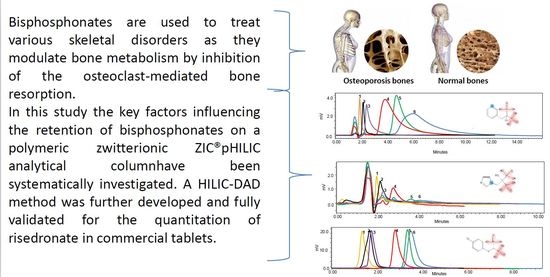
Insights into the Mechanism of Separation of Bisphosphonates by Zwitterionic Hydrophilic Interaction Liquid Chromatography: Application to the Quantitation of Risedronate in Pharmaceuticals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Instrumentation
2.3. Statistical Analysis
2.4. Stock and Working Standard Solutions
2.5. Assay Procedure for the Pharmaceutical Samples
2.6. Accelerated and Long-Term Stability Studies
3. Results and Discussion
3.1. Method Development
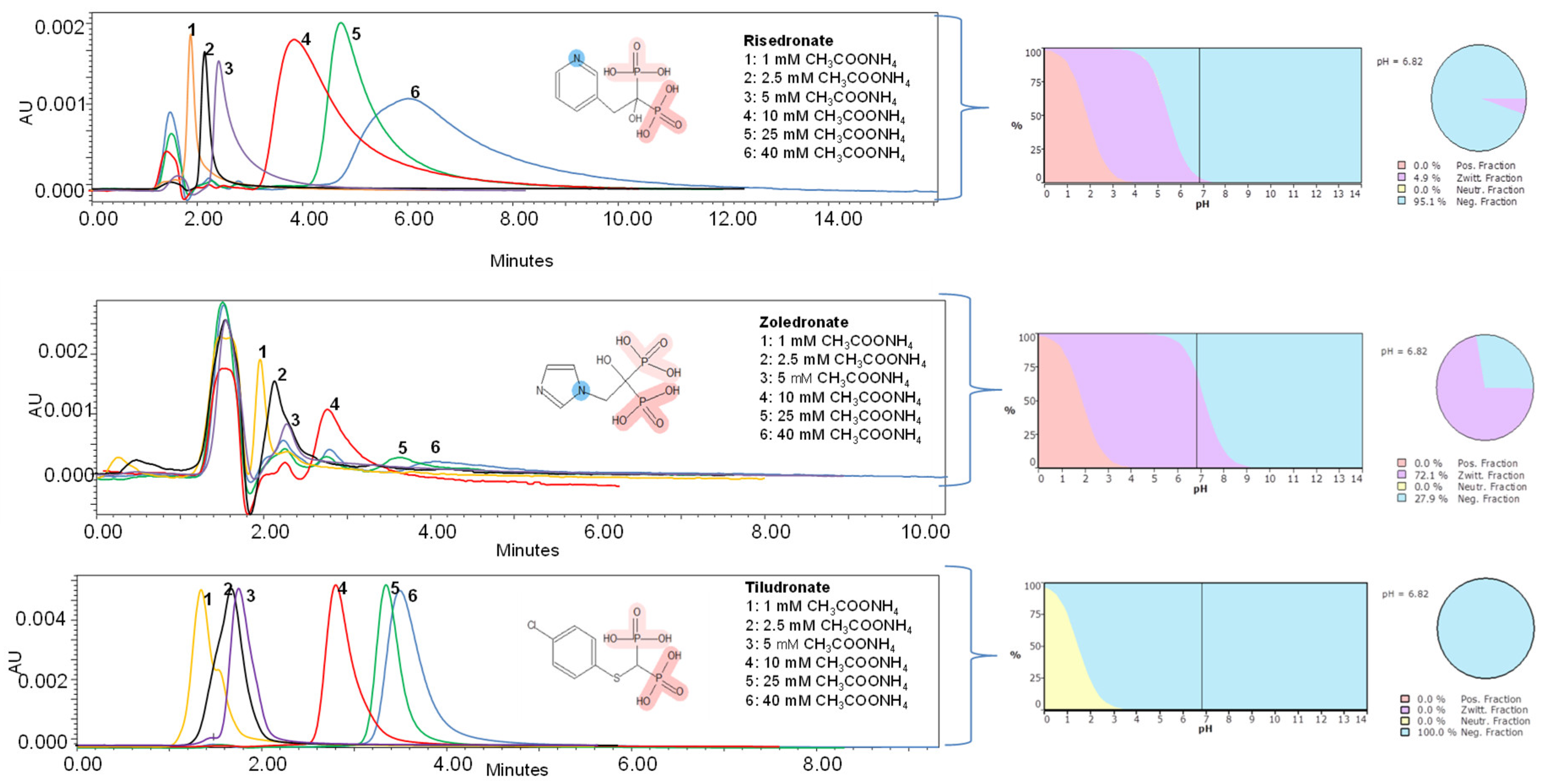
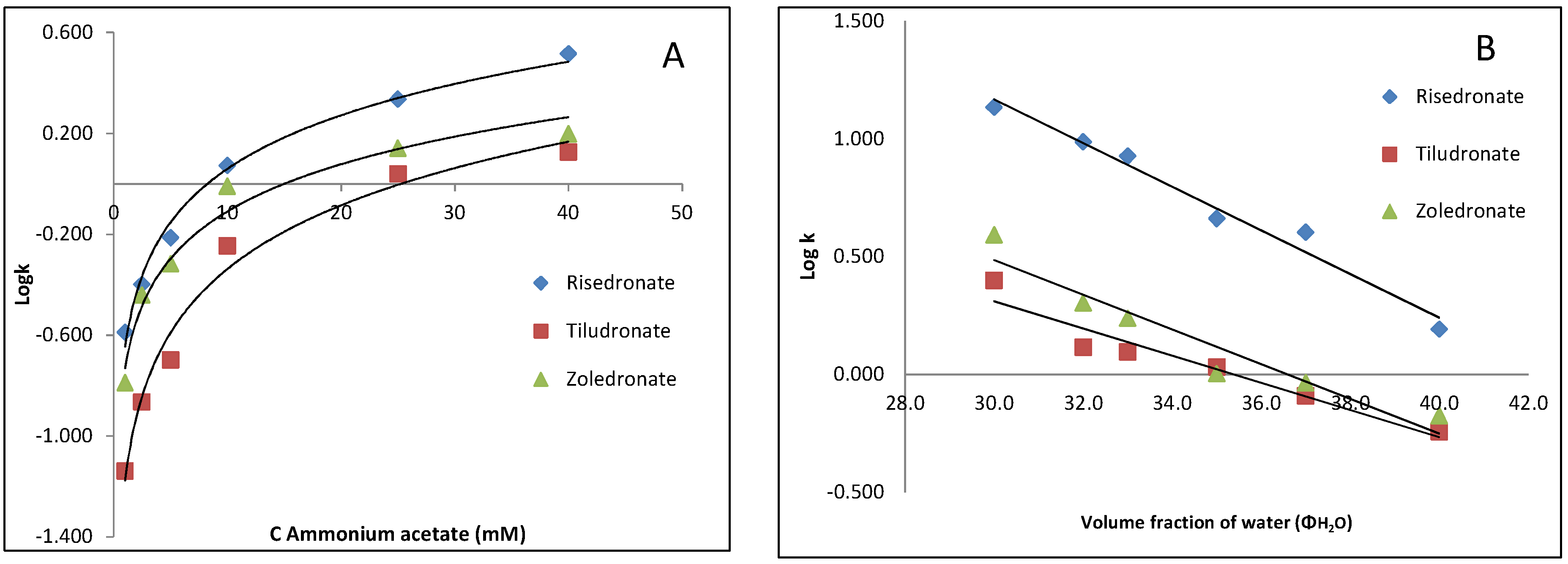
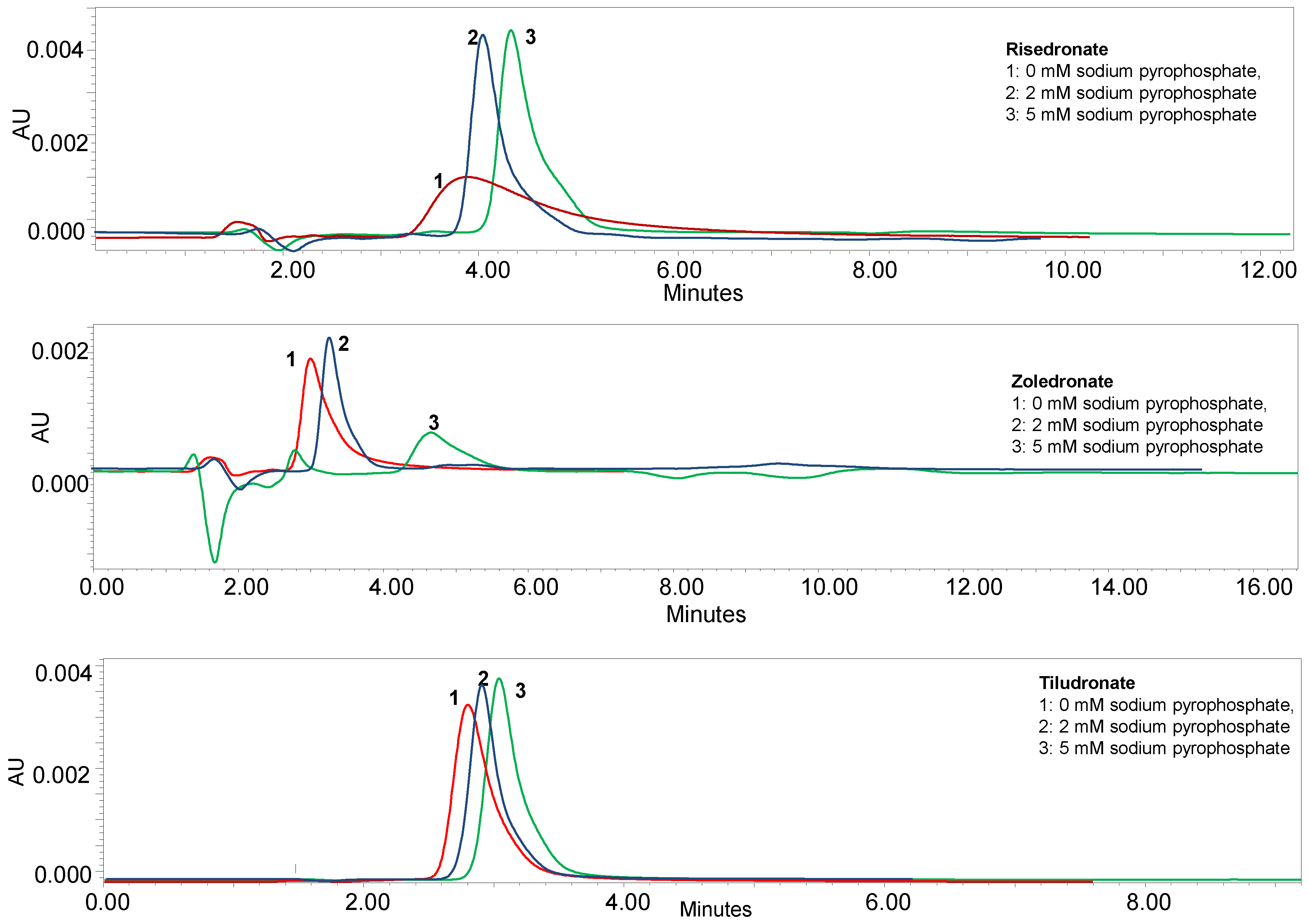
3.1.1. Effect of Chromatographic Parameters on the Bisphosphonates Retention
3.1.2. Optimization of the Chromatographic Parameters for the Quantitation of Risedronate
3.2. Method Validation
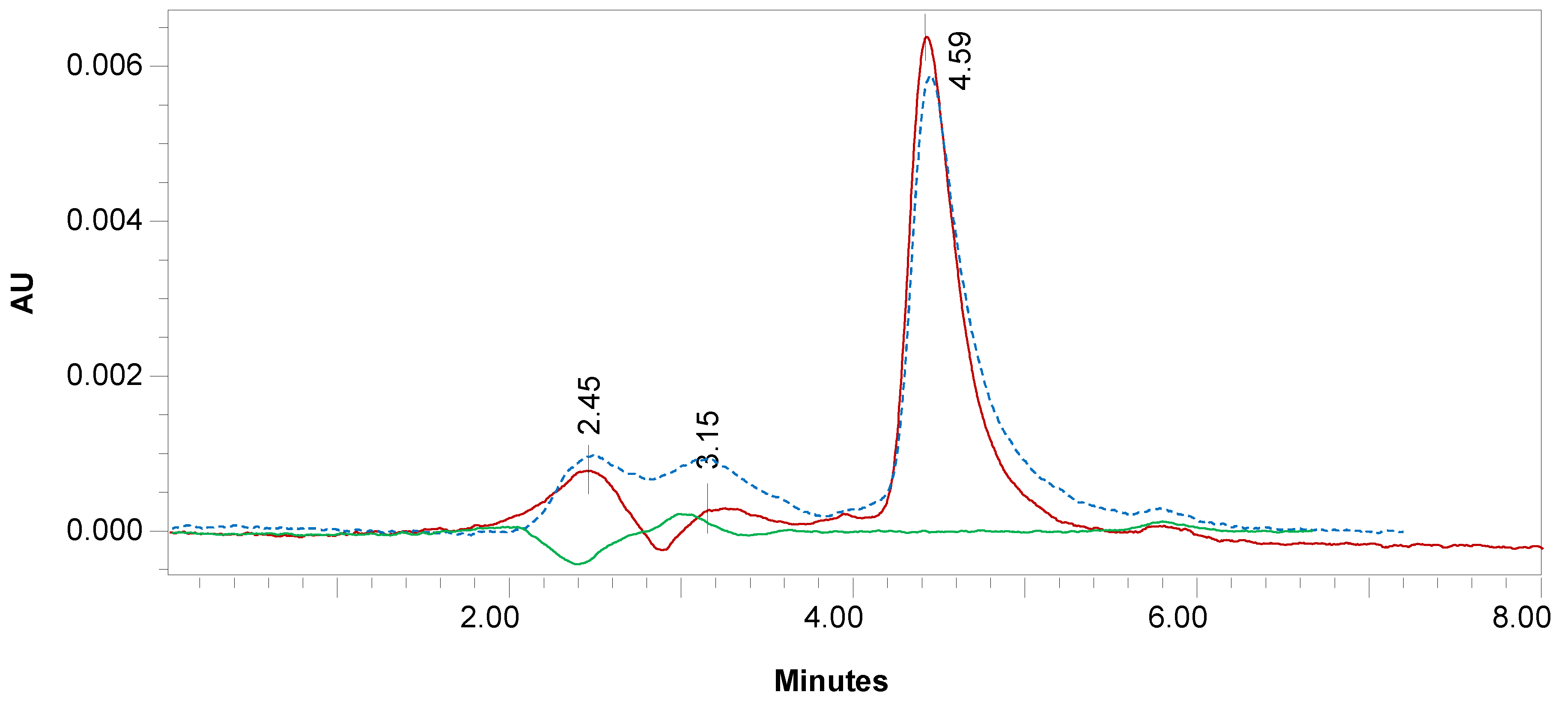
3.2.1. Selectivity
3.2.2. Statistical Analysis of Data
3.2.3. Accelerated and Long-Term Stability Studies
3.3. Analysis of Commercial Tablets
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fleisch, H.; Russell, R.G.; Straumann, F. Effect of pyrophosphate on hydroxyapatite and its implications in calcium homeostasis. Nature 1966, 212, 901–903. [Google Scholar] [CrossRef] [PubMed]
- Drake, M.T.; Clarke, B.L.; Khosla, S. Bisphosphonates: Mechanism of Action and Role in Clinical Practice. Mayo Clin. Proc. 2008, 83, 1032–1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plotkin, L.I.; Aguirre, J.I.; Kousteni, S.; Manolagas, S.C.; Bellido, T. Bisphosphonates and estrogens inhibit osteocyte apoptosis via distinct molecular mechanisms downstream of extracellular signal-regulated kinase activation. J. Biol. Chem. 2005, 280, 7317–7325. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, K.L.; Guo, K.; Dunford, J.E.; Wu, X.; Knapp, S.; Ebetino, F.H.; Rogers, M.J.; Russell, R.G.; Oppermann, U. The molecular mechanism of nitrogen-containing bisphosphonates as antiosteoporosis drugs. Proc. Natl. Acad. Sci. USA 2006, 103, 7829–7834. [Google Scholar] [CrossRef] [PubMed]
- Eid, A.; Atlas, J. The role of bisphosphonates in medical oncology and their association with jaw bone necrosis. Oral. Maxillofac. Surg. Clin. N. Am. 2014, 26, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Wasserzug, O.; Kaffe, I.; Lazarovici, T.S.; Weissman, T.; Yahalom, R.; Fliss, D.M.; Yarom, N. Involvement of the maxillary sinus in bisphosphonate-related osteonecrosis of the jaw: Radiologic aspects. Am. J. Rhinol. Allergy 2017, 31, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Zacharis, C.; Tzanavaras, P. Determination of bisphosphonate active pharmaceutical ingredients in pharmaceuticals and biological material: A review of analytical methods. J. Pharm. Biomed. Anal. 2008, 48, 483–496. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Jiang, Y.; Zhang, D.Q. Simple analysis of four bisphosphonates simultaneously by reversed phase liquid chromatography using n-amylamine as volatile ion-pairing agent. J. Chromatogr. A 2006, 1104, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Vallano, P.T.; Shugarts, S.B.; Kline, W.F.; Woolf, E.J.; Matuszewski, B.K. Determination of risedronate in human urine by column-switching ion-pair high-performance liquid chromatography with ultraviolet detection. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2003, 794, 23–33. [Google Scholar] [CrossRef]
- Kyriakides, D.; Panderi, I. Development and validation of a reversed-phase ion-pair high-performance liquid chromatographic method for the determination of risedronate in pharmaceutical preparations. Anal. Chim. Acta 2007, 584, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Taylor, G.E. Determination of Impurities in Clodronic Acid by Anion-Exchange Chromatography. J. Chromatogr. A 1997, 770, 261–271. [Google Scholar] [CrossRef]
- Hasan, M.; Schumacher, G.; Seekamp, A.; Taedken, T.; Siegmund, W.; Oswalda, S. LC-MS/MS method for the determination of clodronate in human plasma. J. Pharm. Biomed. Anal. 2014, 100, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ruiz, T.; Martínez-Lozano, C.; García-Martínez, M.D. A sensitive post-column photochemical derivatization/fluorimetric detection system for HPLC determination of bisphosphonates. J. Chromatogr. A 2009, 1216, 1312–1318. [Google Scholar] [CrossRef] [PubMed]
- Lapko, V.N.; Miller, P.S.; Sheldon, C.E.; Nachi, R.; Kafonek, C.J. Quantitative analysis of bisphosphonates in biological samples. Bioanalysis 2014, 6, 2931–2950. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, T.; Vicentini, L.; Boschetti, S.; Andreatta, P.; Gatti, R. A novel automated hydrophilic interaction liquid chromatography method using diode-array detector/electrospray ionization tandem mass spectrometry for analysis of sodium risedronate and related degradation products in pharmaceuticals. J. Chromatogr. A 2014, 1365, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.S.; Lapko, V.N.; Lee, J.W.; Basir, Y.J.; Kafonek, C.; Olsen, R.; Briscoe, C. A general approach for the quantitative analysis of bisphosphonates in human serum and urine by high performance liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2006, 20, 3421–3426. [Google Scholar] [CrossRef] [PubMed]
- Raccor, B.S.; Sun, J.; Lawrence, R.F.; Li, L.; Zhang, H.; Somerman, M.J.; Totah, R.A. Quantitation of zoledronic acid in murine bone by liquid chromatography coupled with tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2013, 935, 54–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, A.S.Y.; Ho, E.N.M.; Wan, T.S.M.; Lamb, K.K.H.; Stewart, B.D. Liquid chromatography–mass spectrometry analysis of five bisphosphonates in equine urine and plasma. J. Chromatogr. B 2015, 998–999, 1–7. [Google Scholar] [CrossRef]
- Chen, M.; Liu, K.; Zhong, D.; Chen, X. Trimethylsilyl diazomethane derivatization coupled with solid-phase extraction for the determination of alendronate in human plasma by LC-MS/MS. Anal. Bioanal. Chem. 2012, 402, 791–798. [Google Scholar] [CrossRef]
- Ghassabian, S.; Wright, L.A.; Dejager, A.D.; Smith, M.T. Development and validation of a sensitive solid-phase-extraction (SPE) method using high-performance liquid chromatography/tandem mass spectrometry (LC-MS/MS) for determination of risedronate concentrations in human plasma. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2012, 881–882, 34–41. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, C.; Zhang, Y.; Zhou, L.; Zhong, D.; Chen, X. On-cartridge derivatization coupled with solid-phase extraction for the ultra-sensitive determination of minodronic acid in human plasma by LC-MS/MS method. J. Pharm. Biomed. Anal. 2015, 114, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, E.; Leknes, S.; Wilson, S.R.; Lundanes, E. Liquid chromatography-mass spectrometry platform for both small neurotransmitters and neuropeptides in blood, with automatic and robust solid phase extraction. Sci. Rep. 2015, 5, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Hemström, P.; Irgum, K. Hydrophilic interaction chromatography. J. Sep. Sci. 2006, 29, 1784–1821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Y. Recent progress in the fundamental understanding of hydrophilic interaction chromatography (HILIC). Analyst 2015, 140, 6452–6466. [Google Scholar] [CrossRef] [PubMed]
- Panderi, I.; Malamos, Y.; Machairas, G.; Zaharaki, S. Investigation of the retention mechanism of cephalosporins by zwitterionic hydrophilic interaction liquid chromatography. Chromatographia 2016, 79, 995–1002. [Google Scholar] [CrossRef]
- Alpert, A.J. Electrostatic repulsion hydrophilic interaction chromatography for isocratic 659 separation of charged solutes and selective isolation of phosphopeptides. Anal. Chem. 2008, 80, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Moravcová, D.; Planeta, J. Monolithic Silica Capillary Columns with Improved Retention and Selectivity for Amino Acids. Separations 2018, 5, 48. [Google Scholar] [CrossRef]
- Barth, H.G. Chromatography Fundamentals, Part III: Retention Parameters of Liquid Chromatography. LC-GC 2018, 36, 472–473. [Google Scholar]
- Kanmatareddy, A.; De Borba, B.; Rohrer, J. Evaluation of the USP Risedronate Sodium Assay; Dionex, Application Note 289. Thermo Fisher Scientific: Sunnyvale, CA, USA. Available online: https://assets.thermofisher.com/TFS-Assets/CMD/Application-Notes/AN-289-IC-USP-Risedronate-Sodium-LPN2926-EN.pdf (accessed on September 2016).
- Sinigaglia, L.; Varenna, M.; Casari, S. Pharmacokinetic profile of bisphosphonates in the treatment of metabolic bone disorders. Clin. Cases Miner. Bone Metab. 2007, 4, 30–36. [Google Scholar]
- Schneider, S. Analysis of Risedronate According to USP Using the Agilent 1260 Infinity Bio-Inert Quaternary LC System; Application Note Agilent Technologies, Inc.: Waldbronn, Germany. Available online: https://www.agilent.com/cs/library/applications/5991-2404EN.pdf (accessed on 1 May 2016).
- Greco, G.; Letzel, T. Main interactions and influences of the chromatographic parameters in HILIC separations. J. Chromatogr. Sci. 2013, 51, 684–693. [Google Scholar] [CrossRef]
- Machairas, G.; Panderi, I.; Geballa-Koukoula, A.; Rozou, S.; Antonopoulos, N.; Charitos, C.; Vonaparti, A. Development and validation of a hydrophilic interaction liquid chromatography method for the quantitation of impurities in fixed-dose combination tablets containing rosuvastatin and metformin. Talanta 2018, 183, 131–141. [Google Scholar] [CrossRef] [PubMed]
| Concentration Range, μg mL−1 | Regression Equations a | r b | Standard Deviation | Sr c | |
|---|---|---|---|---|---|
| Slope | Intercept | ||||
| Mean of 3 calibration curves over a period of 1 month | |||||
| 1.5–5 | SRsd = 3.029 × CRsd + 1.76 | ≥0.9991 | 1.7 × 10−3 | 3.2 × 10−2 | ≤0.24 |
| Risedronate | Concentration (μg mL−1) | ||
|---|---|---|---|
| Added concentration | 1.5 | 3.5 | 5 |
| Run 1 (mean ± SD) | 1.4669 ± 0.0051 | 3.556 ± 0.022 | 5.013 ± 0.046 |
| Run 2 (mean ± SD) | 1.4589 ± 0.0033 | 3.567 ± 0.012 | 5.022 ± 0.088 |
| Run 3 (mean ± SD) | 1.4715 ± 0.0044 | 3.5628 ± 0.0091 | 4.973 ± 0.012 |
| Overall mean | 1.4658 | 3.5618 | 5.0031 |
| Intra-day CV(%) a | 0.3 | 0.5 | 0.6 |
| Inter-day CV(%) a | 0.5 | 0.05 | 0.6 |
| Overall accuracy Er% b | −2.3 | 1.8 | 0.1 |
| Degradation Conditions/Time | Time | Concentration (μg mL−1) (Mean ± S.D., n = 3) | % Recovery (Mean ± S.D., n = 3) | Degradation Products Retention Time (min) |
| 1.0 M HCl, 50 °C | 1 day | 3.467 ± 0.038 | 99.0 ± 1.1 | - |
| 2 days | 3.469 ± 0.041 | 99.1 ± 1.2 | - | |
| 8 days | 3.036 ± 0.040 | 86.7 ± 1.1 | <3 | |
| 10 days | 2.736 ± 0.032 | 78.1 ± 0.9 | <3 | |
| 1.0 M NaOH, 25 °C | 1 day | 2.757 ± 0.073 | 78.8 ± 2.1 | <3 |
| 3 % v/v H2O2, 25 °C | 1 h | 3.211 ± 0.042 | 91.7 ± 1.2 | - |
| 2 h | 3.094 ± 0.041 | 88.4 ± 1.1 | <3 | |
| 3 h | 2.895 ± 0.047 | 82.7 ± 1.4 | <3 | |
| Long-Term Stability Studies | Time | Amount (mg) Per Tablet (Mean ± S.D., n = 3) | % Recovery (Mean ± S.D., n = 3) | Degradation Products Retention Time (min) |
| 50 ± 2 °C 15% humidity | 1 month | 32.79 ± 0.51 | 100.9 ± 1.5 | - |
| 3 months | 32.41 ± 0.46 | 99.7 ± 1.3 | - | |
| 50 ± 2 °C 75% humidity | 1 month | 32.38 ± 0.44 | 99.6 ± 1.2 | - |
| 3 months | 25.22 ± 0.63 | 77.6 ± 1.8 | <3 |
| Test | Amount (mg) Per Tablet (Mean ± SD, n = 10) | % Recovery (Mean ± SD, n = 10) |
|---|---|---|
| Quality control | 32.3 ± 0.2 | 99.3 ± 0.7 |
| Content uniformity | 32.6 ± 0.4 | 100.2 ± 1.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panderi, I.; Taxiarchi, E.; Pistos, C.; Kalogria, E.; Vonaparti, A. Insights into the Mechanism of Separation of Bisphosphonates by Zwitterionic Hydrophilic Interaction Liquid Chromatography: Application to the Quantitation of Risedronate in Pharmaceuticals. Separations 2019, 6, 6. https://doi.org/10.3390/separations6010006
Panderi I, Taxiarchi E, Pistos C, Kalogria E, Vonaparti A. Insights into the Mechanism of Separation of Bisphosphonates by Zwitterionic Hydrophilic Interaction Liquid Chromatography: Application to the Quantitation of Risedronate in Pharmaceuticals. Separations. 2019; 6(1):6. https://doi.org/10.3390/separations6010006
Chicago/Turabian StylePanderi, Irene, Eugenia Taxiarchi, Constantinos Pistos, Eleni Kalogria, and Ariadni Vonaparti. 2019. "Insights into the Mechanism of Separation of Bisphosphonates by Zwitterionic Hydrophilic Interaction Liquid Chromatography: Application to the Quantitation of Risedronate in Pharmaceuticals" Separations 6, no. 1: 6. https://doi.org/10.3390/separations6010006