1. Introduction
Medicines obtained from traditional plants are called phytomedicines. The phytomedicines have long been used after several generations of trial and error [
1]. The medicinal plants contain certain chemical compounds responsible for their antimicrobial activity [
2].
Aechmea magdalenae (
Andre) Andre ex Baker, is a plant falling under the family Bromeliaceae, which is a native of Central America. Although the literature availability for the antibacterial activity of this species has been limited, the documented research revealed the use of
A. magdalenae as caustic for wounds [
3]. Therefore, it was hypothesized that plant
A. magdalenae has antibacterial properties. The ethnobotanical approach has also proved to be advantageous while finding new drug candidates.
GC-MS profiling confirmed acetic acid as a high probability (>90%) chemical compound, out of many others, present in the rhizome of
A. magdalenae [
4]. The antibacterial potential of acetic acid was confirmed in our laboratory using agar disc diffusion assay performed on gram negative bacteria [
4]. The agar well diffusion assay was performed to evaluate the antibacterial activity while a 96-well plate assay determined the MIC of the rhizome extract of
A. magdalenae [
4]. Both the agar well diffusion assay and the 96-well plate assay included gram-negative
Escherichia coli (E. coli) and gram-positive
Staphylococcus aureus (S. aureus) bacteria. In this study, we aimed to develop a highly rapid and sensitive ultra-high performance liquid chromatograph-high resolution mass spectrometer (UHPLC-HRMS) method to detect the accurate mass of indicator resazurin and its reduced form, resorufin.
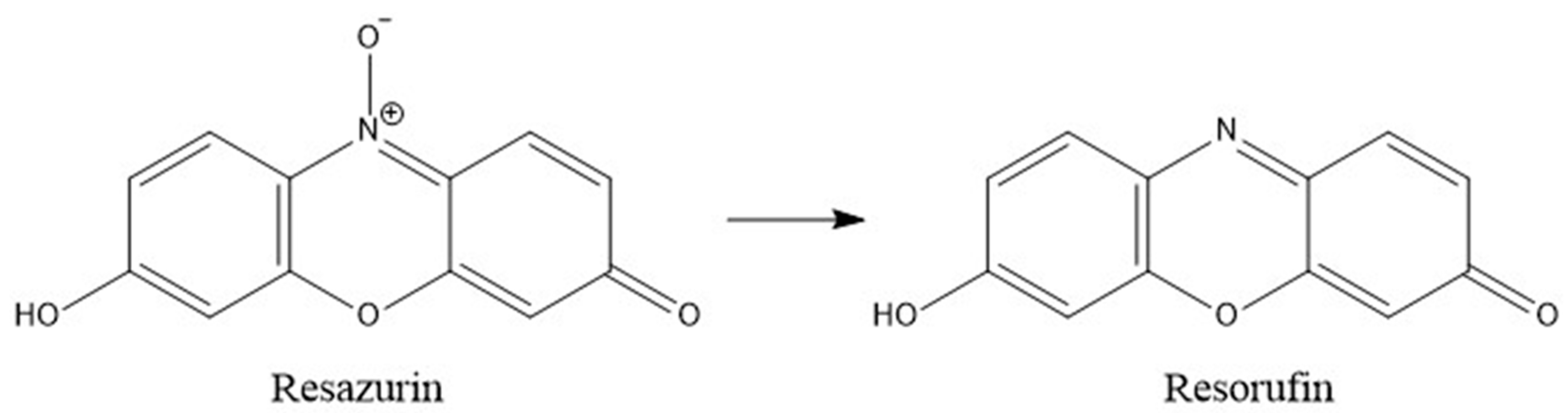
There was a significant mass difference between indicator resazurin and its reduced form, resorufin (
Figure 1). This difference in mass between resazurin’s intact and reduced forms was detected by mass spectrometer in positive ion mode. The MIC of plant
A. magdalenae was determined previously using a 96-well plate assay [
4]. In the present study, a novel rapid LC-MS method was developed that could determine the antibacterial potential of medicinal plants using the mass difference of resazurin and resorufin. The Aechmea plant extract was not too dark, so the MIC was read with naked eye from the 96-well plate before determining the antibacterial potential by using mass spectrometer.
But there are several other medicinal plants in our lab with dark extracts for which the antibacterial activity has yet to be determined. For those plants, determination of antibacterial potential using a 96-well plate would be challenging. In that situation, LC-MS method would determine the antibacterial activity of dark medicinal plant extracts by using the property of mass difference created after reduction of resazurin to resorufin instead of colorimetric analysis. For the plant, A. magdalena, the MIC was already determined before using the 96-well plate assay. But for future dark plant extracts, LC-MS method could be used directly.
3. Results and Discussion
Antimicrobial activity of
A. magdalenae was determined using agar disc diffusion and agar well diffusion assays previously [
4]. Agar disk diffusion method is a standard microbiology practice used for antimicrobial susceptibility testing [
7]. Since GC-MS profiling showed the higher concentration of acetic acid in the Aechmea sample, antibacterial activity of acetic acid was also tested using nutrient agar plates streaked according to 0.5 McFarland adjusted bacteria. The 6 mm disks were impregnated with the varying concentrations of acetic acid starting from the 1% to 5%. The clear areas around the disks after the 24 h incubation period were marked as zones of inhibition [
4].
Agar disk diffusion assay performed using different concentrations of acetic acid resulted in the highest zones of inhibition at the concentration of 5%, followed by 2% using gram positive bacteria (
S. aureus). Therefore, the antibacterial activity of acetic acid was best between the concentration of 5% and 1%. Below this concentration, the acetic acid solution was not bactericidal [
4].
Similar results were obtained in a study conducted by testing antimicrobial activity of acetic acid against a multidrug resistant bacteria panel including
S. aureus. The acetic acid was bactericidal against
S. aureus in varying zones of inhibition starting from the concentration of 0.5% to 2.5% [
8]. Agar well diffusion technique was used to determine the antibacterial potential of
Aechmea magdalenae. Three 6 mm holes were made in the bacteria-coated agar plates [
9]. A specific volume of the roto-vaped and reconstituted plant extract was filled in the wells, followed by the addition of 20% DMSO and 1 mg/mL gentamicin. The plates were para filmed and placed in the incubator for 24 h at 37 °C. The clear areas around the wells were measured as zones of inhibition [
4].
Agar well diffusion assay resulted in smaller zones of inhibition in gram negative bacteria (
E. coli) as compared to gram positive bacteria (
S. aureus). These results indicated that the
A. magdalenae extract was more potent against
S. aureus than
E. coli [
4]. The results produced by another such study also demonstrated higher antibacterial activity of methanolic plant extracts against
S. aureus as compared to
E. coli, which is in accordance with our results [
4,
6].
Resazurin solution (0.1%) was prepared fresh just before the experiment. The full scan LC-MS analysis of the freshly prepared 0.1% resazurin used as one of the controls showed both resazurin as well as resorufin peaks. The retention time (RT) of resazurin peak was 1.17 with the area of 35,435. The RT of resorufin was 1.22 and the area of the peak was 554,082 (
Figure 2). The results demonstrated the presence of both resazurin (
m/
z 230) and resorufin (
m/
z 214) in the control sample taken from freshly prepared resazurin.
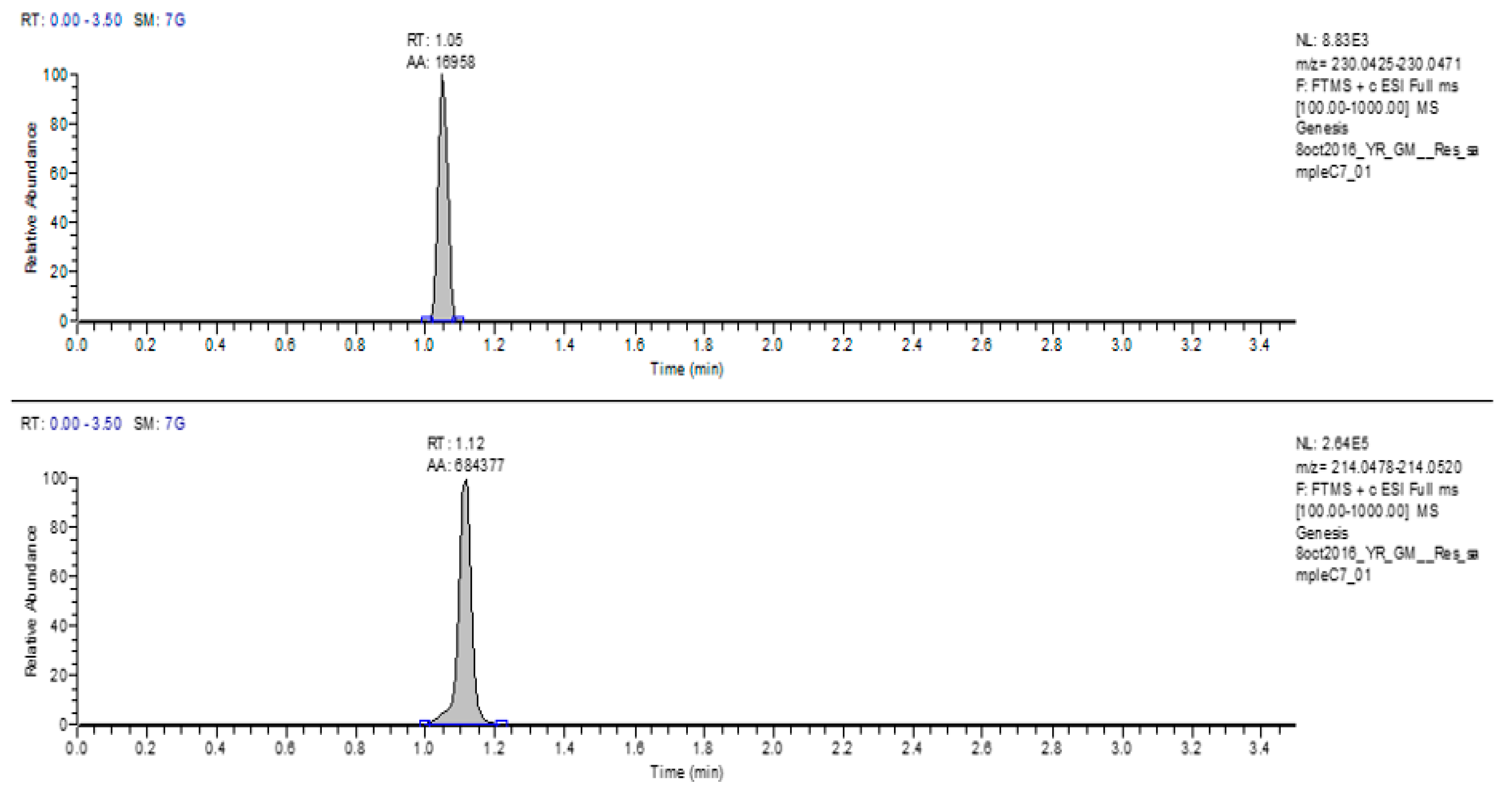
The second resazurin control (0.1%) was prepared 24 h before the analysis and was placed in the incubator at 37 °C. The incubated resazurin control sample was collected after 24 h for LC-MS assay. The full scan LC-MS of the incubated resazurin control also revealed both resazurin as well as resorufin peaks. The RT and the area of the incubated resazurin (
m/
z 230) were 1.05 and 32,151 respectively, while the RT of resorufin (
m/
z 214) was 1.11 and area was 557,304 (
Figure 3).
In the living systems, resazurin is converted into resorufin in their mitochondria where NADPH or NADH acts as a reducing agent in the presence of enzyme NADPH dehydrogenase or NADH dehydrogenase [
10]. The
A. magdalenae extract in the 96-well plate resulted in the reduction of indicator resazurin to resorufin in the presence of both types of bacteria. The samples taken from 96-well plate were forwarded for LC-MS analysis.
The full-scan spectrum showed a complete absence of a resazurin peak (
m/
z 230) with the bacterial growth as shown in
Figure 4. The absence of a resazurin peak meant a full conversion of resazurin (
m/
z 230) into resorufin (
m/
z 214) in the 96-well plates due to bacterial activity. But this conversion was very well demonstrated by mass spectrometer—by producing resorufin peak instead of resazurin peak in the samples taken from the bacterial growth wells. Moreover, the retention time of the resorufin peak from the sample in
Figure 4 was consistent with the resorufin peak generated from the incubated resazurin control in
Figure 3.
The second set of samples for mass spectrometer were prepared from the wells with assumed bacterial inhibition. A 0.1% incubated resazurin solution prepared in water was used as control. The full scan LC-MS spectrum demonstrated the appearance of both resazurin (
m/
z 230) and resorufin peaks (
m/
z 214) (
Figure 5).
Interestingly, the retention times of both resazurin and resorufin peaks from the above-mentioned results matched with the RT of the incubated resazurin controls. It was deduced that the bacterial growth was inhibited in the wells from where these samples were taken. Therefore, resazurin was not converted to resorufin, instead both resazurin and resorufin were present.
To summarize, the indicator resazurin gets reduced to resorufin due to bacterial growth. This special feature of resazurin was used as a marker in this study. A 0.1% freshly prepared and incubated resazurin solution was used as control. The different wells showing bacterial growth and inhibition were subjected for LC-MS analysis. LC-MS results revealed the presence of only resorufin peak, which marked the bacterial growth, whereas the presence of both resazurin and resorufin peaks represented the growth inhibition.
In previous studies, LC-MS has only been used for profiling of the chemical compounds responsible for the antibacterial activity of medicinal plants [
11,
12]. Another study used LC-MS to investigate and identify particularly phenolic compounds in the
Erodium glaucophyllum extract from Tunisian Sahara [
13]. Interestingly, no study has so far used LC-MS for testing the antibacterial potential of a medicinal plant. This study has set some new research parameters where further elaborative studies could be done toward the direct use of mass spectrometry in determining antibacterial activity.
4. Conclusions
The purpose of this study was to develop the method to determine the antibacterial potential of the dark-colored plant extracts when mixed with indicator in a 96-well plate. This LC-MS method was tried on the plant A. magdalenae, for which the MIC was already determined previously using 96-well plate assay. But in the future, the LC-MS method could be directly used for the antibacterial potential analysis of the dark medicinal plant extracts.
To the best of our knowledge, for the first time, LC-MS assay was specifically aimed to measure masses generated due to the reduction of indicator resazurin to resorufin to determine antibacterial activity of a medicinal plant. The development of the assay first involved the method development, followed by mass spectrometric optimization of the individual masses of resazurin, as well as resorufin in their native form and, then, determination of masses created in the samples.