Advancements in Non-Invasive Biological Surface Sampling and Emerging Applications
Abstract
1. Introduction
2. Skin Sampling
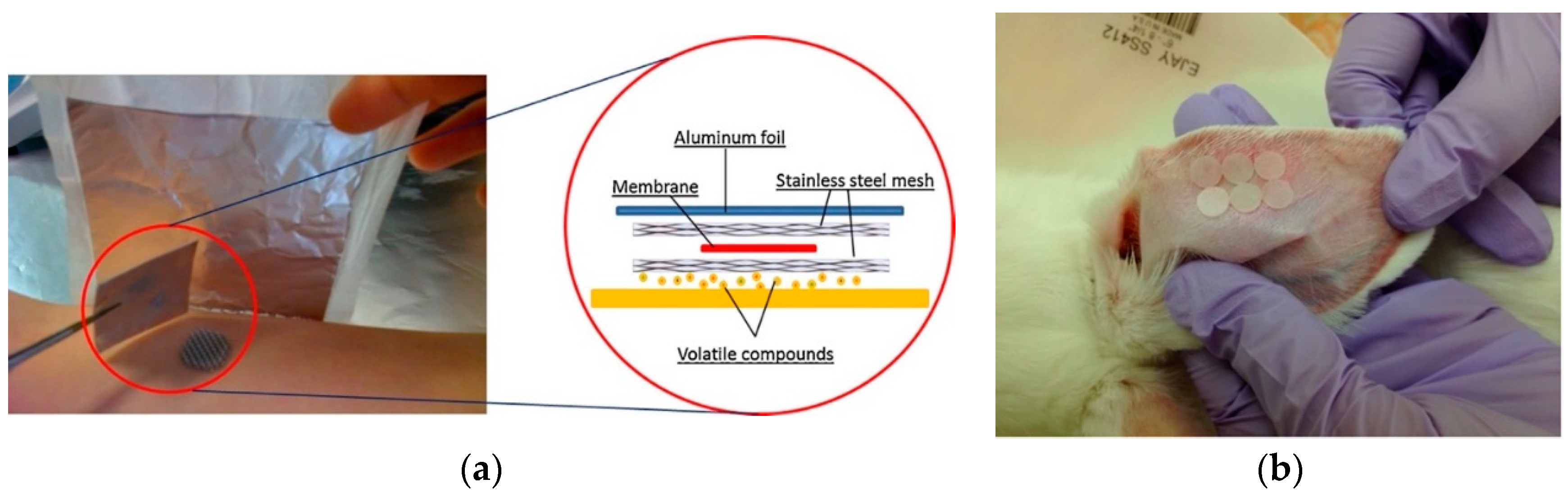
2.1. Direct Contact Type Sampling
2.1.1. Polydimethylsiloxane (PDMS)
2.1.2. Agarose Hydrogel
2.1.3. Microneedle Arrays
2.2. Headspace Sampling
2.2.1. Conventional SPME Fibers
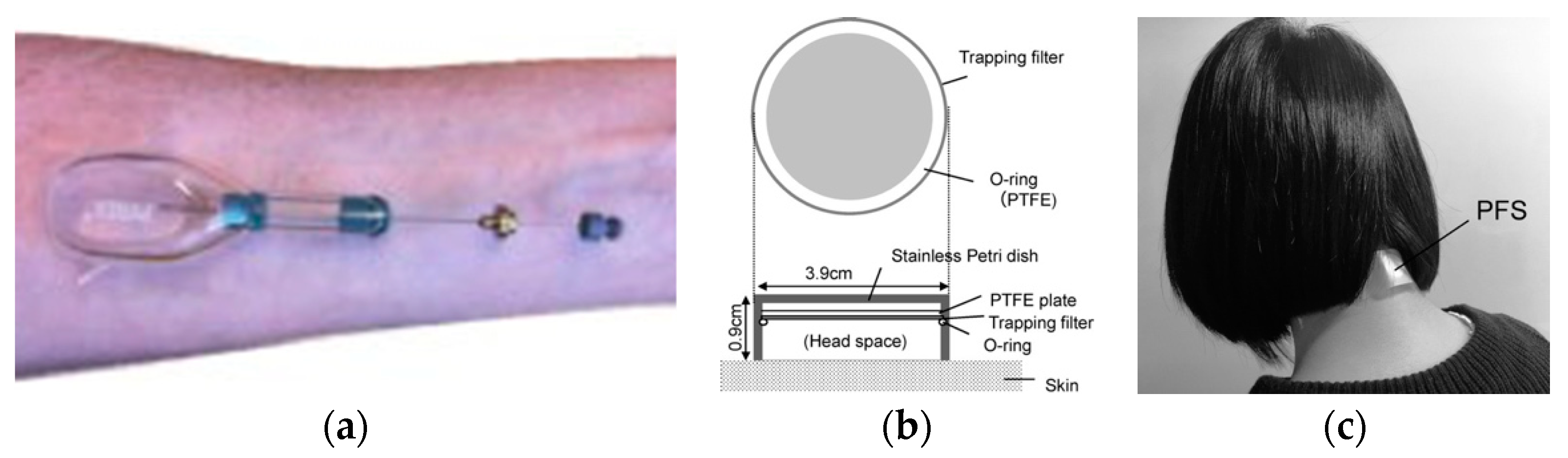
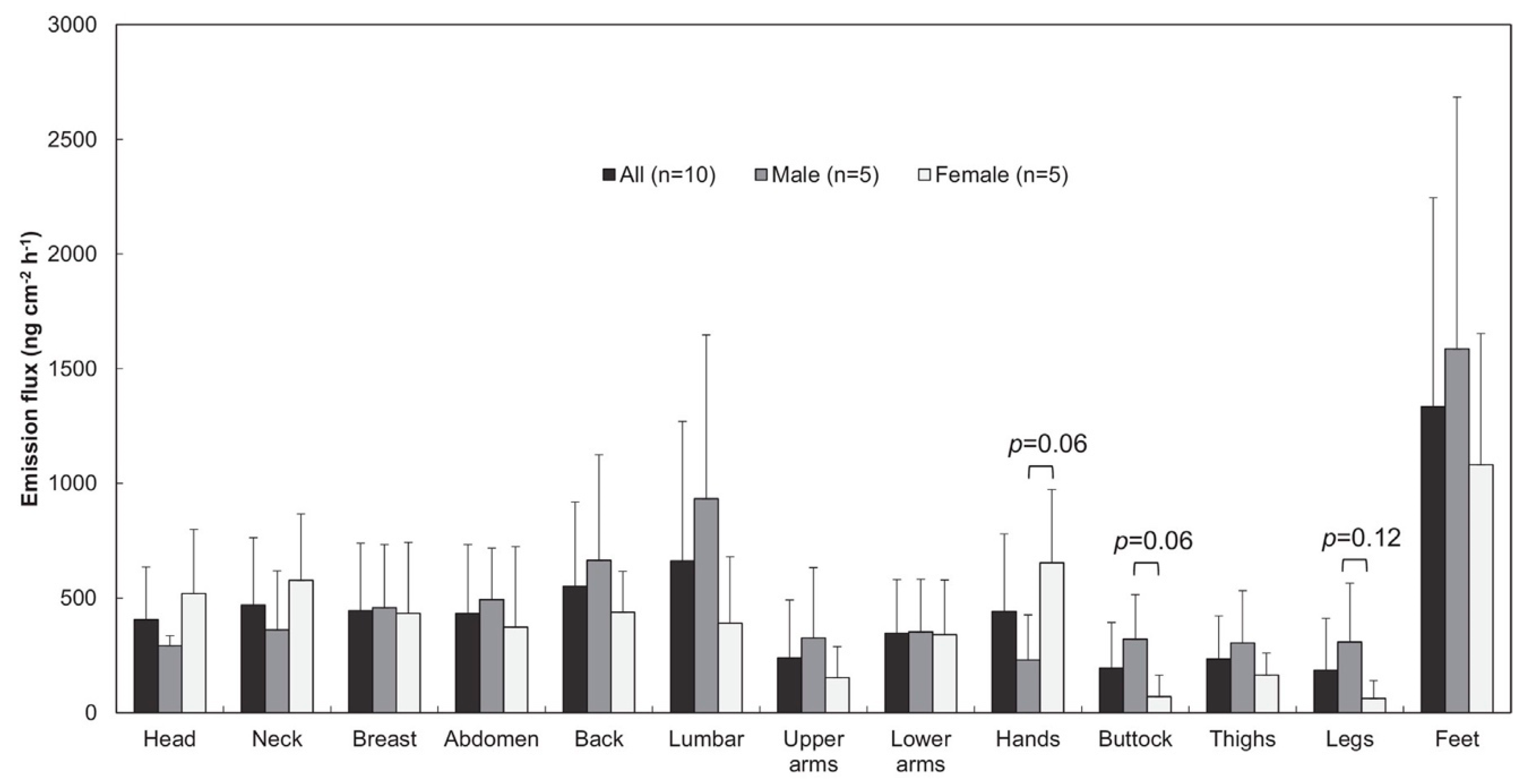
2.2.2. Passive Flux Samplers
2.2.3. Other Wearable Headspace Extractive Samplers
3. Oral Fluid and Ocular Surface Sampling
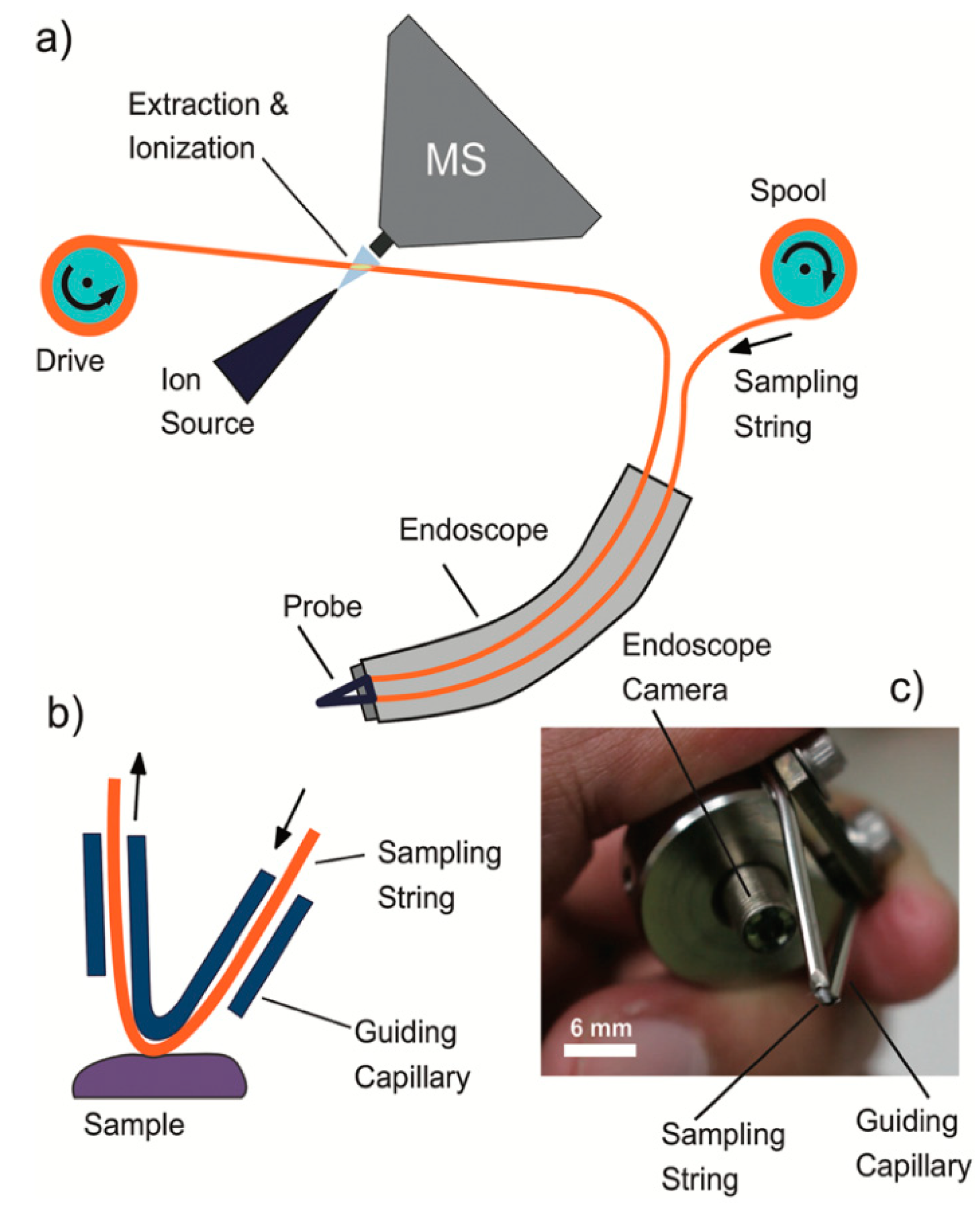
3.1. Saliva Sampling
3.2. Oral Tissue Sampling
3.3. Ocular Surface Sampling
4. Extractive Patches for Imaging Applications
5. Perspectives in Future Directions and Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shim, B.S.; Chen, W.; Doty, C.; Xu, C.; Kotov, N.A. Smart Electronic Yarns and Wearable Fabrics for Human Biomonitoring made by Carbon Nanotube Coating with Polyelectrolytes. Nano Lett. 2008, 8, 4151–4157. [Google Scholar] [CrossRef] [PubMed]
- Amjadi, M.; Pichitpajongkit, A.; Lee, S.; Ryu, S.; Park, I. Highly Stretchable and Sensitive Strain Sensor Based on Silver Nanowire–Elastomer Nanocomposite. ACS Nano 2014, 8, 5154–5163. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Emaminejad, S.; Nyein, H.Y.Y.; Challa, S.; Chen, K.; Peck, A.; Fahad, H.M.; Ota, H.; Shiraki, H.; Kiriya, D.; et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 2016, 529, 509–514. [Google Scholar] [CrossRef]
- Amjadi, M.; Kyung, K.-U.; Park, I.; Sitti, M. Stretchable, Skin-Mountable, and Wearable Strain Sensors and Their Potential Applications: A Review. Adv. Funct. Mater. 2016, 26, 1678–1698. [Google Scholar] [CrossRef]
- Trung, T.Q.; Lee, N.-E. Flexible and Stretchable Physical Sensor Integrated Platforms for Wearable Human-Activity Monitoringand Personal Healthcare. Adv. Mater. 2016, 28, 4338–4372. [Google Scholar] [CrossRef] [PubMed]
- Penn, D.J.; Oberzaucher, E.; Grammer, K.; Fischer, G.; Soini, H.A.; Wiesler, D.; Novotny, M.V.; Dixon, S.J.; Xu, Y.; Brereton, R.G. Individual and gender fingerprints in human body odour. J. R. Soc. Interface 2007, 4, 331–340. [Google Scholar] [CrossRef]
- Nemes, P.; Vertes, A. Ambient mass spectrometry for in vivo local analysis and in situ molecular tissue imaging. Trac.-Trends Anal. Chem. 2012, 34, 22–34. [Google Scholar] [CrossRef]
- Dormont, L.; Bessière, J.-M.; Cohuet, A. Human Skin Volatiles: A Review. J. Chem. Ecol. 2013, 39, 569–578. [Google Scholar] [CrossRef]
- Andrisic, L.; Dudzik, D.; Barbas, C.; Milkovic, L.; Grune, T.; Zarkovic, N. Short overview on metabolomics approach to study pathophysiology of oxidative stress in cancer. Redox Biol. 2017, 14, 47–58. [Google Scholar] [CrossRef]
- Noël, F.; Piérard-Franchimont, C.; Piérard, G.E.; Quatresooz, P. Sweaty skin, background and assessments. Int. J. Dermatol. 2012, 51, 647–655. [Google Scholar] [CrossRef]
- Prada, A.; Furton, K.G. Recent Advances in Solid-Phase Microextraction for Forensic Applications. In Comprehensive Sampling and Sample Preparation; Pawliszyn, J., Ed.; Academic Press: Oxford, UK, 2012; pp. 877–891. [Google Scholar]
- Curran, A.M.; Prada, P.A.; Furton, K.G. Canine human scent identifications with post-blast debris collected from improvised explosive devices. Forensic Sci. Int. 2010, 199, 103–108. [Google Scholar] [CrossRef] [PubMed]
- DeGreeff, L.E.; Furton, K.G. Collection and identification of human remains volatiles by non-contact, dynamic airflow sampling and SPME-GC/MS using various sorbent materials. Anal. Bioanal. Chem. 2011, 401, 1295–1307. [Google Scholar] [CrossRef] [PubMed]
- Prugnolle, F.; Lefèvre, T.; Renaud, F.; Møller, A.P.; Missé, D.; Thomas, F. Infection and body odours: Evolutionary and medical perspectives. Infect. Genet. Evol. 2009, 9, 1006–1009. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-H.; Jahan, S.A.; Kabir, E. A review of breath analysis for diagnosis of human health. TrAC Trends Anal. Chem. 2012, 33, 1–8. [Google Scholar] [CrossRef]
- Santonico, M.; Lucantoni, G.; Pennazza, G.; Capuano, R.; Galluccio, G.; Roscioni, C.; La Delfa, G.; Consoli, D.; Martinelli, E.; Paolesse, R.; et al. In situ detection of lung cancer volatile fingerprints using bronchoscopic air-sampling. Lung Cancer 2012, 77, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Saudan, C.; Baume, N.; Robinson, N.; Avois, L.; Mangin, P.; Saugy, M. Testosterone and doping control. Br. J. Sports Med. 2006, 40, i21–i24. [Google Scholar] [CrossRef] [PubMed]
- Geyer, H.; Schänzer, W.; Thevis, M. Anabolic agents: Recent strategies for their detection and protection from inadvertent doping. Br. J. Sports Med. 2014, 48, 820–826. [Google Scholar] [CrossRef]
- Xu, X.; Weisel, C.P. Dermal uptake of chloroform and haloketones during bathing. J. Expo. Anal. Environ. Epidemiol. 2005, 15, 289–296. [Google Scholar] [CrossRef]
- Jiřík, V.; Machaczka, O.; Miturová, H.; Tomášek, I.; Šlachtová, H.; Janoutová, J.; Velická, H.; Janout, V. Air Pollution and Potential Health Risk in Ostrava Region—A Review. Cent. Eur. J. Public Health 2016, 24, S4–S17. [Google Scholar] [CrossRef]
- Vineis, P.; Chadeau-Hyam, M.; Gmuender, H.; Gulliver, J.; Herceg, Z.; Kleinjans, J.; Kogevinas, M.; Kyrtopoulos, S.; Nieuwenhuijsen, M.; Phillips, D.H.; et al. The exposome in practice: Design of the EXPOsOMICS project. Int. J. Hyg. Environ. Health 2017, 220, 142–151. [Google Scholar] [CrossRef]
- Taylor, R.P.; Polliack, A.A.; Bader, D.L. The Analysis of Metabolites in Human Sweat: Analytical Methods and Potential Application to Investigation of Pressure Ischaemia of Soft Tissues. Ann. Clin. Biochem. Int. J. Biochem. Lab. Med. 1994, 31, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Kutyshenko, V.P.; Molchanov, M.; Beskaravayny, P.; Uversky, V.N.; Timchenko, M.A. Analyzing and Mapping Sweat Metabolomics by High-Resolution NMR Spectroscopy. PLoS ONE 2011, 6, e28824. [Google Scholar] [CrossRef] [PubMed]
- Shirreffs, S.M.; Maughan, R.J. Whole body sweat collection in humans: An improved method with preliminary data on electrolyte content. J. Appl. Physiol. 1997, 82, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, M.; Wysocki, C.J.; Leyden, J.J.; Spielman, A.I.; Sun, X.; Preti, G. Analyses of volatile organic compounds from human skin. Br. J. Dermatol. 2008, 159, 780–791. [Google Scholar] [CrossRef]
- Calderón-Santiago, M.; Priego-Capote, F.; Jurado-Gámez, B.; Luque de Castro, M.D. Optimization study for metabolomics analysis of human sweat by liquid chromatography–tandem mass spectrometry in high resolution mode. J. Chromatogr. A 2014, 1333, 70–78. [Google Scholar] [CrossRef]
- Kintz, P.; Cirimele, V.; Ludes, B. Detection of Cannabis in Oral Fluid (Saliva) and Forehead Wipes (Sweat) from Impaired Drivers. J. Anal. Toxicol. 2000, 24, 557–561. [Google Scholar] [CrossRef][Green Version]
- Wang, L.; Pi, Z.; Liu, S.; Liu, Z.; Song, F. Targeted metabolome profiling by dual-probe microdialysis sampling and treatment using Gardenia jasminoides for rats with type 2 diabetes. Sci. Rep. 2017, 7, 10105. [Google Scholar] [CrossRef]
- Poole, J.J.; Grandy, J.J.; Yu, M.; Boyaci, E.; Gómez-Ríos, G.A.; Reyes-Garcés, N.; Bojko, B.; Heide, H.V.; Pawliszyn, J. Deposition of a Sorbent into a Recession on a Solid Support to Provide a New, Mechanically Robust Solid-Phase Microextraction Device. Anal. Chem. 2017, 89, 8021–8026. [Google Scholar] [CrossRef]
- Bruheim, I.; Liu, X.; Pawliszyn, J. Thin-Film Microextraction. Anal. Chem. 2003, 75, 1002–1010. [Google Scholar] [CrossRef]
- Jiang, R.; Pawliszyn, J. Thin-film microextraction offers another geometry for solid-phase microextraction. TrAC Trends Anal. Chem. 2012, 39, 245–253. [Google Scholar] [CrossRef]
- Souza-Silva, É.A.; Gionfriddo, E.; Shirey, R.; Sidisky, L.; Pawliszyn, J. Methodical evaluation and improvement of matrix compatible PDMS-overcoated coating for direct immersion solid phase microextraction gas chromatography (DI-SPME-GC)-based applications. Anal. Chim. Acta 2016, 920, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Risticevic, S.; Pawliszyn, J. Solid-Phase Microextraction in Targeted and Nontargeted Analysis: Displacement and Desorption Effects. Anal. Chem. 2013, 85, 8987–8995. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Cudjoe, E.; Bojko, B.; Abaffy, T.; Pawliszyn, J. A non-invasive method for in vivo skin volatile compounds sampling. Anal. Chim. Acta 2013, 804, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Schivo, M.; Aksenov, A.A.; Pasamontes, A.; Cumeras, R.; Weisker, S.; Oberbauer, A.M.; Davis, C.E. A rabbit model for assessment of volatile metabolite changes observed from skin: A pressure ulcer case study. J. Breath Res. 2017, 11, 016007. [Google Scholar] [CrossRef]
- Martin, H.J.; Reynolds, J.C.; Riazanskaia, S.; Thomas, C.L.P. High throughput volatile fatty acid skin metabolite profiling by thermal desorption secondary electrospray ionisation mass spectrometry. Analyst 2014, 139, 4279–4286. [Google Scholar] [CrossRef]
- Lang, T.; Justenhoven, C.; Winter, S.; Baisch, C.; Hamann, U.; Harth, V.; Ko, Y.-D.; Rabstein, S.; Spickenheuer, A.; Pesch, B.; et al. The earwax-associated SNP c.538G>A (G180R) in ABCC11 is not associated with breast cancer risk in Europeans. Breast Cancer Res. Treat 2011, 129, 993–999. [Google Scholar] [CrossRef]
- Martin, H.J.; Turner, M.A.; Bandelow, S.; Edwards, L.; Riazanskaia, S.; Thomas, C.L.P. Volatile organic compound markers of psychological stress in skin: A pilot study. J. Breath Res. 2016, 10, 046012. [Google Scholar] [CrossRef][Green Version]
- Turner, M.A.; Bandelow, S.; Edwards, L.; Patel, P.; Martin, H.J.; Wilson, I.D.; Thomas, C.L.P. The effect of a paced auditory serial addition test (PASAT) intervention on the profile of volatile organic compounds in human breath: A pilot study. J. Breath Res. 2013, 7, 017102. [Google Scholar] [CrossRef]
- Stevens, D.; Cornmell, R.; Taylor, D.; Grimshaw, S.G.; Riazanskaia, S.; Arnold, D.S.; Fernstad, S.J.; Smith, A.M.; Heaney, L.M.; Reynolds, J.C.; et al. Spatial variations in the microbial community structure and diversity of the human foot is associated with the production of odorous volatiles. FEMS Microbiol. Ecol. 2015, 91, 1–11. [Google Scholar] [CrossRef]
- McHugh, D.J. Issue 288 of FAO fisheries technical paper. In Production and Utilization of Products from Commercial Seaweeds; Food and Agriculture Organization of the United Nations: Rome, Italy, 1987; p. 189. [Google Scholar]
- Dutkiewicz, E.P.; Lin, J.-D.; Tseng, T.-W.; Wang, Y.-S.; Urban, P.L. Hydrogel Micropatches for Sampling and Profiling Skin Metabolites. Anal. Chem. 2014, 86, 2337–2344. [Google Scholar] [CrossRef]
- Dutkiewicz, E.P.; Chiu, H.-Y.; Urban, P.L. Micropatch-arrayed pads for non-invasive spatial and temporal profiling of topical drugs on skin surface: Skin analysis. J. Mass Spectrom. 2015, 50, 1321–1325. [Google Scholar] [CrossRef] [PubMed]
- Dutkiewicz, E.P.; Hsieh, K.-T.; Wang, Y.-S.; Chiu, H.-Y.; Urban, P.L. Hydrogel Micropatch and Mass Spectrometry-Assisted Screening for Psoriasis-Related Skin Metabolites. Clin. Chem. 2016, 62, 1120–1128. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-C.; Park, J.-H.; Prausnitz, M.R. Microneedles for drug and vaccine delivery. Adv. Drug Deliv. Rev. 2012, 64, 1547–1568. [Google Scholar] [CrossRef] [PubMed]
- Tuan-Mahmood, T.-M.; McCrudden, M.T.C.; Torrisi, B.M.; McAlister, E.; Garland, M.J.; Singh, T.R.R.; Donnelly, R.F. Microneedles for intradermal and transdermal drug delivery. Eur. J. Pharm. Sci. 2013, 50, 623–637. [Google Scholar] [CrossRef] [PubMed]
- Bhargav, A.; Muller, D.A.; Kendall, M.A.F.; Corrie, S.R. Surface Modifications of Microprojection Arrays for Improved Biomarker Capture in the Skin of Live Mice. ACS Appl. Mater. Interfaces 2012, 4, 2483–2489. [Google Scholar] [CrossRef]
- Coffey, J.W.; Corrie, S.R.; Kendall, M.A.F. Early circulating biomarker detection using a wearable microprojection array skin patch. Biomaterials 2013, 34, 9572–9583. [Google Scholar] [CrossRef]
- Lee, K.T.; Muller, D.A.; Coffey, J.W.; Robinson, K.J.; McCarthy, J.S.; Kendall, M.A.F.; Corrie, S.R. Capture of the Circulating Plasmodium falciparum Biomarker HRP2 in a Multiplexed Format, via a Wearable Skin Patch. Anal. Chem. 2014, 86, 10474–10483. [Google Scholar] [CrossRef]
- Yeow, B.; Coffey, J.W.; Muller, D.A.; Grøndahl, L.; Kendall, M.A.F.; Corrie, S.R. Surface Modification and Characterization of Polycarbonate Microdevices for Capture of Circulating Biomarkers, Both in Vitro and in Vivo. Anal. Chem. 2013, 85, 10196–10204. [Google Scholar] [CrossRef]
- Muller, D.A.; Corrie, S.R.; Coffey, J.; Young, P.R.; Kendall, M.A. Surface Modified Microprojection Arrays for the Selective Extraction of the Dengue Virus NS1 Protein as a Marker for Disease. Anal. Chem. 2012, 84, 3262–3268. [Google Scholar] [CrossRef]
- Corrie, S.R.; Fernando, G.J.P.; Crichton, M.L.; Brunck, M.E.G.; Anderson, C.D.; Kendall, M.A.F. Surface-modified microprojection arrays for intradermal biomarker capture, with low non-specific protein binding. Lab Chip 2010, 10, 2655–2658. [Google Scholar] [CrossRef]
- Ng, K.W.; Lau, W.M.; Williams, A.C. Towards pain-free diagnosis of skin diseases through multiplexed microneedles: Biomarker extraction and detection using a highly sensitive blotting method. Drug Deliv. Transl. Res. 2015, 5, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Bernier, U.R.; Kline, D.L.; Barnard, D.R.; Schreck, C.E.; Yost, R.A. Analysis of Human Skin Emanations by Gas Chromatography/Mass Spectrometry. 2. Identification of Volatile Compounds That Are Candidate Attractants for the Yellow Fever Mosquito (Aedes aegypti). Anal. Chem. 2000, 72, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Soini, H.A.; Bruce, K.E.; Klouckova, I.; Brereton, R.G.; Penn, D.J.; Novotny, M.V. In Situ Surface Sampling of Biological Objects and Preconcentration of Their Volatiles for Chromatographic Analysis. Anal. Chem. 2006, 78, 7161–7168. [Google Scholar] [CrossRef] [PubMed]
- Riazanskaia, S.; Blackburn, G.; Harker, M.; Taylor, D.; Thomas, C.L.P. The analytical utility of thermally desorbed polydimethylsilicone membranes for in-vivo sampling of volatile organic compounds in and on human skin. Analyst 2008, 133, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Pawliszyn, J. Headspace solid-phase microextraction. Anal. Chem. 1993, 65, 1843–1852. [Google Scholar] [CrossRef]
- Camarasu, C.C. Headspace SPME method development for the analysis of volatile polar residual solvents by GC-MS. J. Pharm. Biomed. Anal. 2000, 23, 197–210. [Google Scholar] [CrossRef]
- Rocha, S.M.; Ramalheira, V.; Barros, A.A.C.; Delgadillo, I.; Coimbra, M.A. Headspace solid phase microextraction (SPME) analysis of flavor compounds in wines. Effect of the matrix volatile composition in the relative response factors in a wine model. J. Agric. Food Chem. 2001, 49, 5142–5151. [Google Scholar] [CrossRef]
- Pini, G.F.; de Brito, E.S.; García, N.H.P.; Valente, A.L.P.; Augusto, F. A Headspace Solid Phase Microextraction (HS-SPME) method for the chromatographic determination of alkylpyrazines in cocoa samples. J. Braz. Chem. Soc. 2004, 15, 267–271. [Google Scholar] [CrossRef]
- Zhang, Z.-M.; Cai, J.-J.; Ruan, G.-H.; Li, G.-K. The study of fingerprint characteristics of the emanations from human arm skin using the original sampling system by SPME-GC/MS. J. Chromatogr. B 2005, 822, 244–252. [Google Scholar] [CrossRef]
- Sekine, Y.; Toyooka, S.; Watts, S.F. Determination of acetaldehyde and acetone emanating from human skin using a passive flux sampler—HPLC system. J. Chromatogr. B 2007, 859, 201–207. [Google Scholar] [CrossRef]
- Duffy, E.; Jacobs, M.R.; Kirby, B.; Morrin, A. Probing skin physiology through the volatile footprint: Discriminating volatile emissions before and after acute barrier disruption. Exp. Dermatol. 2017, 26, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Duffy, E.; Albero, G.; Morrin, A. Headspace Solid-Phase Microextraction Gas Chromatography-Mass Spectrometry Analysis of Scent Profiles from Human Skin. Cosmetics 2018, 5, 62. [Google Scholar] [CrossRef]
- Gómez-Ríos, G.A.; Reyes-Garcés, N.; Bojko, B.; Pawliszyn, J. Biocompatible Solid-Phase Microextraction Nanoelectrospray Ionization: An Unexploited Tool in Bioanalysis. Anal. Chem. 2016, 88, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- Mirabelli, M.F.; Wolf, J.-C.; Zenobi, R. Direct Coupling of Solid-Phase Microextraction with Mass Spectrometry: Sub-pg/g Sensitivity Achieved Using a Dielectric Barrier Discharge Ionization Source. Anal. Chem. 2016, 88, 7252–7258. [Google Scholar] [CrossRef]
- Gómez-Ríos, G.A.; Liu, C.; Tascon, M.; Reyes-Garcés, N.; Arnold, D.W.; Covey, T.R.; Pawliszyn, J. Open Port Probe Sampling Interface for the Direct Coupling of Biocompatible Solid-Phase Microextraction to Atmospheric Pressure Ionization Mass Spectrometry. Anal. Chem. 2017, 89, 3805–3809. [Google Scholar] [CrossRef]
- Mosquera, J.; Scholtens, R.; Ogink, N. Using Passive Flux Samplers to determine the ammonia emission from mechanically ventilated animal houses. In Proceedings of the 2003 ASAE Annual Meeting, Las Vegas, NV, USA, 27–30 July 2003; American Society of Agricultural and Biological Engineers: San Jose, MI, USA, 2003. [Google Scholar]
- Larios, A.D.; Brar, S.K.; Ramírez, A.A.; Godbout, S.; Sandoval-Salas, F.; Palacios, J.H.; Dubé, P.; Delgado, B.; Giroir-Fendler, A. Parameters determining the use of zeolite 5A as collector medium in passive flux samplers to estimate N2O emissions from livestock sources. Environ. Sci. Pollut. Res. 2017, 24, 12136–12143. [Google Scholar] [CrossRef]
- Larios, A.D.; Kaur Brar, S.; Avalos Ramírez, A.; Godbout, S.; Sandoval-Salas, F.; Palacios, J.H. Challenges in the measurement of emissions of nitrous oxide and methane from livestock sector. Rev. Environ. Sci. Biotechnol. 2016, 15, 285–297. [Google Scholar] [CrossRef]
- Debbagh, M.; Adamchuk, V.; Madramootoo, C.; Whalen, J. Development of a Wireless Sensor Network for Passive in situ Measurement of Soil CO2 Gas Emissions in the Agriculture Landscape. In Proceedings of the 14th International Conference on Precision Agriculture, Montreal, QC, Canada, 24–27 June 2018. [Google Scholar]
- Fujii, M.; Shinohara, N.; Lim, A.; Otake, T.; Kumagai, K.; Yanagisawa, Y. A study on emission of phthalate esters from plastic materials using a passive flux sampler. Atmos. Environ. 2003, 37, 5495–5504. [Google Scholar] [CrossRef]
- Kimura, K.; Sekine, Y.; Furukawa, S.; Takahashi, M.; Oikawa, D. Measurement of 2-nonenal and diacetyl emanating from human skin surface employing passive flux sampler—GCMS system. J. Chromatogr. B 2016, 1028, 181–185. [Google Scholar] [CrossRef]
- Furukawa, S.; Sekine, Y.; Kimura, K.; Umezawa, K.; Asai, S.; Miyachi, H. Simultaneous and multi-point measurement of ammonia emanating from human skin surface for the estimation of whole body dermal emission rate. J. Chromatogr. B 2017, 1053, 60–64. [Google Scholar] [CrossRef]
- Sekine, Y.; Sato, S.; Kimura, K.; Sato, H.; Nakai, S.; Yanagisawa, Y. Detection of tobacco smoke emanating from human skin surface of smokers employing passive flux sampler—GCMS system. J. Chromatogr. B 2018, 1092, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Wang, X.; Li, X.; Inlora, J.; Wang, T.; Liu, Q.; Snyder, M. Dynamic Human Environmental Exposome Revealed by Longitudinal Personal Monitoring. Cell 2018, 175, 277–291. [Google Scholar] [CrossRef] [PubMed]
- Roodt, A.P.; Naudé, Y.; Stoltz, A.; Rohwer, E. Human skin volatiles: Passive sampling and GC × GC-ToFMS analysis as a tool to investigate the skin microbiome and interactions with anthropophilic mosquito disease vectors. J. Chromatogr. B 2018, 1097, 83–93. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, S.G.; Kincl, L.D.; Anderson, K.A. Silicone Wristbands as Personal Passive Samplers. Environ. Sci. Technol. 2014, 48, 3327–3335. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, E.; Queiroz, J.A. The role of alternative specimens in toxicological analysis. Biomed. Chromatogr. 2008, 22, 795–821. [Google Scholar] [CrossRef]
- De Almeida, P.D.V.; Grégio, A.M.T.; Machado, M.A.N.; De Lima, A.A.S.; Azevedo, L.R. Saliva composition and functions: A comprehensive review. J. Contemp. Dent. Pract. 2008, 9, 72–80. [Google Scholar]
- Gröschl, M.; Köhler, H.; Topf, H.-G.; Rupprecht, T.; Rauh, M. Evaluation of saliva collection devices for the analysis of steroids, peptides and therapeutic drugs. J. Pharm. Biomed. Anal. 2008, 47, 478–486. [Google Scholar] [CrossRef]
- Higashi, T. Salivary Hormone Measurement Using LC/MS/MS: Specific and Patient-Friendly Tool for Assessment of Endocrine Function. Biol. Pharm. Bull. 2012, 35, 1401–1408. [Google Scholar] [CrossRef]
- Bessonneau, V.; Boyaci, E.; Maciazek-Jurczyk, M.; Pawliszyn, J. In vivo solid phase microextraction sampling of human saliva for non-invasive and on-site monitoring. Anal. Chim. Acta 2015, 856, 35–45. [Google Scholar] [CrossRef]
- Chen, L.C.; Naito, T.; Tsutsui, S.; Yamada, Y.; Ninomiya, S.; Yoshimura, K.; Takeda, S.; Hiraoka, K. In vivo endoscopic mass spectrometry using a moving string sampling probe. Analyst 2017, 142, 2735–2740. [Google Scholar] [CrossRef]
- Semba, R.D.; Enghild, J.J.; Venkatraman, V.; Dyrlund, T.F.; Van Eyk, J.E. The Human Eye Proteome Project: Perspectives on an emerging proteome. Proteomics 2013, 13, 2500–2511. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S. Diagnosis of infectious diseases of the eye. Eye 2012, 26, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Stamer, W.D.; Williams, A.M.; Pflugfelder, S.; Coupland, S.E. Accessibility to and Quality of Human Eye Tissue for Research: A Cross-Sectional Survey of ARVO Members. Invest. Ophthalmol. Vis. Sci. 2018, 59, 4783–4792. [Google Scholar] [CrossRef] [PubMed]
- Liou, Y.-W.; Chang, K.-Y.; Lin, C.-H. Sampling and profiling caffeine and its metabolites from an eyelid using a watercolor pen based on electrospray ionization/mass spectrometry. Int. J. Mass Spectrom. 2017, 422, 51–55. [Google Scholar] [CrossRef]
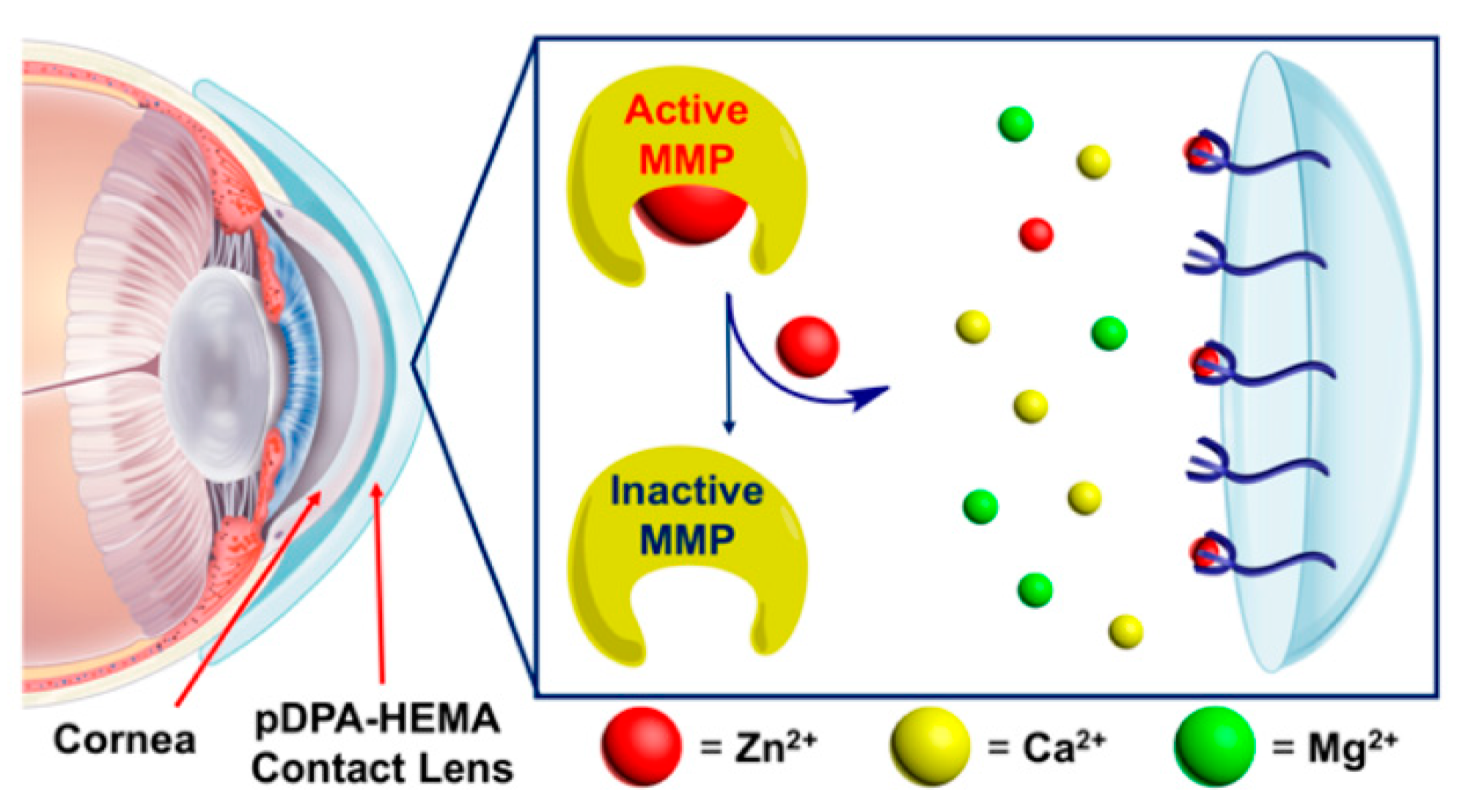
- Lopez, C.; Park, S.; Edwards, S.; Vong, S.; Hou, S.; Lee, M.; Sauerland, H.; Lee, J.-J.; Jeong, K.J. Matrix Metalloproteinase-Deactivating Contact Lens for Corneal Melting. ACS Biomater. Sci. Eng. 2019, 5, 1195–1199. [Google Scholar] [CrossRef]
- Maruyama, S.; Kikuchi, K.; Hirano, T.; Urano, Y.; Nagano, T. A Novel, Cell-Permeable, Fluorescent Probe for Ratiometric Imaging of Zinc Ion. J. Am. Chem. Soc. 2002, 124, 10650–10651. [Google Scholar] [CrossRef]
- Hemalatha, R.G.; Ganayee, M.A.; Pradeep, T. Electrospun Nanofiber Mats as “Smart Surfaces” for Desorption Electrospray Ionization Mass Spectrometry (DESI MS)-Based Analysis and Imprint Imaging. Anal. Chem. 2016, 88, 5710–5717. [Google Scholar] [CrossRef]
- Li, W.; Chen, X.; Wang, Z.; Wong, Y.E.; Wu, R.; Hung, Y.-L.W.; Chan, T.-W.D. Tissue imaging with in situ solid-phase extraction micro-funnel based spray ionization mass spectrometry. Eur. J. Mass Spectrom. 2018, 24, 66–73. [Google Scholar] [CrossRef]




| Peak No | m/z* (IT) | m/z* (FT – ICR) | Metabolite Formula | Metabolite Name | Predicted b m/z* | MS/MS | Compared with Standard |
|---|---|---|---|---|---|---|---|
| 1 | 89.0 | 89.02438 | C3H6O3 | lactic acid | 89.02442 | + | + |
| 2 | 93.0 | 93.04578 | C5H6N2 | fragment of uronic acid | 93.04582 | + | + |
| 3 | 104.0 | 104.03530 | C3H7NO3 | serine | 104.03532 | + | + |
| 4 | 118.0 | 118.05089 | C4H9NO3 | threonine | 118.05097 | + | + |
| 5 | 128.0 | 128.03530 | C5H7NO3 | pyroglutamic acid | 128.03532 | + | + |
| 6 | 131.0 | 131.08259 | C5H12N2O2 | ornithine a | 131.08260 | + | - |
| 7 | 137.0 | 137.03561 | C6H6N2O2 | urocanic acid | 137.03565 | + | + |
| 8 | 154.0 | 154.06218 | C6H9N3O2 | histidine a | 154.06220 | + | - |
| 9 | 179.0 | 179.05731 | C7H8N4O2 | paraxanthine a | 179.05745 | - | - |
| Method | Materials | Body Part | Analytes | Sampling Time | Instrumentations | Comments |
|---|---|---|---|---|---|---|
| Patch type [34] | PDMS | Upper back, forearms, back thigh | VOCs | 1 h | GC–MS | “sandwich membrane”, minimal contamination |
| Patch type [36] | PDMS | Armpit | Fatty acid metabolites, VOCs | 30 min | TD–SESI–MS/TD–GC–MS | suitable for automation |
| Patch type [38] | PDMS | Forehead | VOCs | 30 min | TD–GC–MS | complementary to breath analysis |
| Patch type [35] | PDMS | Ear | Rabbit skin metabolites, ulcer metabolites | 30 min | GC–MS | rabbit model study |
| Patch type [40] | PDMS | Foot | VOCs | 30 min | TD–GC–MS | complementary to bacterial mapping |
| Patch type [42] | Agarose hydrogel | Lower arm | Skin metabolites | 1 min–3 h | nanoDESI–MS | direct mass spectrometry |
| Patch type [44] | Agarose hydrogel | Upper and lower limbs, abdomen, back | Psoriatic skin metabolites | 20 min | nanoDESI–MS | direct mass spectrometry |
| Patch type [43] | Agarose hydrogels | Lower arm | Topical drug metabolites, nicotine and scopolamine metabolites | 10 min | nanoDESI–MS | direct mass spectrometry |
| Microneedle [53] | Polylactic acid | Mouse skin | Skin biomarkers | 1 h | microplate UV/VIS spectrophotometry, densitometric analysis | limited to biomarkers with known antibodies, can only sample from specific skin depth |
| Headspace [63] | DVB/carboxen/PDMS (Stableflex) | Volar forearm | VOCs | 15 min | GC–MS | glass housing |
| Headspace [64] | DVB/carboxen/PDMS (Stableflex) | Volar forearm | Skin and fragrance-derived VOCs | 5–40 min | GC–MS | glass housing |
| Passive sampling [77] | PDMS | Wrist, ankle | Skin VOCs, mosquito semiochemicals | 4 h | GC × GC–TOFMS | controlled environment is recommended for good repeatability |
| Passive flux sampling [73] | MonoTrap® DCC18 | Forearm, thigh, calf, forehead, neck, abdomen | 2-nonenal, diacetyl | 7 h | GC–MS | flux flow knowledge is required for quantitation |
| Passive flux sampling [74] | Conditioned cellulose paper | see Figure 4 | Ammonia | 1 h | Ion chromatography | flux flow knowledge is required for quantitation |
| Passive flux sampling [75] | MonoTrap® DCC18 | Forearm, back of the hand | VOCs | 1 h | GC–MS | flux flow knowledge is required for quantitation |
| Passive sampling [78] | Silicone | Wrist | PAHs, environmental chemicals | 2–24 h | GC–MS | low repeatability |
| Method | Biocompatibility | Non-Invasiveness | Ease-of-Use | Commercial Availability | Sampler to Instrument Coupling | Real Time Monitoring |
|---|---|---|---|---|---|---|
| PDMS patches | ☆☆☆ | ☆☆☆ | ☆☆☆ | Yes c | Yes f | No |
| Hydrogel patches | ☆☆☆ | ☆☆☆ | ☆☆☆ | No | Yes | No |
| Microneedle arrays | ☆☆ | ☆ | ☆☆ | No | No | No |
| SPME headspace sampler | ☆☆ a | ☆☆☆ | ☆ | Yes d | Yes | No |
| Passive flux sampler | ☆☆ a | ☆☆☆ | ☆☆☆ | Yes e | No | No |
| Wearable headspace sampling | ☆☆☆ | ☆☆☆ | ☆☆☆ | No | Yes f | No |
| TFME sampling | ☆☆☆ | ☆☆☆ | ☆☆☆ | Yes e | Yes | No |
| String type sampler | ☆☆☆ | ☆☆☆ | ☆ | No | Yes | Yes |
| Brush type sampler | ☆☆b | ☆☆ | ☆☆ | Not applicable | Yes | No |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nalbant, A.A.; Boyacı, E. Advancements in Non-Invasive Biological Surface Sampling and Emerging Applications. Separations 2019, 6, 52. https://doi.org/10.3390/separations6040052
Nalbant AA, Boyacı E. Advancements in Non-Invasive Biological Surface Sampling and Emerging Applications. Separations. 2019; 6(4):52. https://doi.org/10.3390/separations6040052
Chicago/Turabian StyleNalbant, Atakan Arda, and Ezel Boyacı. 2019. "Advancements in Non-Invasive Biological Surface Sampling and Emerging Applications" Separations 6, no. 4: 52. https://doi.org/10.3390/separations6040052
APA StyleNalbant, A. A., & Boyacı, E. (2019). Advancements in Non-Invasive Biological Surface Sampling and Emerging Applications. Separations, 6(4), 52. https://doi.org/10.3390/separations6040052






