Abstract
Presently, results from a study carried out in this area using the essential oil from the Calyptranthes concinna species, a representative from the Myrtaceae family, are reported. The essential oil was obtained by hydrodistillation and gas chromatography coupled to mass spectrometry was used to identify its chemical constituents. Antibacterial activity was determined using the broth microdilution method, thus obtaining the Minimal Inhibitory Concentration (MIC) value, from which the subinhibitory concentration (MIC/8) was derived. The C. concinna essential oil presented antibacterial activity against both standard and multiresistant bacteria. In addition, the oil demonstrated an antibiotic activity potentiation against Staphylococcus aureus and Escherichia coli when in combination with the antibiotic gentamicin, reducing the MIC from 141.38 μg/mL and 208.63 μg/mL to 64 μg/mL and 128 μg/mL, respectively. Conclusions: Findings from the present study suggest this oil is promising in terms of its antimicrobial activity.
1. Introduction
Microorganismal resistance, with its main consequence being a difficulty in treating pathologies, increases healthcare costs and mortality rates associated with infections [1], being considered a growing problem for public healthcare around the world [2].
An alternative for tackling this problem is the use of natural products, such as essential oils, in substitution or in combination with antibiotics, as these products possess antimicrobial properties [3,4]. Moreover, these products can be obtained from several botanical families.
The indiscriminate use of antibiotics occurs in different proportions around the world and is associated with factors such as self-medication and inadequate prescription. Indiscriminate use is responsible for the high bacterial resistance report rates that have increased each year [5]. The need for the development of increasingly potent antimicrobials has grown due to diverse needs, such as those presented in the public healthcare field, where the emergence of microbial resistance is a factor reducing the quality of life of individuals and which considerably increases the probability of hospital infection [6].
Myrtaceae is a family of angiosperms composed of approximately 140 genera. These species are trees or shrubs, producing fruits which are mostly edible [7,8,9]. Most of their representatives are important essential oil sources [9].
The Calyptranthes genus, a member of the Myrtaceae family, is composed of approximately 100 species. The biological activity from this genus has been attributed to compounds present in its structure such as benzopyrene or chromene [9,10,11], examples of which include antibacterial activity [12,13], anti-inflammatory activity [14], antiparasitic activity [15,16], antitumor activity [12], antinociceptive activity [14] and antispasmodic activity [12,16], among others.
With the aforementioned in mind, the present study aimed to evaluate the antimicrobial and bacterial resistance modifying potential of the Calyptranthes concinna DC essential oil against standard and multiresistant Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa strains.
2. Materials and Methods
2.1. Botanical Material Collection
Plant material collection of Calyptranthes concinna leaves was carried out in the Butuguara Private Natural Heritage Reserve (Reserva Particular do Patrimônio Natural Butuguara; RPPN), in the municipality of Palmeira-PR, Southern Brazil (25° 20.884′ S and 049° 47.258′ W). The collection was carried out under a license issued by the Environmental Institute of Paraná (No. 284/11). An exsiccate was prepared and deposited in the Spiritist Integrated Faculties Herbarium (Herbário das Faculdades Integradas Espírita), registered under collection number HFIE 8.820 and SISGEN n° AC30A0A.
2.2. Essential Oil Extraction
Extraction of the Calyptranthes concinna essential oil was carried out using the hydrodistillation method in a Clevenger apparatus for a period of 2.5 h, using 50 g of dry leaves in 1 L of distilled water with 3 replicates [17]. The leaves were dried using an electric dryer FANEM, Model 320 SE with air circulation at 40 °C for 24 h. The leaf water content was determined using 20 g of leaves that were dried to a constant weight using an electric dryer FANEM, Model 320 SE with air circulation at 65 °C, in triplicates. Following extraction, the samples were collected and kept in a freezer until further analysis. The yield obtained from the C. concinna essential oil was 0.26% of the dry leaf mass.
2.3. Chemical Composition Determination
Identification of the Calyptranthes concinna essential oil chemical constituents was performed by gas chromatography coupled to mass spectrometry (GC/MS). The essential oil was diluted in dichloromethane at a 1% proportion and 1.0 μL of the solution was injected at a 1:20 flow division in an Agilent 6890 chromatograph (Palo Alto, CA, USA) coupled to an Agilent 5973N selective mass detector. The injector was maintained at 250 °C. Constituent separation was obtained using a HP-5MS capillary column (5%-phenyl-95%-dimethylpolysiloxane, 30 m × 0.25 mm × 0.25 μm) with helium as the carrier gas (1.0 mL min−1). The oven temperature was programmed from 60 to 240 °C at a rate of 3 °C min−1. The mass detector was operated in the electronic ionization mode (70 eV), at a rate of 3.15 sweeps s−1 with mass bands from 40 to 450 u. The transfer line was maintained at 260 °C, the ion source at 230 °C and the analyser (quadrupole) at 150 °C.
For quantification, the diluted samples were injected into an Agilent 7890A chromatograph (Palo Alto, CA, USA) equipped with a flame ionization detector (FID), operated at 280 °C. The same column and analytical conditions described above were used except for the carrier gas, which for this was hydrogen, at a flow rate of 1.5 mL min−1. The percentage composition was obtained using the electronic integration of the DIC signal divided by each component’s total area (area%).
Chemical constituent identification was obtained by comparing their mass spectra with those of spectra databases [18,19], as well as by their linear retention indices, calculated from the injection of a homologous hydrocarbon series (C7–C26) and compared with data from the literature [20].
2.4. Drugs and Reagents
Gentamicin and Oxacillin (Sigma Co., St. Louis, MO, USA) were the antibiotics used in the experiments. Both drugs were dissolved in sterile distilled water until reaching a concentration of 1024 μg/mL. Ten milligrams from each essential oil used in the study was weighed into separate tubes and diluted in 1 mL of dimethyl sulfoxide (DMSO) and sterile distilled water until reaching a concentration of 1024 μg/mL.
To read the experiments, the resazurin sodium reagent (Sigma-Aldrich, St. Louis, MO, USA) was used as a colorimetric indicator of bacterial growth through the oxidation-reduction method [21,22].
2.5. Microbial Strains
The microorganisms used in the experiments were obtained from the Laboratory of Microbiology and Molecular Biology (Laboratório de Microbiologia e Biologia Molecular; LMBM) of the Regional University of Cariri (Universidade Regional do Cariri; URCA). The standard strains used were Escherichia coli ATCC 25922, Staphylococcus aureus ATCC 25923 and Pseudomonas aeruginosa ATCC 9027. The multiresistant strains used were S. aureus 10, P. aeruginosa 24 and E. coli 06.
2.6. Minimum Inhibitory Concentration Determination
The Minimum Inhibitory Concentration (MIC) was determined by the broth microdilution method adapted from [23]. For the procedures, inocula from the 24 h growth cultures grown on petri dishes in Heart Infusion Agar—HIA (Difco Laboratories Ltd., Franklin Lakes, NJ, USA) were prepared. A loop from each strain was suspended in 0.9% saline solution and compared to the McFarland turbidity scale (1 × 105 CFU/mL) at 0.5.
Eppendorf® tubes containing 1.5 mL of solution were prepared. From this solution, 10% corresponded to the bacterial inoculum and the remainder of the solution was supplemented with 10% Brain Heart Infusion Broth (BHI) culture medium (1350 μL). Subsequently, 100 μL were added to a 96-well microdilution plate; thereafter, a 1:1 serial microdilution was performed up until the penultimate cavity, with the last well being used as a microbial growth control. Concentrations ranged from 512 to 0.5 μg/mL. The plates were then taken to a microbial growth incubator for 24 h at 37 °C.
For reading the MIC, 20 uL of resazurin solution were used and the solution was observed for any colour changes following an oxidation-reduction reaction at room temperature during 2 h. A colour change from blue to pink is interpreted as the occurrence of bacterial growth [21,22]. The procedures were performed in triplicates.
2.7. Essential Oil Modulating Effect on the Activity of Clinically Used Antibiotics
To verify if the essential oils could modify the action of antibiotics used against multiresistant bacteria, the method proposed by [24] was used. The compounds were evaluated at a subinhibitory concentration (MIC/8) so there would be no bacterial growth inhibition by direct action.
For these procedures, Eppendorf® tubes with a volume of 1500 μL of the solution were prepared, 10% of which corresponded to the bacterial inoculum (150 μL) and the essential oils (MIC/8 μL). The control solution was prepared in Eppendorf® tubes containing 1350 μL BHI and 150 μL of the bacterial suspension. Subsequently, the plates were filled with 100 μL of the solution and microdiluted using 100 μL of the antibiotics at a 1:1 ratio up to the penultimate cavity; the last well was used as a bacterial growth control. All procedures were performed in triplicates and readings were performed using resazurin.
2.8. Statistical Analysis
The results obtained in the tests were analysed using the geometric mean in a two-way ANOVA with Bonferroni’s post hoc test (p < 0.05 considered as significant).
3. Results
Chemical Composition Determination
In the C. concinna DC. chemical analysis, the following compounds were identified at greater proportions; elemicin (17.9%), alpha-cadinol (8.5%), Globulol (7.7%), cis-beta-guanene (7.4%), alpha-pinene (6.6%) and beta-pinene (6.2%), described in Table 1.

Table 1.
Chemical composition of the Calyptranthes concinna Essential Oil (EOCc).
Results obtained from the Minimal Inhibitory Concentration (MIC) experiments using representatives from the Myrtaceae family are described in Table 2.

Table 2.
Minimum inhibitory concentration of the EOCc (μg/mL).
The C. concinna essential oil presented antibacterial activity against S. aureus ATCC (203.18 μg/mL) standard strains, in addition to showing antibacterial activity against Pseudomonas aeruginosa for both the standard and multi-resistant strains obtaining MIC values of 406.37 μg/mL and 645.08 μg/mL, respectively.
The essential oil modulatory effect was tested in association with the antibiotics gentamicin and oxacillin in order to evaluate a possible interaction between them, to verify if a synergistic or antagonistic activity exists, that is, if the substances in association would improve or impair antimicrobial action. A synergistic effect was observed in the association with the aminoglycoside gentamicin obtaining a MIC reduction from 141.4 to 64 μg/mL against S. aureus and from 208.6 to 6 μg/mL against E. coli (Figure 1).
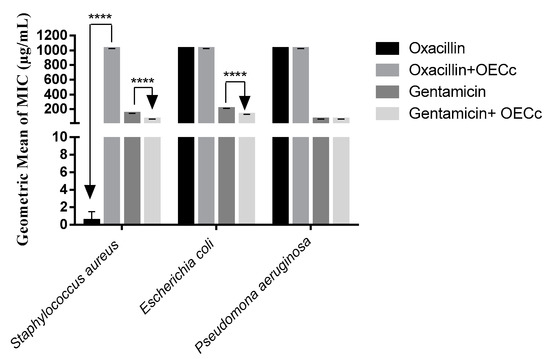
Figure 1.
Potentiating effect of the Calyptranthes concinna Essential Oil (EOCc) against antibiotics (p < 0.05).
4. Discussion
The study by the authors of [10] also reported a significant concentration of monoterpenes, such as pinenes occuring in greater quantity, in addition to sesquiterpenes and eudesmol isomers. According to the author, this characteristic is frequent in essential oils collected from Brazil, these being commercially important volatile compounds [25]. The authors of [26] studied the chemical composition of essential oils from the Calyptranthes genus and identified a high concentration of cyclic sesquiterpenes such as Caryophyllene, Germacran and Cadinanol, in accordance with the present study, which are considered common compounds in Myrtaceae.
When comparing the antibacterial activity of the C. concinna essential oil with other plants from the same genus, Calyptranthes pallens demonstrated an antibacterial effect against S. aureus with a MIC of 39 μg/mL, obtaining similar results against P. aeruginosa (MIC = 625 μg/mL) [27]. However, studies with Calyptranthes microphylla did not demonstrate any antibacterial effect against the same bacteria using the disk diffusion method [28]. These different results may be due the different chemical composition among these species, as well as the different methodologies used.
The antimicrobial activity of the analysed essential oil may be associated with the fact essential oils can alter cell membrane structure and permeability [29], especially due to the hydrophobic nature of compounds present in essential oils, which cause alterations in the enzymatic system and genetic material loss [30]. This is the first study portraying the C. concinna antibacterial activity.
Aminoglycosides are antibiotics which inhibit protein synthesis by altering the conformation of the bacterial ribosome [31,32]. Resistance to aminoglycosides and other drugs has been a major threat to public health, where its main mechanism of resistance may be due to enzymatic inactivation or antibiotic efflux [33]. Increased antibacterial activity or resistance reversal classify these products as antibiotic activity modifiers [34].
An antagonistic effect against S. aureus was observed in the association with the antibiotic oxacillin. The antagonistic effect of essential oils may be explained by these compounds binding to the active site the antibiotic would occupy or even due to possible antibiotic chelation mechanisms, thus diminishing the antibiotic’s spectrum of action [1,35].
Despite the possibility of using this natural product to potentiate antibiotic activity, some limitations must be overcome, such as the use of pharmaceutical nanoformulations as nanospheres, liposomes and complexes with cyclodextrin, as well as bioactivity evaluations using in vivo models.
5. Conclusions
Natural product research has gained prominence over time with the obtained results stimulating in-depth studies and investments in these. The possibility of using essential oils for antimicrobial treatment may reduce both microbial resistance and the cost these processes demand. The essential oil under study showed clinically relevant antimicrobial activity for in vivo treatment, in addition to potentiating the action of aminoglycosides.
Therefore, the results presented herein will hopefully contribute to the growth of essential oil studies and stimulate more in-depth experiments for confirmation of their therapeutic potential.
Author Contributions
Conceptualization, H.D.M.C.; methodology, M.d.S.C., N.J.S.A. and T.S.d.F.; validation, F.A.B.d.C.; formal analysis, L.E.d.S. and H.D.M.C.; investigation, W.d.A., C.D. and C.C.; resources, L.E.d.S. and H.D.M.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors are grateful to the follow Brazilian Research agencies by the laboratorial and finatial support: FUNCAP, CAPES, CNPq and FINEP.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Coutinho, H.D.M.; Freitas, M.A.; Gondim, C.N.F.L.; Albuquerque, R.S.; Ferreira, J.V.A.; Andrade, J.C. Atividade antimicrobiana in vitro de Geraniol e Cariofileno sobre Staphylococcus aureus. Rev. Cuba Plantas Med. 2015, 20, 98–105. [Google Scholar]
- Silva, A.C.; Iacuzio, R.; Cândido, T.J.S.; Rodrigues, M.X.; Silva, N.C.C. Resistência antimicrobiana de Salmonella spp., Staphylococcus aureus e Escherichia coli isolados de carcaças de frangos: Resistência a antibióticos e óleos essenciais. Rev. Bras. Agropecu. Sustent. 2018, 8, 95–103. [Google Scholar] [CrossRef]
- Costa, B.L. Avaliação da Composição Química e das Atividades Antimicrobiana e Antioxidante dos Extratos de Eugenia uniflora. UNB 2015, 1–45. [Google Scholar]
- Cirino, I.C.S. Modulação da Resistência à Drogas por óleos essenciais em linhagens de Staphylococcus aureus. UFPB 2014, 1–79. [Google Scholar]
- Loureiro, R.J.; Roque, F.; Rodrigues, A.T.; Herdeiro, M.T.; Ramalheira, E. O uso de antibióticos e as resistências bacterianas: Breves notas sobre a sua evolução. Rev. Port. Saúde Public. 2016, 34, 77–84. [Google Scholar] [CrossRef]
- Costa, A.L.P.; Silva Junior, A.C.S. Resistência bacteriana aos antibióticos e Saúde Pública: Uma breve revisão de literatura. Estação Científica 2017, 7, 45–57. [Google Scholar] [CrossRef]
- Azevedo, S.G. Caracterização química e atividades biológicas dos óleos essenciais de folhas de Eugenia spp. (Myrtaceae) ocorrentes na Amazônia de terra firme. UFAM 2014, 1–122. [Google Scholar]
- Martins, A.S. Atividade antimicrobiana dos óleos essenciais de espécies do gênero Eugenia (Myrtaceae). UNB 2015, 1–44. [Google Scholar]
- Dexheimer, G.M. Efeito do Extrato de Calyptranthes grandifolia O. Berg (Myrtaceae) na Expressão do TNF- α NF-ƙβ E p38 α em Células de Adenocarcinoma Colorretal (CACO-2). UFT 2015, 1–90. [Google Scholar]
- Ramos, M.F.S.; Siani, A.C.; Souza, M.C.; Rosas, E.C.; Henriques, M.D.G.M. Avaliação da atividade antiinflamatória dos óleos essenciais de cinco espécies de Myrtaceae. Rev. Fitos 2013, 2, 58–66. [Google Scholar]
- Cunha, F.A.B.; Pinho, A.I.; Santos, J.F.S.; Sobral-Souza, C.E.; Albuquerque, R.S.; Matias, E.F.F.; Leite, N.L.; Tintino, S.R.; Costa, J.G.M.; Boligon, A.A.; et al. Cytoprotective effect of Eugenia uniflora L. against the waste contaminant mercury chloride. Arab. J. Chem. 2016, 18, 1–7. [Google Scholar] [CrossRef]
- Dexheimer, G.M.; Pozzobon, A. Atividade biológica de plantas da família Myrtaceae: Revisão sistemática de artigos entre 1989 e 2015. Rev. Cuba Plantas Med. 2017, 22, 1–22. [Google Scholar]
- Limberger, R.P.; Apel, M.A.; Sobral, M.; Schapoval, E.S.; Henriques, A.T. Investigação da atividade antimicrobiana do óleo volátil de espécies da família Myrtaceae. Rev. Bras. Farm. 1998, 79, 49–52. [Google Scholar]
- Passos, L.O.; Pina, L.T.S.; de Jesus, A.M.; de Melo, M.S.; Bispo, R.M.; Alves, P.B.; de Lima, P.C.N.; de Souza, V.R.M.; Silva, G.H.; Júnior, L.J.Q.; et al. A New Β-triketone and antinociceptive effect from the essential oil of the leaves of Calyptranthes restingae Sobral (Myrtaceae). Med. Aromat. Plants 2016, 5, 2167-0412. [Google Scholar]
- Cruz, A.V.M.; Kaplan, M.A.C. Uso medicinal de espécies das famílias Myrtaceae e Melastomataceae no Brasil. Floram 2012, 11, 47–52. [Google Scholar]
- Mors, W.B.; Rizzini, C.T.; Pereira, N.A. Medicinal Plants of Brazil, 1st ed.; Reference Publications: Chicago, MI, USA, 2000; p. 501. [Google Scholar]
- Wasicky, R. Uma modificação do aparelho de clevenger para extração de óleos essenciais. Rev. Bras. Cien Farm. 1963, 1, 77–81. [Google Scholar]
- Wiley Registry of Mass Spectral Data, 6th ed.; Wiley Interscience: New York, NY, USA, 1994.
- NIST Chemistry Webbook. Available online: http://webbook.nist.gov (accessed on 12 June 2018).
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Sales, G.W.P.; Batista, A.H.M.; Rocha, L.Q.; Nogueira, M.A.P. Efeito antimicrobiano e modulador do óleo essencial extraído da casca de frutos da Hymenaea courbaril L. Rev. Ciênc Farm. Apl. 2014, 35, 709–715. [Google Scholar]
- Salvat, A.; Antonnacci, L.; Fortunato, R.H.; Suarez, E.Y.; Godoy, H.M. Screening of some plants from North Argentin for their antimicrobial activity. Lett. Appl. Microbiol. 2001, 32, 293–297. [Google Scholar] [CrossRef]
- Javadpour, M.M.; Juban, M.M.; Lo, W.C.; Bishop, S.M.; Alberty, J.B.; Cowell, S.M.; Becker, C.L.; McLaughlin, M.L. De novo antimicrobial peptides with low mammalian cell toxicity. J. Med. Chem. 1996, 39, 3107–3113. [Google Scholar] [CrossRef]
- Coutinho, H.D.M.; Costa, J.G.M.; Siqueira-Junior, J.P.; Lima, E.O.A. Atividade anti-estafilocócica in vitro de Hyptis martiusii Benth contra estirpes de Staphylococcus aureus resistentes à meticilina. Rev. Bras. Farmacogn. 2008, 1, 670–675. [Google Scholar] [CrossRef]
- Ootani, M.A.; Aguiar, R.W.; Ramos, A.C.C.; Brito, D.R.; Silva, J.B.; Cajazeira, J.P. Use of essential oil in agriculture. J. Biotechnol. Biodiv. 2013, 4, 162-1. [Google Scholar] [CrossRef]
- Linberger, R.P.; Simões-Pires, C.A.; Sobral, M.; Menut, C.; Bessiere, J.-M.; Henriques, A.T. Essential oils from Calyptranthes concinna, C. lucida and C. rubella (Myrtaceae). Braz. J. Pharm. Sci. 2002, 38, 355–360. [Google Scholar]
- Bansal, A.; Boehme, A.K.; Eiter, L.C.; Schmidt, J.M.; Setzer, W.M.; Vincent, M.A. Chemical composition and bioactivity of the leaf oil of Calyptranthes pallens (Poir.) Griseb. from Abaco Island, Bahamas. Nat. Prod. Commun. 2006, 1, 303–306. [Google Scholar] [CrossRef]
- Tenorio, A.I.; Vargas, D.; Espinosa, A.; Díaz, A.; Gupta, M.P. Chemical composition of leaf essential oils of Calyptranthes microphylla B. Holts & ML, Myrcia aff fosteri Croat and Eugenia octopleura Krug & Urb from Panama. J. Essent. Oil Res. 2011, 23, 29–33. [Google Scholar]
- Mohamed, A.A.; Ali, S.I.; El-Baz, F.K. Antioxidant and antibacterial activities of crude extracts and essential oils of Syzygium cumini leaves. PLoS ONE 2013, 8, 6069. [Google Scholar] [CrossRef]
- Ferraz, E.O.; Vieira, M.A.R.; Ferreira, M.I.; Fernandes Junior, A.; Marques, M.O.M.; Minatel, I.O.; Albano, M.; Sambo, P.; Lima, G.P.P. Seasonality effects on chemical composition, antibacterial activity and essential oil yield of three species of Nectandra. PLoS ONE 2018, 13, 0204132. [Google Scholar] [CrossRef]
- Canton, M.; Onofre, S.B. Interferência de extratos da Baccharis dracunculifolia DC., Asteraceae, sobre a atividade de antibióticos usados na clínica. Rev. Bras. Farmacogn. 2010, 20, 348–354. [Google Scholar] [CrossRef]
- Jana, S.; Deb, J.K. Molecular understanding of aminoglycoside action and resistance. Appl. Microb. Biotechnol. 2006, 70, 140–150. [Google Scholar] [CrossRef]
- Coutinho, H.D.M.; Costa, J.G.M.; Lima, E.O.; Falcão-Silva, V.S.; Siqueira-Júnior, J.P. Enhancement of the antibiotic activity against a multiresistant Escherichia coli by Mentha arvensis L. and chlorpromazine. Chemotherapy 2008, 54, 328–330. [Google Scholar] [CrossRef]
- Coutinho, H.D.M.; Costa, J.G.M.; FalCão-silVa, V.S.; siqueira-Júnior, J.P.; LiMa, E.O. Fruits to potentiate the antibiotic activity: The effect of Eugenia uniflora and Eugenia jambolanum L. against MRSA. Acta Aliment 2011, 41, 67–72. [Google Scholar] [CrossRef]
- Oliveira, F.S.; Freitas, T.S.; Cruz, R.P.D.; Costa, M.D.S.; Pereira, R.L.S.; Quintans-Júnior, L.J.; Andrade, T.A.; Menezes, P.D.P.; Sousa, B.M.H.; Nunes, P.S.; et al. Evaluation of the antibacterial and modulatory potential of α-bisabolol, β-cyclodextrin and α-bisabolol/β-cyclodextrin complex. Biomed. Pharmacother. 2017, 92, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).