I.; et al. Anti-inflammatory effects of crocin and crocetin in rat brain microglial cells. Eur. J. Pharmacol. 2010, 648, 110–116. [Google Scholar] [CrossRef]
- Koo, H.-J.; Lim, K.-H.; Jung, H.-J.; Park, E.-H. Anti-inflammatory evaluation of gardenia extract, geniposide and genipin. J. Ethnopharmacol. 2006, 103, 496–500. [Google Scholar] [CrossRef]
- He, S.-Y.; Qian, Z.-Y.; Tang, F.-T.; Wen, N.; Xu, G.-L.; Sheng, L. Effect of crocin on experimental atherosclerosis in quails and its mechanisms. Life Sci. 2005, 77, 907–921. [Google Scholar] [CrossRef] [PubMed]
- Aung, H.H.; Wang, C.Z.; Ni, M.; Fishbein, A.; Mehendale, S.R.; Xie, J.T.; Shoyama, A.Y.; Yuan, C.S. Crocin from crocus sativus possesses significant anti-proliferation effects on human colorectal cancer cells. Exp. Oncol. 2007, 29, 175–180. [Google Scholar] [PubMed]
- Yorgun, M.A. Effects of crocin on diabetic maculopathy: A placebo-controlled randomized clinical trial. Am. J. Ophthalmol. 2019, 204, 141–142. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim-Habibi, M.-B.; Amininasad, M.; Ebrahim-Habibi, A.; Sabbghian, M.; Nemat-Gorgani, M. Fibrillation of α-lactalbumin: Effect of crocin and safranal, two natural small molecules from crocus sativus. Biopolymers 2010, 93, 854–865. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Mang, Y.; Shen, F.; Xie, J.; Su, W. Homogenate extraction of gardenia yellow pigment from Gardenia Jasminoides ellis fruit using response surface methodology. J. Food Sci. Technol. 2014, 51, 1575–1581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Dong, S.; Chen, F. Ultrasonic-assisted extraction of gardenia yellow from fructus Gardeniae. Adv. Mat. Res. 2012, 396, 1075–1078. [Google Scholar] [CrossRef]
- Budisa, N.; Schulze-Makuch, D. Supercritical carbon dioxide and its potential as a life-sustaining solvent in a planetary environment. Life 2014, 4, 331–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karasu, S.; Bayram, Y.; Ozkan, K.; Sagdic, O. Extraction optimization crocin pigments of saffron (crocus sativus) using response surface methodology and determination stability of crocin microcapsules. J. Food Meas. Charact. 2019, 13, 1515–1523. [Google Scholar] [CrossRef]
- del Pilar Sánchez-Camargoa, A.; Mendiolab, J.A.; Ibáñez, E. Gas expanded-liquids. In Supercritical and Other High-Pressure Solvent Systems: For Extraction, Reaction and Material Processing; Hunt, A.J., Attard, T.M., Eds.; Royal Society of Chemistry: London, UK, 2018; pp. 512–529. [Google Scholar]
- Rodríguez-Pérez, C.; Mendiola, J.A.; Quirantes-Piné, R.; Ibáñez, E.; Segura-Carretero, A. Green downstream processing using supercritical carbon dioxide: CO2-expanded ethanol and pressurized hot water extractions for recovering bioactive compounds from Moringa oleifera leaves. J. Supercrit. Fluids 2016, 116, 90–100. [Google Scholar] [CrossRef]
- Paudel, A.; Jessop, M.J.; Stubbins, S.H.; Champagne, P.; Jessop, P.G. Extraction of lipids from microalgae using CO2-expanded methanol and liquid CO2. Bioresour. Technol. 2015, 184, 286–290. [Google Scholar] [CrossRef]
- Al-Hamimi, S.; Mayoral, A.A.; Cunico, L.P.; Charlotta Turner, C. Carbon dioxide expanded ethanol extraction: Solubility and extraction kinetics of α-pinene and cis-verbenol. Anal. Chem. 2016, 88, 4336–4345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- del-Valle, J.M.; Martín, Á.; Cocero, M.J.; de la Fuente, J.C.; de la Cruz-Quiroz, R. Supercritical CO2 extraction of solids using aqueous ethanol as static modifier is a two-step mass transfer process. J. Supercrit. Fluids 2019, 143, 179–190. [Google Scholar] [CrossRef]
- Kawamura, H.; Mishima, K.; Sharmin, T.; Ito, S.; Kawakami, R.; Kato, T.; Misumi, M.; Suetsugu, T.; Orii, H.; Kawano, H.; et al. Ultrasonically enhanced extraction of luteolin and apigenin from the leaves of Perilla frutescens (L.) Britt. using liquid carbon dioxide and ethanol. Ultrason. Sonochem. 2016, 29, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, S.; Tashiro, H.; Ono, K.; Sharmin, T.; Kato, T.; Irie, K.; Mishima, K.; Satho, T.; Aida, T.M.; Mishima, K. Rapid production of liposomes using high pressure carbon dioxide and direct ultrasonication. J. Supercrit. Fluids 2020, 160, 104782. [Google Scholar] [CrossRef]
- Mishima, K.; Kawakami, R.; Yokota, H.; Harada, T.; Kato, T.; Kawamura, H.; Matsuyama, K.; Mustofa, S.; Hasanah, F.; Siregar, Y.D.I.; et al. Extraction of Luteolin and Apigenin from leaves of Perilla frutescens (L.) Britt. with Liquid Carbon Dioxide. Solvent Extr. Res. Dev. Jpn. 2014, 21, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Cristea, D.; Bareau, I.; Vilarem, G. Identification and quantitative HPLC analysis of the main flavonoids present in weld (Reseda luteola L.). Dye. Pigm. 2003, 57, 267–272. [Google Scholar] [CrossRef]
- Hojnik, M.; Škerget, M.; Knez, Ž. Isolation of chlorophylls from stinging nettle (Urticadioica L.). Sep. Purif. Technol. 2007, 57, 37–46. [Google Scholar] [CrossRef]
- Chemat, F.; Zill-e-Huma; KamranKhan, M. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef]
- Jessop, P.G.; Subramaniam, B. Gas-expanded liquids. Chem. Rev. 2007, 107, 2666–2694. [Google Scholar] [CrossRef]
- Nerome, H.; Ito, M.; Machmudah, S.; Wahyudiono; Kanda, H.; Goto, M. Extraction of phytochemicals from saffron by supercritical carbondioxide with water and methanol as entrainer. J. Supercrit. Fluids 2016, 107, 377–383. [Google Scholar] [CrossRef]


| Time (min) | Methanol (vol%) |
|---|---|
| 0 | 5 |
| 25 | 40 |
| 40 | 60 |
| 50 | 90 |
| 60 | 90 |
| 65 | 5 |
| 75 | 5 |
| Parameter | Crocin-1 | Crocin-2 | |
|---|---|---|---|
| Linearity | Concentration range of crocin-1 standards (μg/mL) | 2.25–36.0 | 0.125–2.0 |
| Correlation coefficient of linearity equation | 0.9995 | 0.9997 | |
| Intercept of linearity equation | 17439 | −8999.8 | |
| Slope of linearity equation | 2.0 × 108 | 4.0 × 108 | |
| Limit of detection (μg/mL) | 0.34 | 0.01 | |
| Limit of quantification (μg/mL) | 1.13 | 0.04 | |
| Precision | Intra-day (RSD, % of peak area, n = 3) | ||
| Day 1 | 2.74 | 1.84 | |
| Day 2 | 2.75 | 0.53 | |
| Day 3 | 1.09 | 1.52 | |
| Inter-day (RSD, % of peak area n = 3) | 3.47 | 1.05 | |
| Extraction Methods | Extraction Conditions | Concentration | ||||||
|---|---|---|---|---|---|---|---|---|
| Solvent | Modifier | T (°C) | P (MPa) | t (min) | US ampl. (%) | Crocin-1 (μg/mL) | Crocin-2 (μg/mL) | |
| Solid–liquid | Methanol | - | 25 | 0.1 | 60 | - | b 10.43 ± 0.31 | c 0.37 ± 0.02 |
| Ethanol | - | 25 | 0.1 | 60 | - | a 0.76 ± 0.17 | a 0.08 ± 0.01 | |
| Water | - | 25 | 0.1 | 60 | - | b 6.93 ± 0.21 | b 0.22 ± 0.002 | |
| Con. UAE | Methanol | - | 40 | 0.1 | 40 | - | b 12.08 ± 0.35 | a 0.46 ± 0.02 |
| Ethanol | - | 40 | 0.1 | 40 | - | a 3.36 ± 0.31 | c 0.17 ± 0.01 | |
| Water | - | 40 | 0.1 | 40 | - | b 10.22 ± 0.35 | c 0.33 ± 0.01 | |
| CXLs | Liquid CO2 | Ethanol | 25 | 10 | 60 | - | a 1.64 ± 0.23 | a 0.11 ± 0.03 |
| Liquid CO2 | Water | 25 | 10 | 60 | - | b 7.84 ± 0.32 | b,c 0.25 ± 0.02 | |
| Liquid CO2 | 50% Ethanol | 25 | 10 | 60 | - | b,c 11.57 ± 0.12 | c,d 0.42 ± 0.03 | |
| Liquid CO2 | 80% Ethanol | 25 | 10 | 60 | - | b,c 10.77 ± 0.29 | c,d 0.41 ± 0.01 | |
| CXLs + DS | Liquid CO2 | Ethanol | 25 | 10 | 60 | 20% | a 2.63 ± 0.13 | a 0.13 ± 0.01 |
| Liquid CO2 | Water | 25 | 10 | 60 | 20% | b 8.25 ± 0.34 | b 0.26 ± 0.02 | |
| Liquid CO2 | 50% Ethanol | 25 | 10 | 60 | 20% | b,c,d 12.91 ± 0.75 | d 0.52 ± 0.03 | |
| Liquid CO2 | 80% Ethanol | 25 | 10 | 60 | 20% | d 13.63 ± 0.52 | d,e 0.51 ± 0.05 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakai, H.; Ono, K.; Tokunaga, S.; Sharmin, T.; Aida, T.M.; Mishima, K. Extraction of Natural Pigments from Gardenia Jasminoides J.Ellis Fruit Pulp Using CO2-Expanded Liquids and Direct Sonication. Separations 2021, 8, 1. https://doi.org/10.3390/separations8010001
Sakai H, Ono K, Tokunaga S, Sharmin T, Aida TM, Mishima K. Extraction of Natural Pigments from Gardenia Jasminoides J.Ellis Fruit Pulp Using CO2-Expanded Liquids and Direct Sonication. Separations. 2021; 8(1):1. https://doi.org/10.3390/separations8010001
Chicago/Turabian StyleSakai, Hiroki, Kento Ono, Shinichi Tokunaga, Tanjina Sharmin, Taku Michael Aida, and Kenji Mishima. 2021. "Extraction of Natural Pigments from Gardenia Jasminoides J.Ellis Fruit Pulp Using CO2-Expanded Liquids and Direct Sonication" Separations 8, no. 1: 1. https://doi.org/10.3390/separations8010001
APA StyleSakai, H., Ono, K., Tokunaga, S., Sharmin, T., Aida, T. M., & Mishima, K. (2021). Extraction of Natural Pigments from Gardenia Jasminoides J.Ellis Fruit Pulp Using CO2-Expanded Liquids and Direct Sonication. Separations, 8(1), 1. https://doi.org/10.3390/separations8010001





