Determination of Hexabromocyclododecanes in Fish Using Modified QuEChERS Method with Efficient Extract Clean-Up Prior to Liquid Chromatography–Tandem Mass Spectrometry
Abstract
1. Introduction
2. Materials and Methods
2.1. Standards and Reagents
2.2. Fish Samples
2.3. Lipid and Moisture Determination
2.4. Sample Preparation
2.5. Instrumental Analysis
3. Results and Discussion
3.1. Sample Preparation
3.2. Method Performance
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Onogbosele, C.O.; Scrimshaw, M.D. Hexabromocyclododecane and Hexachlorocyclohexane: How Lessons Learnt Have Led to Improved Regulation. Crit. Rev. Environ. Sci. Technol. 2014, 44, 1423–1442. [Google Scholar] [CrossRef]
- Stockholm Convention. Stockholm Convention on Persistent Organic Pollutants (POPs). Available online: http://chm.pops.int/2013 (accessed on 13 April 2020).
- European Union. Directive 2013/39/EU of the European Parliament and of the Council of 12 August 2013 Amending Directives 2000/60/EC and 2008/105/EC as Regards Priority Substances in the Field of Water Policy. Off. J. Eur. Union 2013, 226, 1–17. [Google Scholar]
- European Union. Directive 2000/60/EC of the European Parliament and of the Council, Establishing a Framework for Community Action in the Field of Water Policy. Off. J. Eur. Union 2000, 327, 1–72. [Google Scholar]
- Budakowski, W.; Tomy, G. Congener-Specific Analysis of Hexabromocyclododecane by High-Performance Liquid Chromatography/Electrospray Tandem Mass Spectrometry. Rapid Commun. Mass Spectrom. 2003, 17, 1399–1404. [Google Scholar] [CrossRef]
- Janák, K.; Covaci, A.; Voorspoels, S.; Becher, G. Hexabromocyclododecane in Marine Species from the Western Scheldt Estuary: Diastereoisomer- and Enantiomer-Specific Accumulation. Environ. Sci. Technol. 2005, 39, 1987–1994. [Google Scholar] [CrossRef]
- Tomy, G.T.; Halldorson, T.; Danell, R.; Law, K.; Arsenault, G.; Alaee, M.; MacInnis, G.; Marvin, C.H. Refinements to the Diastereoisomer-Specific Method for the Analysis of Hexabromocyclododecane. Rapid Commun. Mass Spectrom. 2005, 19, 2819–2826. [Google Scholar] [CrossRef]
- Morris, S.; Bersuder, P.; Allchin, C.R.; Zegers, B.; Boon, J.P.; Leonards, P.E.G.; de Boer, J. Determination of the Brominated Flame Retardant, Hexabromocyclodocane, in Sediments and Biota by Liquid Chromatography-Electrospray Ionisation Mass Spectrometry. TrAC Trends Anal. Chem. 2006, 25, 343–349. [Google Scholar] [CrossRef]
- Frederiksen, M.; Vorkamp, K.; Bossi, R.; Rigét, F.; Dam, M.; Svensmark, B. Method Development for Simultaneous Analysis of HBCD, TBBPA, and Dimethyl-TBBPA in Marine Biota from Greenland and the Faroe Islands. Int. J. Environ. Anal. Chem. 2007, 87, 1095–1109. [Google Scholar] [CrossRef]
- Zhou, S.N.; Reiner, E.J.; Marvin, C.; Kolic, T.; Riddell, N.; Helm, P.; Dorman, F.; Misselwitz, M.; Brindle, I.D. Liquid Chromatography–Atmospheric Pressure Photoionization Tandem Mass Spectrometry for Analysis of 36 Halogenated Flame Retardants in Fish. J. Chromatogr. A 2010, 1217, 633–641. [Google Scholar] [CrossRef]
- Hu, X.; Hu, D.; Song, Q.; Li, J.; Wang, P. Determinations of Hexabromocyclododecane (HBCD) Isomers in Channel Catfish, Crayfish, Hen Eggs and Fish Feeds from China by Isotopic Dilution LC–MS/MS. Chemosphere 2011, 82, 698–707. [Google Scholar] [CrossRef] [PubMed]
- Ten Dam, G.; Pardo, O.; Traag, W.; van der Lee, M.; Peters, R. Simultaneous Extraction and Determination of HBCD Isomers and TBBPA by ASE and LC–MSMS in Fish. J. Chromatogr. B 2012, 898, 101–110. [Google Scholar] [CrossRef]
- Anastassiades, M.; Lehotay, S.J.; Stajnbaher, D.; Schenck, F.J. Fast and Easy Multiresidue Method Employing Acetonitrile Extraction/Partitioning and “Dispersive Solid-Phase Extraction” for the Determination of Pesticide Residues in Produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef]
- Lankova, D.; Kockovska, M.; Lacina, O.; Kalachova, K.; Pulkrabova, J.; Hajslova, J. Rapid and Simple Method for Determination of Hexabromocyclododecanes and Other LC–MS–MS-Amenable Brominated Flame Retardants in Fish. Anal. Bioanal. Chem. 2013, 405, 7829–7839. [Google Scholar] [CrossRef]
- Tavoloni, T.; Stramenga, A.; Stecconi, T.; Siracusa, M.; Bacchiocchi, S.; Piersanti, A. Single Sample Preparation for Brominated Flame Retardants in Fish and Shellfish with Dual Detection: GC-MS/MS (PBDEs) and LC-MS/MS (HBCDs). Anal. Bioanal. Chem. 2020, 412, 397–411. [Google Scholar] [CrossRef]
- Norli, H.R.; Christiansen, A.; Deribe, E. Application of QuEChERS Method for Extraction of Selected Persistent Organic Pollutants in Fish Tissue and Analysis by Gas Chromatography Mass Spectrometry. J. Chromatogr. A 2011, 1218, 7234–7241. [Google Scholar] [CrossRef]
- Molina-Ruiz, J.M.; Cieslik, E.; Cieslik, I.; Walkowska, I. Determination of Pesticide Residues in Fish Tissues by Modified QuEChERS Method and Dual-d-SPE Clean-Up Coupled to Gas Chromatography-Mass Spectrometry. Environ. Sci. Pollut. Res. Int. 2015, 22, 369–378. [Google Scholar] [CrossRef]
- Tölgyessy, P.; Miháliková, Z.; Matulová, M. Determination of Selected Chlorinated Priority Substances in Fish Using QuEChERS Method with Dual dSPE Clean-Up and Gas Chromatography. Chromatographia 2016, 79, 1561–1568. [Google Scholar] [CrossRef]
- Morrison, S.A.; Sieve, K.K.; Ratajczak, R.E.; Bringolf, R.B.; Belden, J.B. Simultaneous Extraction and Cleanup of High-Lipid Organs from White Sturgeon (Acipenser Transmontanus) for Multiple Legacy and Emerging Organic Contaminants Using QuEChERS Sample Preparation. Talanta 2016, 146, 16–22. [Google Scholar] [CrossRef]
- Cloutier, P.L.; Fortin, F.; Groleau, P.E.; Brousseau, P.; Fournier, M.; Desrosiers, M. QuEChERS Extraction for Multi-Residue Analysis of PCBs, PAHs, PBDEs and PCDD/Fs in Biological Samples. Talanta 2017, 165, 332–338. [Google Scholar] [CrossRef]
- Han, L.; Matarrita, J.; Sapozhnikova, Y.; Lehotay, S.J. Evaluation of a Recent Product to Remove Lipids and Other Matrix Co-Extractives in the Analysis of Pesticide Residues and Environmental Contaminants in Foods. J. Chromatogr. A 2016, 1449, 17–29. [Google Scholar] [CrossRef]
- Tölgyessy, P.; Nagyová, S. Rapid Sample Preparation Method with High Lipid Removal Efficiency for Determination of Sulphuric Acid Stable Organic Compounds in Fish Samples. Food Anal. Methods 2018, 11, 2485–2496. [Google Scholar] [CrossRef]
- Nagyová, S.; Tölgyessy, P. Validation Including Uncertainty Estimation of a GC–MS/MS Method for Determination of Selected Halogenated Priority Substances in Fish Using Rapid and Efficient Lipid Removing Sample Preparation. Foods 2019, 8, 101. [Google Scholar] [CrossRef]
- Xu, W.; Wang, X.; Cai, Z. Analytical Chemistry of the Persistent Organic Pollutants Identified in the Stockholm Convention: A Review. Anal. Chim. Acta 2013, 790, 1–13. [Google Scholar] [CrossRef]
- Tölgyessy, P.; Miháliková, Z. Rapid Determination of Total Lipids in Fish Samples Employing Extraction/Partitioning with Acetone/Ethyl Acetate Solvent Mixture and Gravimetric Quantification. Food Control 2016, 60, 44–49. [Google Scholar] [CrossRef]
- European Commission. Guidance Document on Analytical Quality Control and Validation Procedures for Pesticides Residues Analysis in Food and Feed, SANTE/11813/2017. Supersedes SANTE/11945/2015. Implemented by 01/01/2018. Available online: https://ec.europa.eu/food/sites/food/files/plant/docs/pesticides_mrl_guidelines_wrkdoc_2017-11813.pdf (accessed on 29 May 2020).

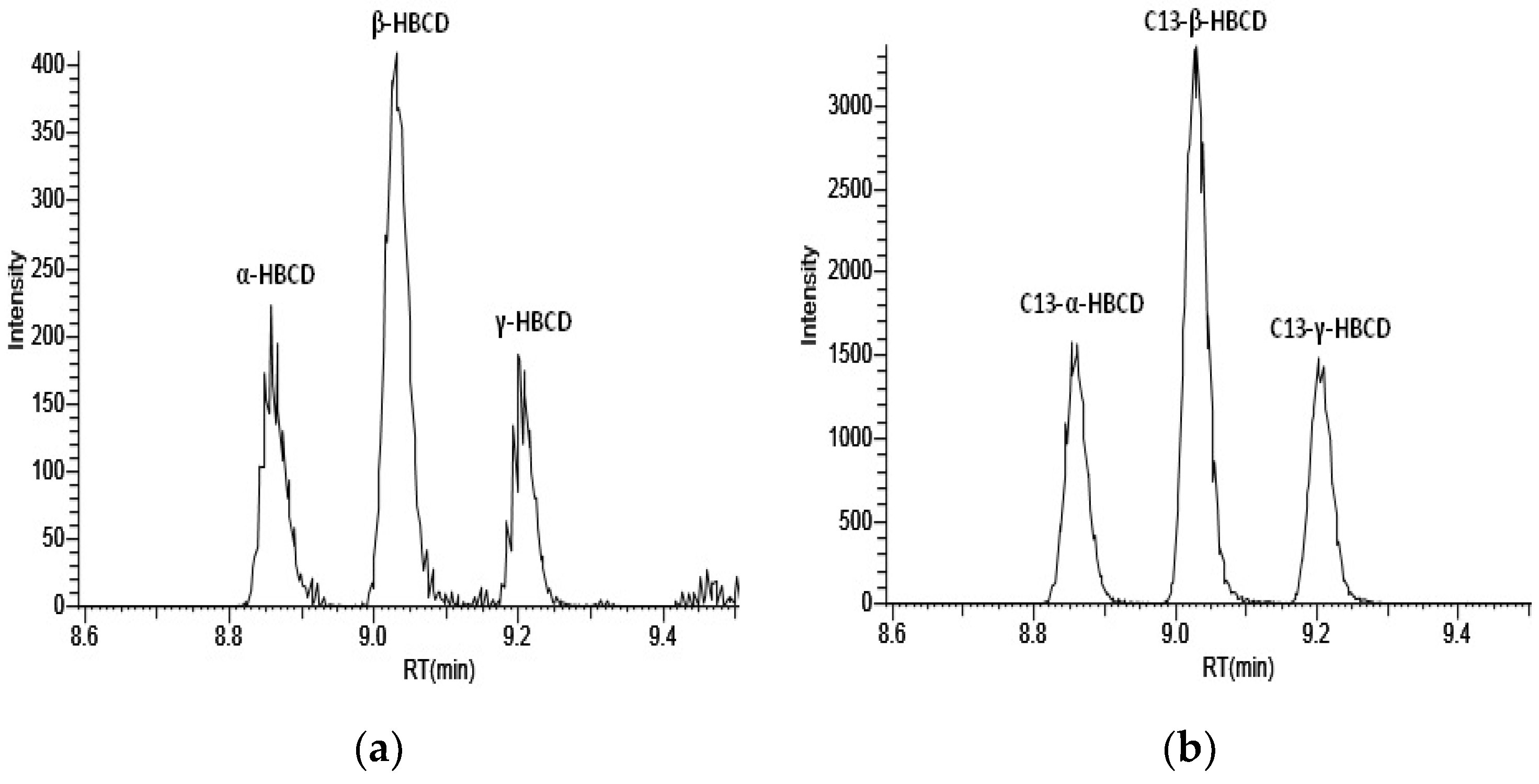
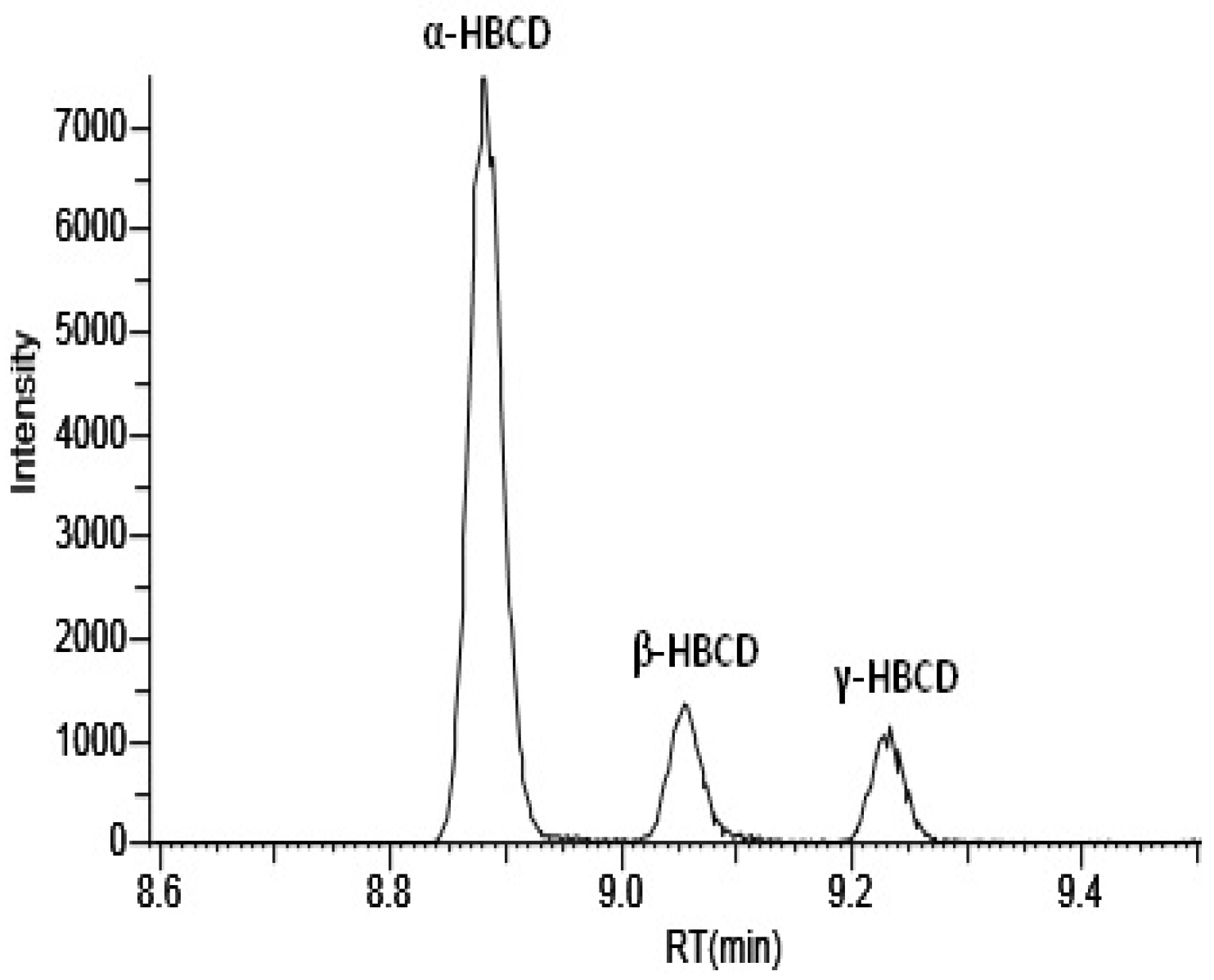
| Step | Time (min) | Mobile Phase A (%) | Mobile Phase B (%) | Flow Rate (mL min−1) |
|---|---|---|---|---|
| 1 | 0 | 90 | 10 | 0.3 |
| 2 | 0.5 | 50 | 50 | 0.3 |
| 3 | 7.5 | 0 | 100 | 0.4 |
| 4 | 10.0 | 0 | 100 | 0.5 |
| 5 | 10.4 | 0 | 100 | 0.5 |
| 6 | 10.5 | 90 | 10 | 0.5 |
| 7 | 13.0 | 90 | 10 | 0.3 |
| 8 | 13.5 | 90 | 10 | 0.3 |
| Analytes | Precursor Ion (m/z) | Transition 1 a/CE (m/z)/(V) | Transition 2/CE (m/z)/(V) | RF Lens (V) |
|---|---|---|---|---|
| α-, β-, γ-HBCD | 641 | 81/10 | 79/10 | 115 |
| C13: α-, β-, γ-HBCD | 653 | 81/10 | 79/10 | 127 |
| Ref. | Fish Homogenate Mass | Water Addition | Extractant | Added Salts | Crude Extract Volume | Clean-Up |
|---|---|---|---|---|---|---|
| [14] | 5 g | 10 mL | 10 mL MeCN + 0.2 mL formic acid | 1 g NaCl + 4 g MgSO4 | 2 mL | dSPE: 0.1 g C18 + 0.02 g PSA + 0.3 g MgSO4 0.2-µm nylon filter |
| [15] | 20 g | 5 mL | 15 mL ethyl acetate | 3 g NaCl + 6 g MgSO4 | 10 mL | SPE: Extrelut NT-3 (+H2SO4), Isolute silica columns GPC |
| This work | 5 g | 10 mL | 10 mL MeCN | 1 g NaCl + 4 g MgSO4 | 2 mL | DLLME: 8 mL 0.5 M CH3COONa + 100 µL CHCl3 Acid digestion: 1 mL 18.4 M H2SO4 |
| Analyte | Linear Range (ng g−1) | R2 | LOD (ng g−1) | LOQ (ng g−1) | Recovery (RSD) a | |
|---|---|---|---|---|---|---|
| 0.5 ng g−1 (%) | 5 ng g−1 (%) | |||||
| α-HBCD | 0.1–50 | 0.9989 | 0.075 | 0.25 | 89 (5.8) | 98 (3.0) |
| β-HBCD | 0.1–50 | 0.9994 | 0.045 | 0.15 | 100 (7.4) | 99 (2.0) |
| γ-HBCD | 0.1–50 | 0.9988 | 0.076 | 0.25 | 102 (7.1) | 98 (3.4) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okšová, L.; Tölgyessy, P. Determination of Hexabromocyclododecanes in Fish Using Modified QuEChERS Method with Efficient Extract Clean-Up Prior to Liquid Chromatography–Tandem Mass Spectrometry. Separations 2020, 7, 44. https://doi.org/10.3390/separations7030044
Okšová L, Tölgyessy P. Determination of Hexabromocyclododecanes in Fish Using Modified QuEChERS Method with Efficient Extract Clean-Up Prior to Liquid Chromatography–Tandem Mass Spectrometry. Separations. 2020; 7(3):44. https://doi.org/10.3390/separations7030044
Chicago/Turabian StyleOkšová, Linda, and Peter Tölgyessy. 2020. "Determination of Hexabromocyclododecanes in Fish Using Modified QuEChERS Method with Efficient Extract Clean-Up Prior to Liquid Chromatography–Tandem Mass Spectrometry" Separations 7, no. 3: 44. https://doi.org/10.3390/separations7030044
APA StyleOkšová, L., & Tölgyessy, P. (2020). Determination of Hexabromocyclododecanes in Fish Using Modified QuEChERS Method with Efficient Extract Clean-Up Prior to Liquid Chromatography–Tandem Mass Spectrometry. Separations, 7(3), 44. https://doi.org/10.3390/separations7030044





