Detection of Microbial Nitroreductase Activity by Monitoring Exogenous Volatile Organic Compound Production Using HS-SPME-GC-MS
Abstract
1. Introduction
2. Materials and Reagents
2.1. Instrumentation
2.2. Nitroreductase Activity Study across 51 Microorganisms
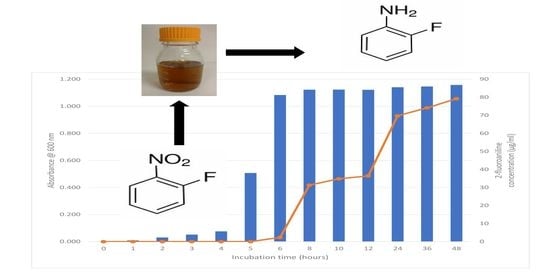
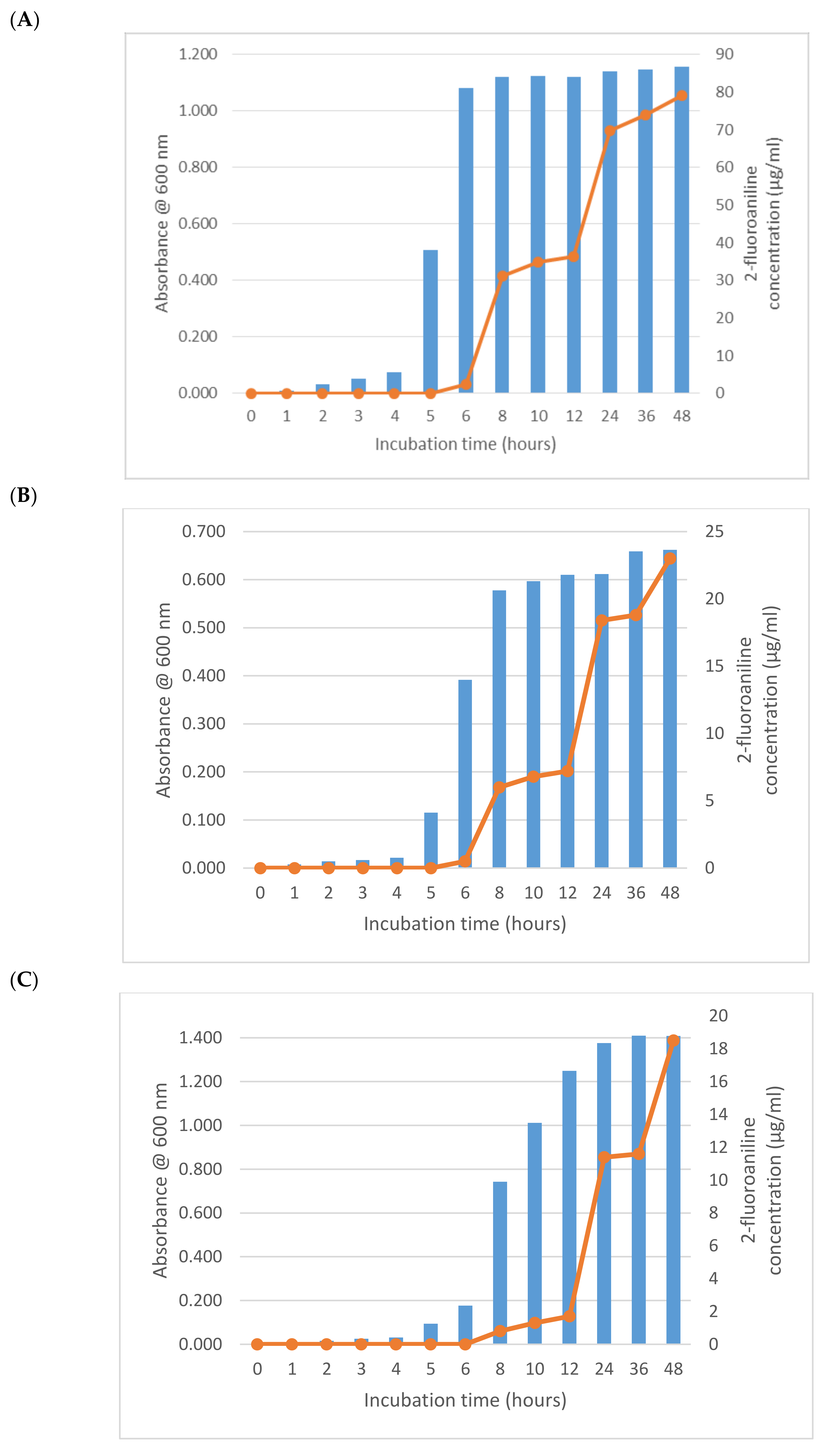
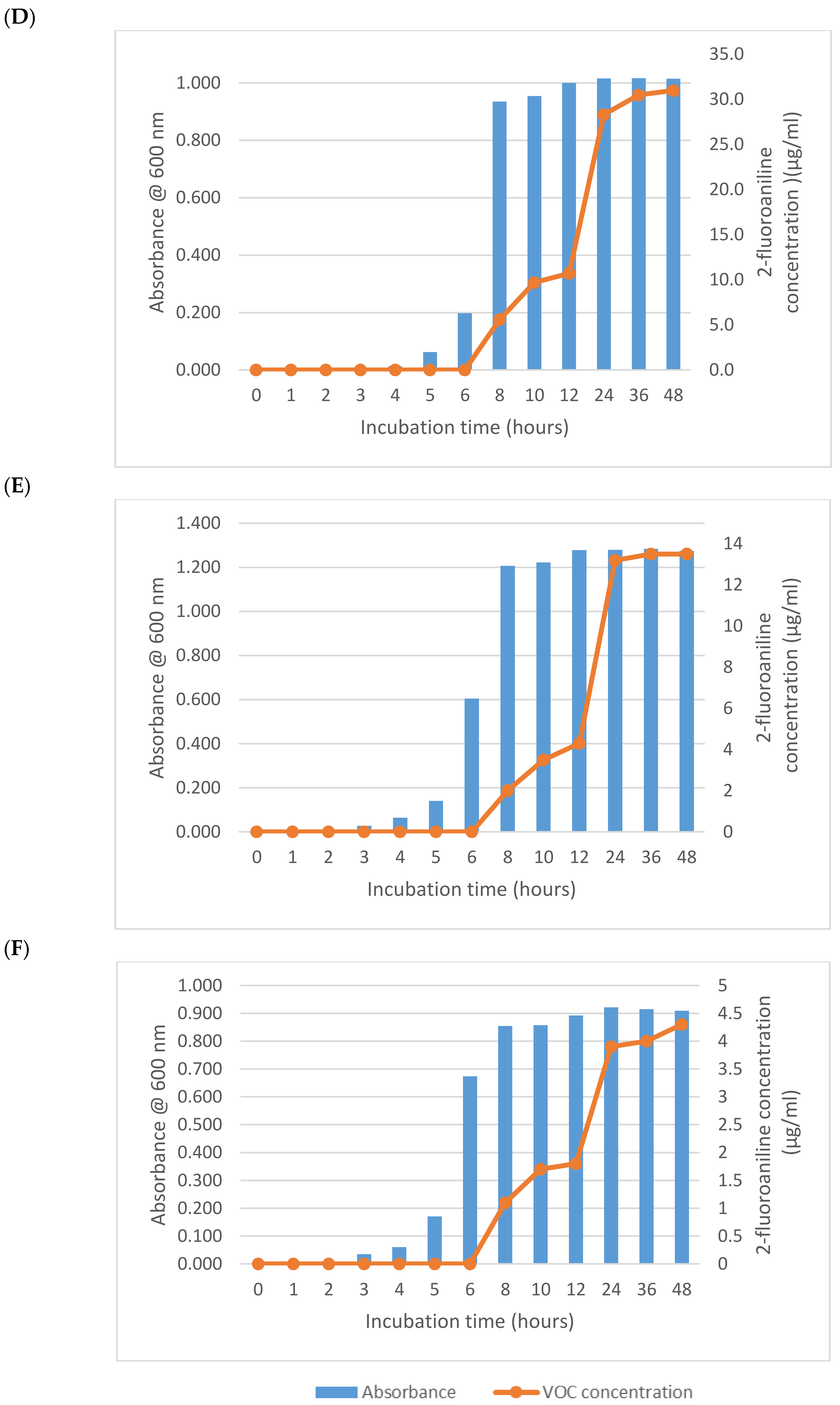
2.3. Incubation Time Study
2.4. Initial Inoculum Study
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Orenga, S.; James, A.L.; Manafi, M.; Perry, J.D.; Pincus, D.H. Enzymatic substrates in microbiology. J. Microbiol. Methods 2009, 79, 139–155. [Google Scholar] [CrossRef]
- Manafi, M. New developments in chromogenic and fluorogenic culture media. Int. J. Food Microbiol. 2000, 60, 205–218. [Google Scholar] [CrossRef]
- Manafi, M.; Kneifel, W.; Bascomb, S. Fluorogenic and chromogenic substrates used in bacterial diagnostics. Microbiol. Rev. 1991, 55, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Váradi, L.; Luo, J.L.; Hibbs, D.E.; Perry, J.D.; Anderson, R.J.; Orenga, S.; Groundwater, P.W. Methods for the detection and identification of pathogenic bacteria: Past, present, and future. Chem. Soc. Rev. 2017, 46, 4818–4832. [Google Scholar] [CrossRef] [PubMed]
- Bahroun, N.H.; Perry, J.D.; Stanforth, S.P.; Dean, J.R. Use of exogenous volatile organic compounds to detect Salmonella in milk. Anal. Chim. Acta 2018, 1028, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Guízar, S.; Sykes, H.; Perry, J.D.; Schwalbe, E.C.; Stanforth, S.P.; Perez-Perez, M.C.I.; Dean, J.R. A chromatographic approach to distinguish Gram-positive from Gram-negative bacteria using exogenous volatile organic compound metabolites. J. Chromatogr. A 2017, 1501, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.; Stephenson, D.; Sykes, H.E.; Perry, J.D.; Stanforth, S.P.; Dean, J.R. Detection of β-alanyl aminopeptidase as a biomarker for Pseudomonas aeruginosa in the sputum of patients with cystic fibrosis using exogenous volatile organic compound evolution. RSC Adv. 2020, 10, 10634–10645. [Google Scholar] [CrossRef]
- Roldán, M.D.; Pérez-Reinado, E.; Castillo, F.; Moreno-Vivián, C. Reduction of polynitroaromatic compounds: The bacterial nitroreductases. FEMS Microbiol. Rev. 2008, 32, 474–500. [Google Scholar] [CrossRef]
- Asnis, R.E. The reduction of Furacin by cell-free extracts of Furacin-resistant and parent-susceptible strains of Escherichia coli. Arch. Biochem. Biophys. 1957, 66, 208–216. [Google Scholar] [CrossRef]
- James, A.L.; Perry, J.D.; Jay, C.; Monget, D.; Rasburn, J.W.; Gould, F.K. Fluorogenic substrates for the detection of microbial nitroreductases. Lett. Appl. Microbiol. 2001, 33, 403–408. [Google Scholar] [CrossRef]
- Nokhbeh, M.; Boroumandi, S.; Pokorny, N.; Koziarz, P.; Paterson, E.; Lambert, I.B. Identification and characterization of SnrA, an inducible oxygen-insensitive nitroreductase in Salmonella enterica serovar Typhimurium TA1535. Mutat. Res. Mol. Mech. Mutagen. 2002, 508, 59–70. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Bennett, G.N.; Song, H.-G. Degradation of 2,4,6-trinitrotoluene by Klebsiella sp. isolated from activated sludge. Biotechnol. Lett. 2002, 24, 2023–2028. [Google Scholar] [CrossRef]
- Somerville, C.C.; Nishino, S.F.; Spain, J.C. Purification and characterization of nitrobenzene nitroreductase from Pseudomonas pseudoalcaligenes JS45. J. Bacteriol. 1995, 177, 3837–3842. [Google Scholar] [CrossRef]
- Anlezark, G.M.; Vaughan, T.E.; Fashola-Stone, E.; Michael, N.P.; Murdoch, H.; Sims, M.A.; Stubbs, S.; Wigley, S.; Minton, N.P. Bacillus amyloliquefaciens orthologue of Bacillus subtilis ywrO encodes a nitroreductase enzyme which activates the prodrug CB 1954. Microbiology 2002, 148, 297–306. [Google Scholar] [CrossRef][Green Version]
- Totty, H.; Ullery, M.; Spontak, J.; Viray, J.; Adamik, M.; Katzin, B.; Dunne, W.M.; Deol, P. A controlled comparison of the BacT/ALERT® 3D and VIRTUO™ microbial detection systems. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 1795–1800. [Google Scholar] [CrossRef]
- Congestrì, F.; Pedna, M.F.; Fantini, M.; Samuelli, M.; Schiavone, P.; Torri, A.; Bertini, S.; Sambri, V. Comparison of ‘time to detection’ values between BacT/ALERT VIRTUO and BacT/ALERT 3D instruments for clinical blood culture samples. Int. J. Infect. Dis. 2017, 62, 1–5. [Google Scholar] [CrossRef]
- The UK Sepsis Trust. Sepsis Manual, 5th ed.; The UK Sepsis Trust: Birmingham, UK, 2019; ISBN 978-0-9928155-0-9. Available online: www.sepsistrust.org (accessed on 1 May 2020).
- Tait, E.; Perry, J.D.; Stanforth, S.P.; Dean, J.R. Identification of volatile organic compounds produced by bacteria using head space—Solid phase microextraction gas chromatography mass spectrometry (HS-SPME-GC-MS). J. Chromatogr. Sci. 2013, 52, 363–373. [Google Scholar] [CrossRef]
- Koder, R.L.; Haynes, C.A.; Rodgers, M.E.; Rodgers, D.W.; Miller, A.-F. Flavin Thermodynamics Explain the Oxygen Insensitivity of Enteric Nitroreductases. Biochemistry 2002, 41, 14197–14205. [Google Scholar] [CrossRef]
- Cellier, M.; Gignoux, A.; James, A.L.; Orenga, S.; Perry, J.D.; Robinson, S.N.; Stanforth, S.P.; Turnbull, G. 2-(Nitroaryl)benzothiazole and benzoxazole derivatives as fluorogenic substrates for the detection of nitroreductase activity in clinically important microorganisms. Bioorg. Med. Chem. Lett. 2015, 25, 5694–5698. [Google Scholar] [CrossRef]
- Larsson, L.; Mårdh, P.A.; Odham, G.; Carlsson, M.L. Diagnosis of bacteraemia by automated head-space capillary gas chromatography. J. Clin. Pathol. 1982, 35, 715–718. [Google Scholar] [CrossRef]
- Allardyce, R.A.; Langford, V.S.; Hill, A.L.; Murdoch, D.R. Detection of volatile metabolites produced by bacterial growth in blood culture media by selected ion flow tube mass spectrometry (SIFT-MS). J. Microbiol. Methods 2006, 65, 361–365. [Google Scholar] [CrossRef]
- Drees, C.; Vautz, W.; Liedtke, S.; Rosin, C.; Althoff, K.; Lippmann, M.; Zimmermann, S.; Legler, T.J.; Yildiz, D.; Perl, T.; et al. GC-IMS headspace analyses allow early recognition of bacterial growth and rapid pathogen differentiation in standard blood cultures. Appl. Microbiol. Biotechnol. 2019, 103, 9091–9101. [Google Scholar] [CrossRef]
- Dolch, M.E.; Janitza, S.; Boulesteix, A.-L.; Graßmann-Lichtenauer, C.; Praun, S.; Denzer, W.; Schelling, G.; Schubert, S. Gram-negative and -positive bacteria differentiation in blood culture samples by headspace volatile compound analysis. J. Biol. Res. 2016, 23, 1–8. [Google Scholar] [CrossRef][Green Version]
- Dolch, M.E.; Hornuss, C.; Klocke, C.; Praun, S.; Villinger, J.; Denzer, W.; Schelling, G.; Schubert, S. Volatile organic compound analysis by ion molecule reaction mass spectrometry for Gram-positive bacteria differentiation. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 3007–3013. [Google Scholar] [CrossRef]
| VOC | Retention Time (min) | Quantitative m/z | Linear Range (µg/mL) | y = mx + c | Correlation Coefficient r2 | N | LOD (µg/mL) | LOQ (µg/mL) |
|---|---|---|---|---|---|---|---|---|
| Aniline | 6.23 | 39, 66, 93 | 0–100 | y = 19574x + 120.3 | 0.996 | 5 | 0.05 | 0.17 |
| 2-Fluoroaniline | 5.96 | 83, 84, 111 | 0–100 | y = 78901x + 346.8 | 0.999 | 5 | 0.01 | 0.03 |
| Microorganism | ID | Gram Designation | Aniline Concentration (µg/mL) | 2-Fluoroaniline Concentration (µg/mL) |
|---|---|---|---|---|
| Escherichia coli | NCTC 12241 | Negative | 55.9 a (55.2, 56.7) b | 75.0 (74.6, 75.3) |
| Escherichia coli | NCTC 8912 | Negative | 35.8 (35.4, 36.2) | 63.9 (65.4, 62.4) |
| Escherichia coli CPE 14 | Clinical | Negative | 13.5 (13.5, 13.6) | 57.8 (57.7, 57.9) |
| Escherichia coli CPE 15 | Clinical | Negative | 36.9 (36.6, 37.2) | 62.2 (62.3, 62.0) |
| Escherichia coli O157 | NCTC 12079 | Negative | 28.1 (26.9, 29.2) | 60.4 (60.4, 60.3) |
| Klebsiella pneumoniae | NCTC 9633 | Negative | 43.0 (42.4, 43.7) | 66.9 (67.4, 66.3) |
| Klebsiella pneumoniae | NCTC 418 | Negative | 44.5 (45.2, 43.8) | 64.2 (64.1, 64.2) |
| Salmonella stanley | Clinical | Negative | 38.9 (38.6, 39.3) | 66.6 (66.5, 66.7) |
| Salmonella london | Clinical | Negative | 45.7 (44.6, 46.9) | 76.9 (76.7, 77.1) |
| Salmonella gallinarum | Clinical | Negative | 41.8 (41.2, 42.5) | 76.8 (77.2, 76.3) |
| Salmonella typhimurium | NCTC 74 | Negative | 9.1 (9.0, 9.2) | 25.0 (24.8, 25.1) |
| Salmonella enteritidis | NCTC 6676 | Negative | 33.6 (32.9, 34.3) | 62.9 (61.9, 63.8) |
| Citrobacter freundii | NCTC 9750 | Negative | 24.8 (24.6, 24.9) | 60.4 (59.5, 61.2) |
| Enterobacter cloacae | NCTC 11936 | Negative | 23.4 (22.0, 24.7) | 73.5 (74.1, 72,8) |
| Enterobacter aerogenes | NCTC 9777 | Negative | 24.0 (24.5, 23.6) | 71.4 (70.9, 71.8) |
| Pseudomonas aeruginosa | NCIMB 8295 | Negative | 1.8 (1.8, 1.8) | 14.0 (14.2, 13.7) |
| Pseudomonas aeruginosa | NCTC 12903 | Negative | 2.2 (2.4, 2.1) | 14.8 (14.9, 14.7) |
| Stenotrophomonas maltophilia | NCTC 10257 | Negative | 22.3 (21.6, 23.1) | 82.3 (82.6, 81.9) |
| Serratia marcescens | NCTC 10211 | Negative | 24.3 (24.2, 24.5) | 38.7 (39.1, 40.2) |
| Serratia odorifera | NCTC 11214 | Negative | 1.9 (1.9, 1.9) | 29.3 (29.1, 29.3) |
| Serratia liquefaciens | NCTC 11361 | Negative | 45.4 (45.7, 45.3) | 76.9 (77.6, 76.1) |
| Shigella sonnei | NCTC 9774 | Negative | 5.4 (5.9, 5.0) | 11.5 (11.9, 11.1) |
| Shigella boydii | NCTC 9327 | Negative | 2.4 (2.8, 2.1) | 8.1 (8.2, 7.9) |
| Shigella flexneri | NCTC 9780 | Negative | 3.8 (3.5, 4.0) | 17.4 (17.1, 17.7) |
| Shigella dysenteriae | NCTC 9730 | Negative | 8.2 (8.2, 8.3) | 31.2 (31.8, 30.6) |
| Proteus vulgaris | NCTC 4175 | Negative | 24.9 (24.5, 25.3) | 63.7 (62.5, 64.8) |
| Proteus mirabilis | NCTC 11938 | Negative | 29.3 (27.9, 30.6) | 69.2 (70.8, 67.6) |
| Cronobacter sakazakii | ATCC 29544 | Negative | 12.6 (13.0, 12.2) | 65.6 (65.9, 65.2) |
| Morganella morganii | Clinical | Negative | 4.5 (5.0, 4.1) | 33.3 (32.0, 34.6) |
| Hafnia alvei | NCTC 8105 | Negative | 19.4 (18.8, 20.1) | 71.3 (71.7, 70.8) |
| Yersinia pseudotuberculosis | NCTC 10275 | Negative | 15.1 (14.2, 16.0) | 34.9 (35.5, 34.3) |
| Yersinia enterocolitica | NCTC 11176 | Negative | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) |
| Providencia rettgeri | NCTC 7475 | Negative | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) |
| Providencia stuartii | NCTC 10318 | Negative | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) |
| Burkholderia cepacia | ATCC 25416 | Negative | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) |
| Acinetobacter baumannii | ATCC 19606 | Negative | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) |
| Staphylococcus aureus | NCTC 12973 | Positive | 2.9 (2.7, 3.1) | 4.0 (4.1, 3.8) |
| Staphylococcus aureus (MRSA) | NCTC 11939 | Positive | 4.7 (4.6, 4.8) | 9.2 (9.4, 8.9) |
| Staphylococcus epidermidis | NCTC 11047 | Positive | 15.3 (15.7, 14.8) | 13.0 (13.2, 12.7) |
| Listeria monocytogenes | NCTC 11994 | Positive | 11.9 (12.2, 11.7) | 20.6 (20.9, 20.2) |
| Enterococcus faecium | NCTC 7171 | Positive | 7.9 (7.9, 7.9) | 5.1 (5.2, 4.9) |
| Enterococcus faecalis | NCTC 775 | Positive | 11.2 (11.5, 10.9) | 11.8 (12.2, 11.4) |
| Bacillus subtilis | NCTC 8236 | Positive | 14.3 (13.2, 15.2) | 27.3 (27.5, 27.1) |
| Bacillus cereus | NCTC 7464 | Positive | 3.1 (3.1, 3.1) | 14.4 (14.2, 14.5) |
| Streptococcus pyogenes | NCTC 8306 | Positive | 5.4 (5.2, 5.5) | 6.0 (5.8, 6.1) |
| Streptococcus agalactiae | ATCC 27956 | Positive | 9.8 (9.3, 10.3) | 8.8 (8.6, 9.0) |
| Streptococcus pneumoniae | DSMZ 11865 | Positive | 8.8 (8.9, 8.7) | 7.0 (7.2, 6.8) |
| Micrococcus luteus | NCIMB 10474 | Positive | 10.3 (10.6, 10.1) | 11.0 (11.3, 10.7) |
| Corynebacterium diphtheriae | NCTC 10356 | Variable | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) |
| Candida albicans | ATCC 90028 | Fungi | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) |
| Candida glabrata | NCPF 3943 | Fungi | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) |
| Preincubation Inoculum (CFU/mL/mL) | 2-Fluoroaniline Concentration (µg/mL) | |
|---|---|---|
| E. coli NCTC 12241 | B. subtilis NCTC 8236 | |
| 101 | 9.3 a (9.1, 9.5) b | 1.8 (1.6, 2.0) |
| 102 | 25.3 (24.6, 26.0) | 6.2 (6.3, 6.1) |
| 103 | 31.7 (31.1, 32.3) | 11.0 (10.9, 11.2) |
| 104 | 38.3 (38.7, 38.0) | 12.4 (12.7, 12.0) |
| 105 | 48.0 (47.6, 48.4) | 14.1 (13.9, 14.3) |
| 106 | 75.0 (74.6, 75.3) | 33.2 (32.2, 34.1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thompson, R.; Perry, J.D.; Stanforth, S.P.; Dean, J.R. Detection of Microbial Nitroreductase Activity by Monitoring Exogenous Volatile Organic Compound Production Using HS-SPME-GC-MS. Separations 2020, 7, 64. https://doi.org/10.3390/separations7040064
Thompson R, Perry JD, Stanforth SP, Dean JR. Detection of Microbial Nitroreductase Activity by Monitoring Exogenous Volatile Organic Compound Production Using HS-SPME-GC-MS. Separations. 2020; 7(4):64. https://doi.org/10.3390/separations7040064
Chicago/Turabian StyleThompson, Ryan, John D. Perry, Stephen P. Stanforth, and John R. Dean. 2020. "Detection of Microbial Nitroreductase Activity by Monitoring Exogenous Volatile Organic Compound Production Using HS-SPME-GC-MS" Separations 7, no. 4: 64. https://doi.org/10.3390/separations7040064
APA StyleThompson, R., Perry, J. D., Stanforth, S. P., & Dean, J. R. (2020). Detection of Microbial Nitroreductase Activity by Monitoring Exogenous Volatile Organic Compound Production Using HS-SPME-GC-MS. Separations, 7(4), 64. https://doi.org/10.3390/separations7040064