Multiple-stage Precursor Ion Separation and High Resolution Mass Spectrometry toward Structural Characterization of 2,3-Diacyltrehalose Family from Mycobacterium tuberculosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Mass Spectrometry
2.4. Nomenclature
3. Results and Discussion
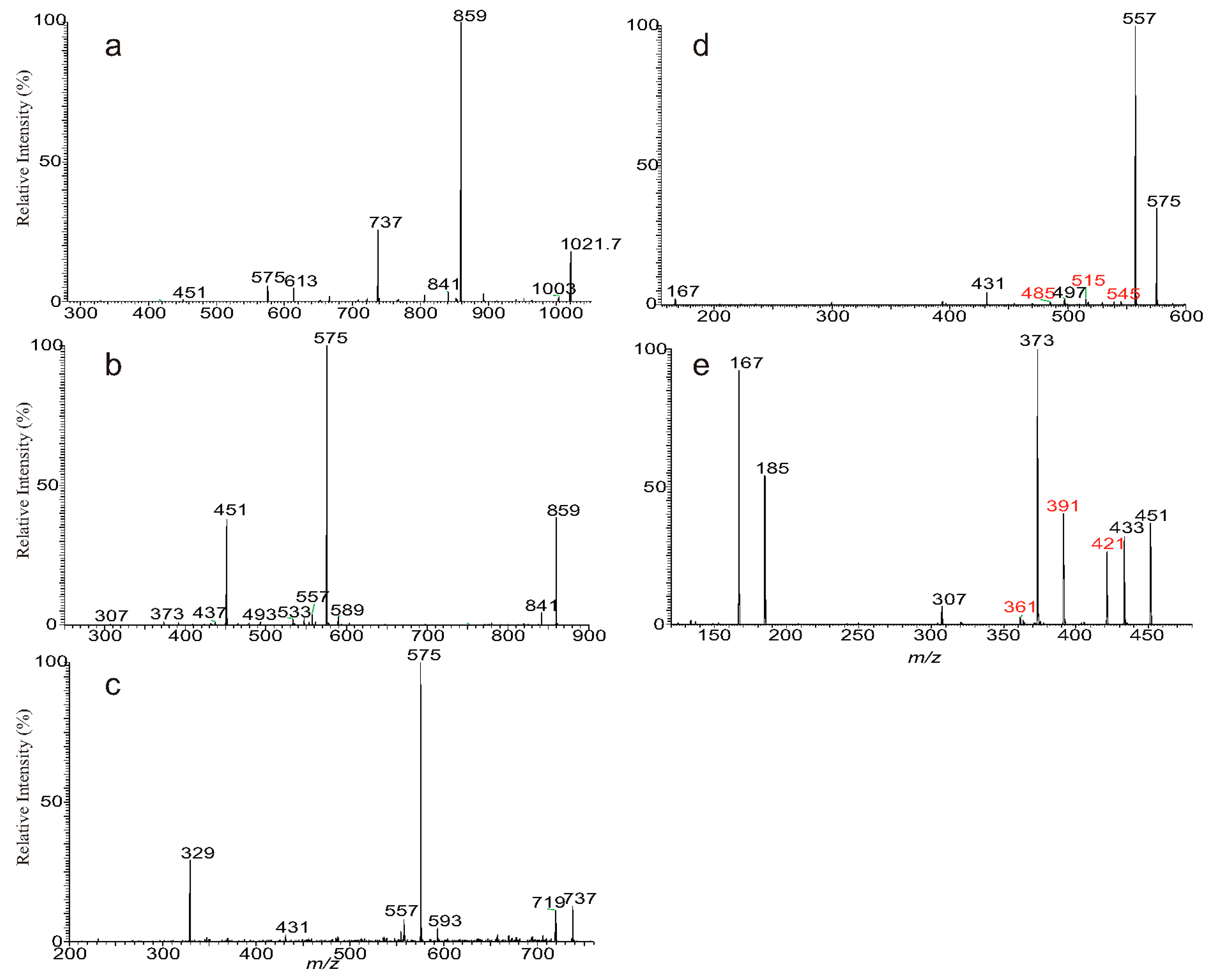
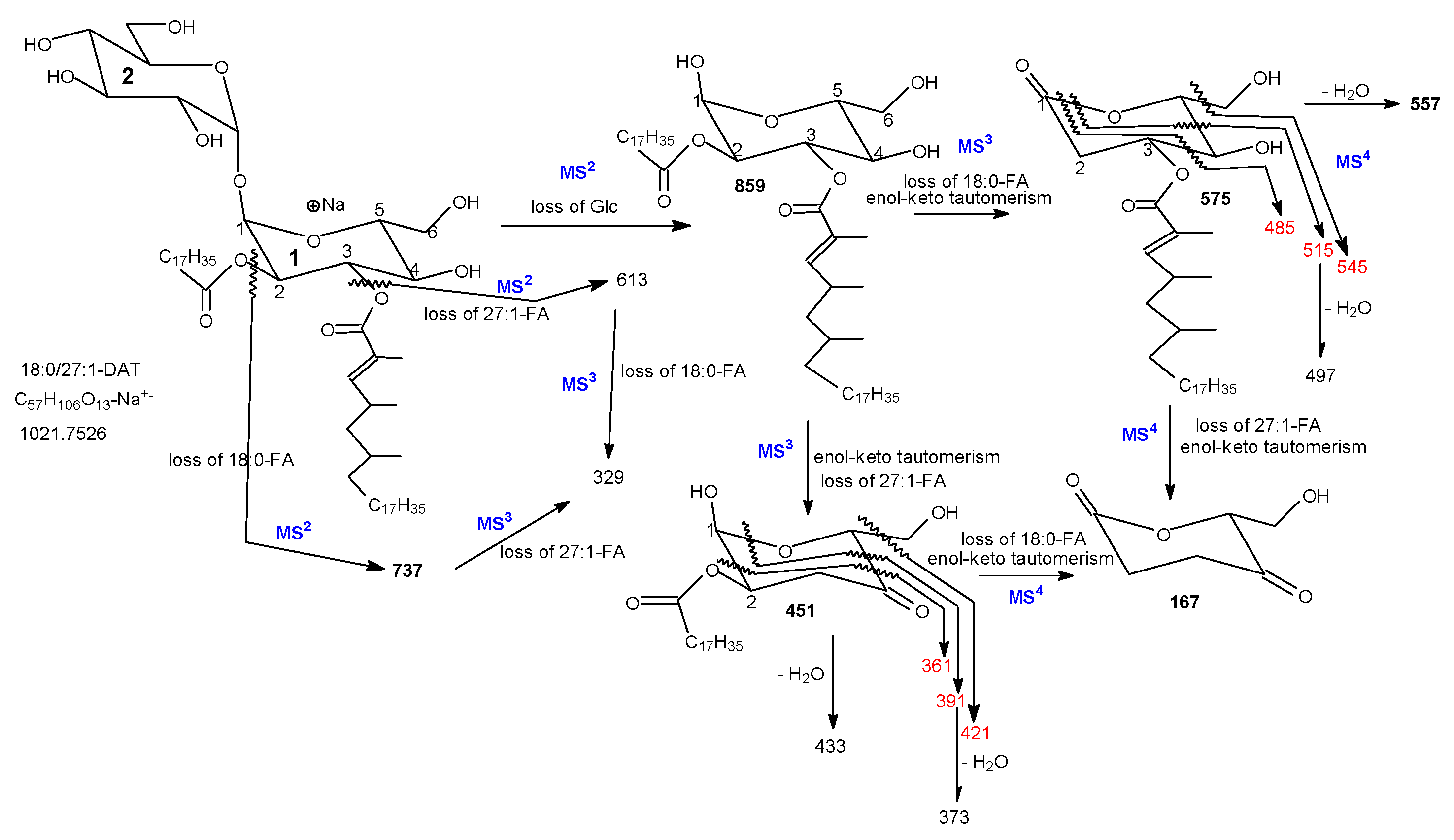
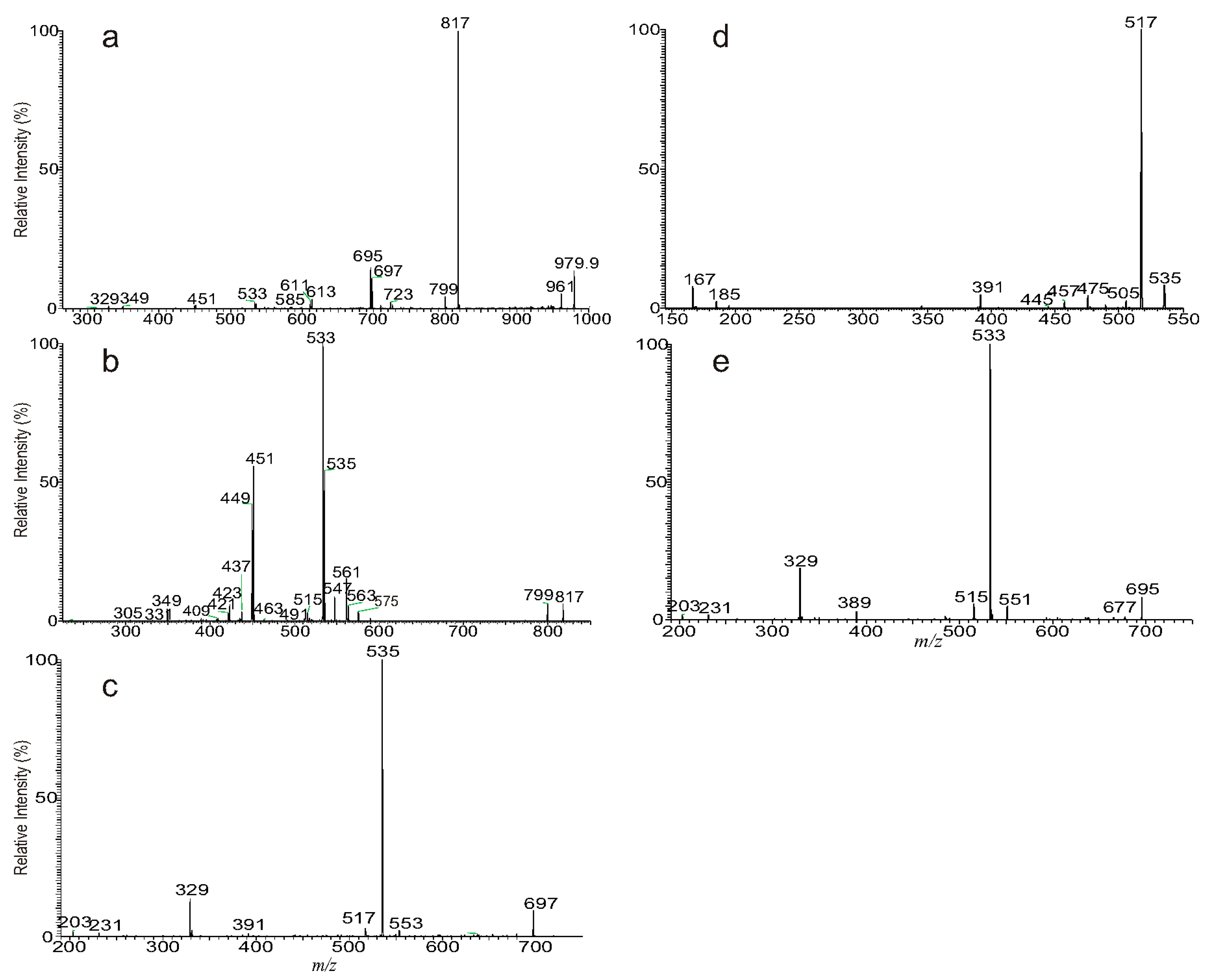
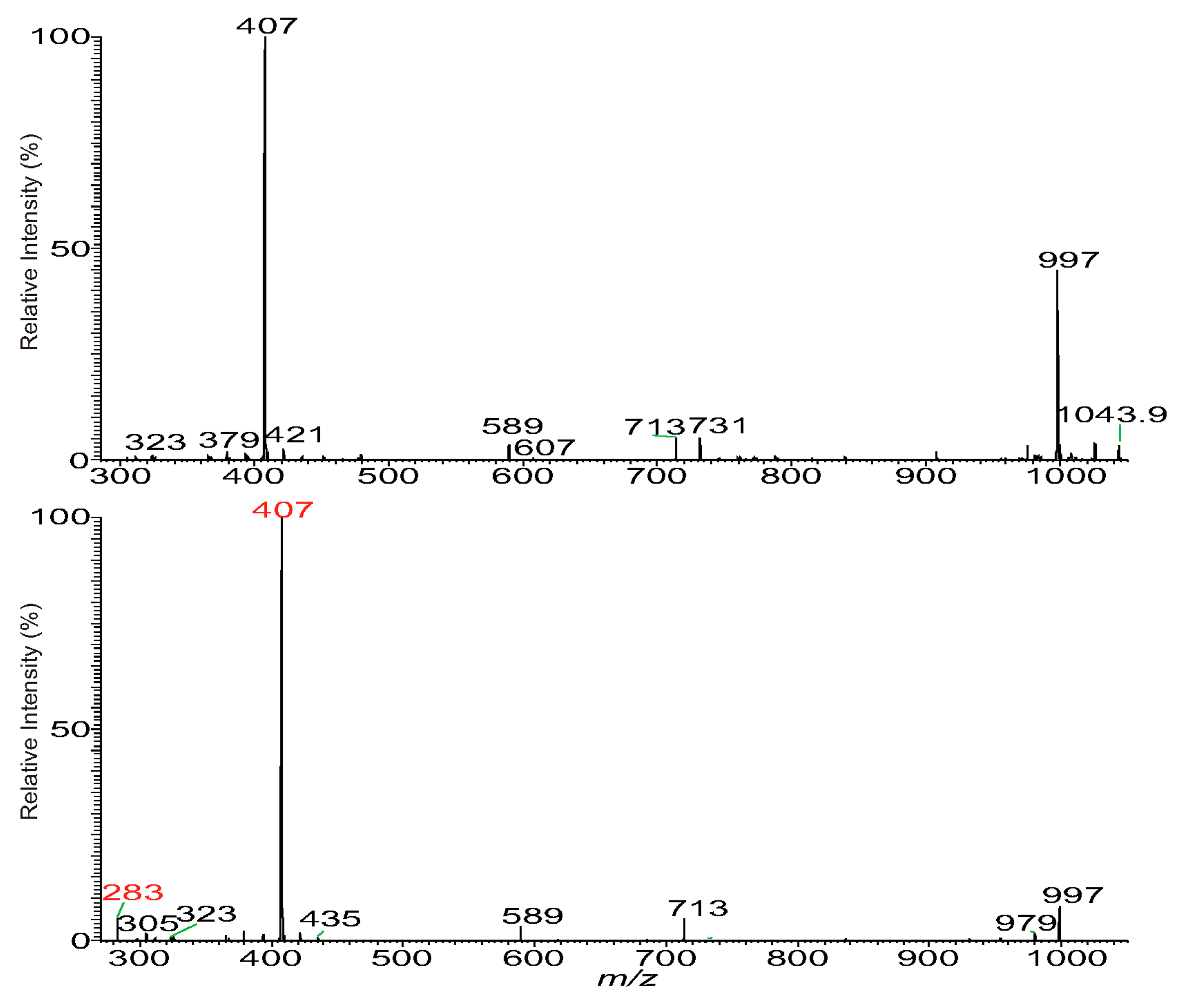
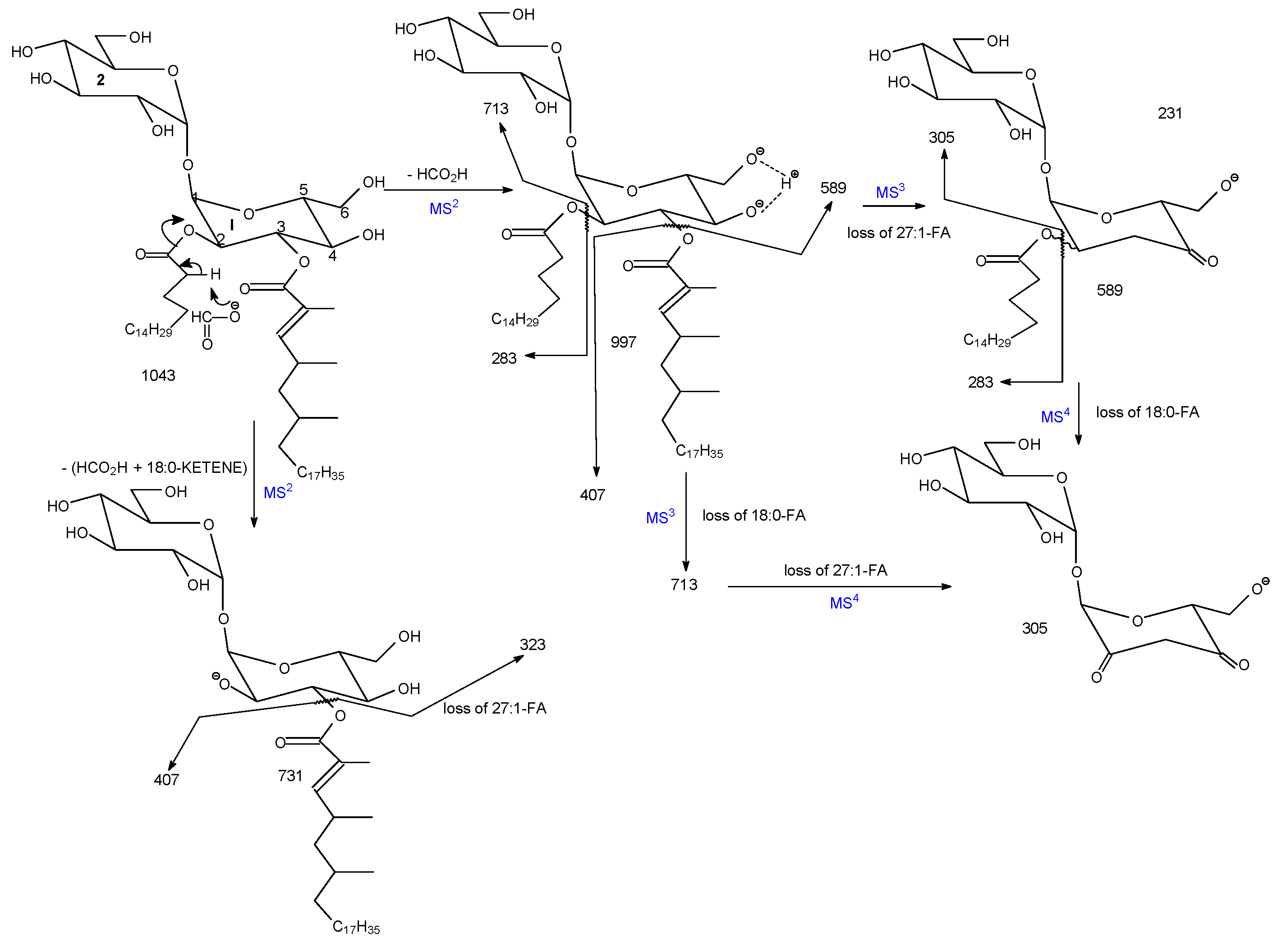
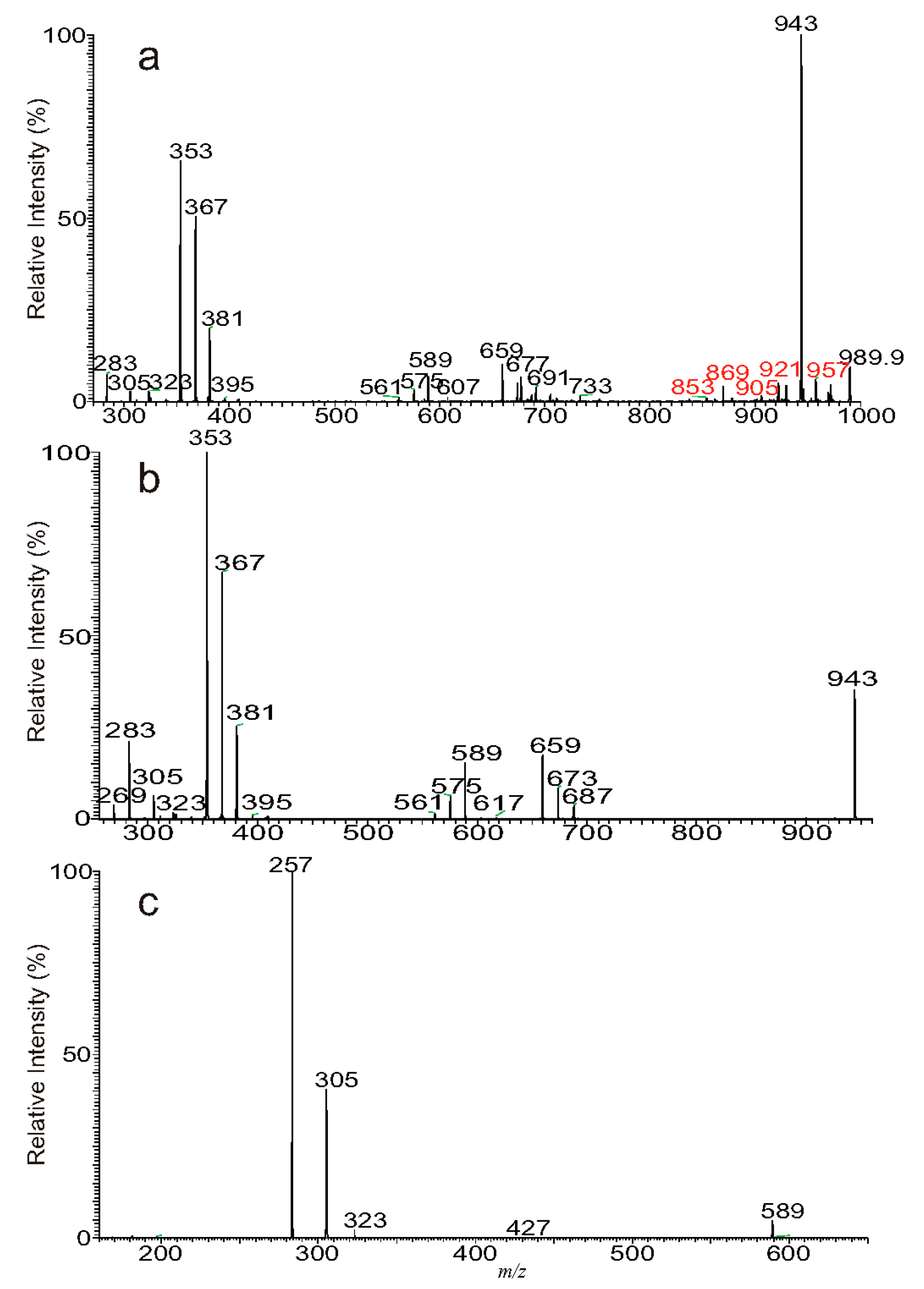
3.1. The Fragmentation Processes of the [M + Na]+ Ions of DAT Revealed by LIT MSn
3.2. LIT MSn on the [M + Na]+ Ions of DAT for Stereoisomer Recognition
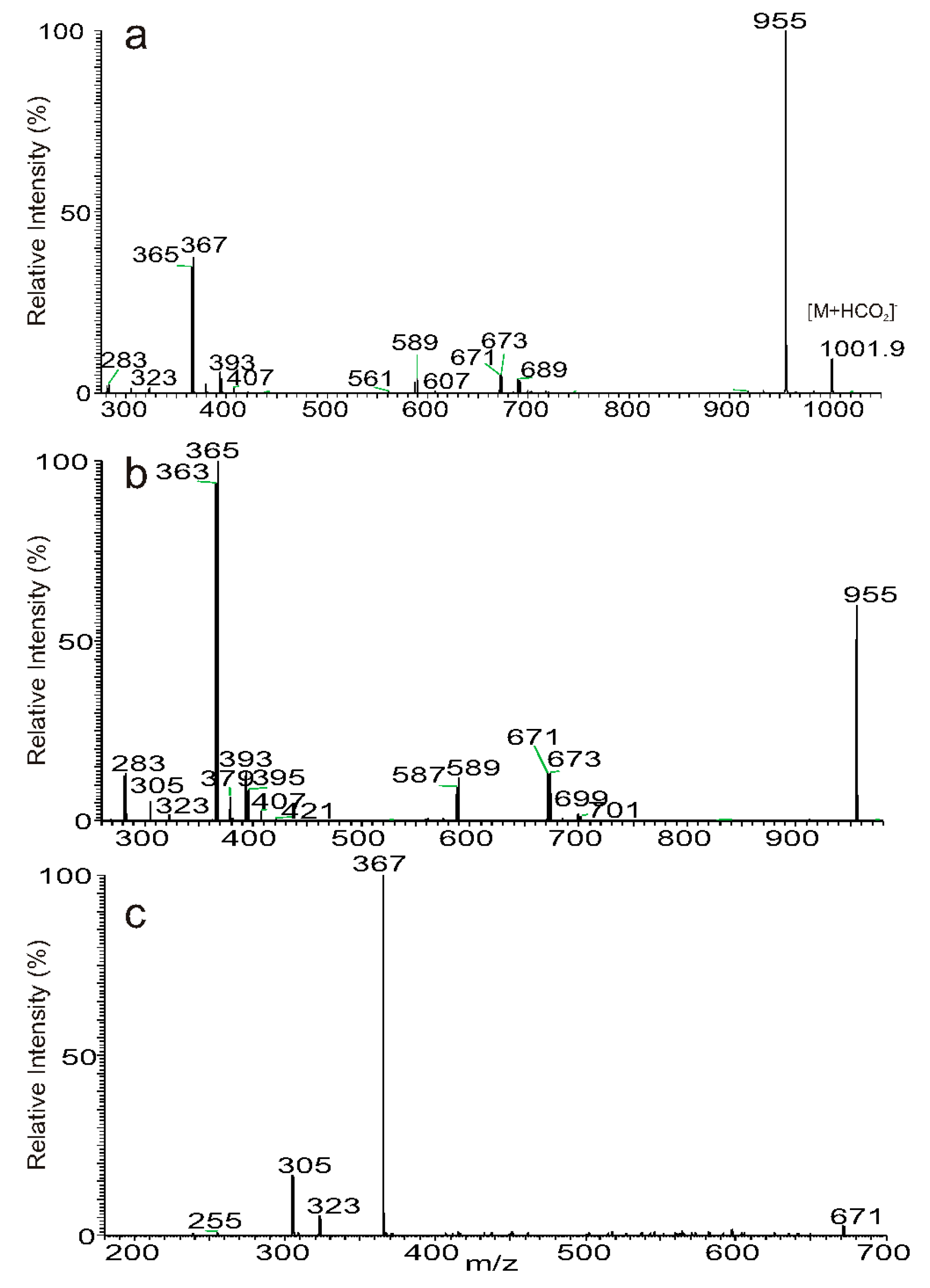
3.3. The Fragmentation Processes of the [M + HCO2]− Ions of DAT Revealed by LIT MSn
3.4. Recognition of Stereoisomers Applying LIT MSn on the [M + HCO2]− Ions
3.5. Characterization of Minor Species Applying LIT MSn on the [M + HCO2]− Ions
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Johnson, J.V.; Yost, R.A.; Kelley, P.E.; Bradford, D.C. Tandem-in-space and tandem-in-time mass spectrometry: triple quadrupoles and quadrupole ion traps. Anal. Chem. 1990, 62, 2162–2172. [Google Scholar] [CrossRef]
- Markarov, A.; Cousijn, E.; Cantebury, J.; Denisov, E.; Thoeing, C.; Lange, O.; Kreutzman, A.; Ayzikov, K.; Damoc, E.; Tabiwang, A.; et al. Extension of Orbitrap capabilities to enable new applications. In Proceedings of the 65th Conference on Mass Spectrometry and Allied Topics, Indianapolis, IN, USA, 4 June 2017. [Google Scholar]
- Vogel, C.; Marcotte, E.M. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat. Rev. Genet. 2012, 13, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Perry, R.H.; Cooks, R.G.; Noll, R.J. Orbitrap mass spectrometry: Instrumentation, ion motion and applications. Mass Spectrom. Rev. 2008, 27, 661–699. [Google Scholar] [CrossRef] [PubMed]
- Senyuva, H.Z.; Gökmen, V.; Sarikaya, E.A. Future perspectives in Orbitrap™-high-resolution mass spectrometry in food analysis: A review. Food Addit. Contam. A 2015, 32, 1568–1606. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, R.E.; Snyder, A.P. Chap. 14—Mass spectrometry-based proteomics techniques for biological identification. In Biological Identification; Schaudies, R.P., Ed.; Woodhead Publishing: Sawston, UK, 2014; pp. 370–430. [Google Scholar]
- Eliuk, S.; Makarov, A. Evolution of Orbitrap Mass Spectrometry Instrumentation. Ann. Rev. Anal. Chem. 2015, 8, 61–80. [Google Scholar] [CrossRef] [PubMed]
- Rhoades, E.R.; Streeter, C.; Turk, J.; Hsu, F.-F. Characterization of Sulfolipids of Mycobacterium tuberculosis H37Rv by Multiple-Stage Linear Ion-Trap High-Resolution Mass Spectrometry with Electrospray Ionization Reveals That the Family of Sulfolipid II Predominates. Biochemistry 2011, 50, 9135–9147. [Google Scholar] [CrossRef] [PubMed]
- Hsu, F.-F. Characterization of Hydroxyphthioceranoic and Phthioceranoic Acids by Charge-Switch Derivatization and CID Tandem Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2016, 27, 622–632. [Google Scholar] [CrossRef]
- Hsu, F.F.; Turk, J.; Owens, R.M.; Rhoades, E.R.; Russell, D.G. Structural Characterization of Phosphatidyl-myo-Inositol Mannosides from Mycobacterium bovis Bacillus Calmette Guerin by Multiple-Stage Quadrupole Ion-Trap Mass Spectrometry with Electrospray Ionization. II. Monoacyl- and Diacyl-PIMs. J. Am. Soc. Mass Spectrom. 2007, 18, 479–492. [Google Scholar] [CrossRef]
- Hsu, F.F.; Turk, J.; Owens, R.M.; Rhoades, E.R.; Russell, D.G. Structural characterization of phosphatidyl-myo-inositol mannosides from Mycobacterium bovis Bacillus Calmette Guerin by multiple-stage quadrupole ion-trap mass spectrometry with electrospray ionization. I. PIMs and lyso-PIMs. J. Am. Soc. Mass Spectrom. 2007, 18, 466–478. [Google Scholar] [CrossRef]
- Flentie, K.N.; Stallings, C.L.; Turk, J.; Minnaard, A.J.; Hsu, F.-F. Characterization of phthiocerol and phthiodiolone dimycocerosate esters of M. tuberculosis by multiple-stage linear ion-trap MS. J. Lipid Res. 2016, 57, 142–155. [Google Scholar] [CrossRef]
- Hoppe, H.C.; de Wet, B.J.; Cywes, C.; Daffe, M.; Ehlers, M.R. Identification of phosphatidylinositol mannoside as a mycobacterial adhesin mediating both direct and opsonic binding to nonphagocytic mammalian cells. Infect. Immun. 1997, 65, 3896–3905. [Google Scholar] [PubMed]
- Howard, N.C.; Marin, N.D.; Ahmed, M.; Rosa, B.A.; Martin, J.; Bambouskova, M.; Sergushichev, A.; Loginicheva, E.; Kurepina, N.; Rangel-Moreno, J.; et al. Mycobacterium tuberculosis carrying a rifampicin drug resistance mutation reprograms macrophage metabolism through cell wall lipid changes. Nat. Microbiol. 2018, 3, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- DaffÉ, M.; Lacave, C.; LanÉElle, M.-A.; Gillois, M.; LanÉElle, G. Polyphthienoyl trehalose, glycolipids specific for virulent strains of the tubercle bacillus. Eur. J. Biochem. 1988, 172, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Minnikin, D.E.; Dobson, G.; Sesardic, D.; Ridell, M. Mycolipenates and Mycolipanolates of Trehalose from Mycobacterium tuberculosis. J. Gen. Microbiol. 1985, 131, 1369–1374. [Google Scholar] [CrossRef] [PubMed]
- Munoz, M.; Laneelle, M.A.; Luquin, M.; Torrelles, J.; Julian, E.; Ausina, V.; Daffe, M. Occurrence of an antigenic triacyl trehalose in clinical isolates and reference strains of Mycobacterium tuberculosis. FEMS Microbiol. Lett. 1997, 157, 251–259. [Google Scholar] [CrossRef]
- Lemassu, A.; Laneelle, M.A.; Daffe, M. Revised structure of a trehalose-containing immunoreactive glycolipid of Mycobacterium tuberculosis. FEMS Microbiol. Lett. 1991, 62, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Ariza, M.A.; Martín-Luengo, F.; Valero-Guillén, P.L. A family of diacyltrehaloses isolated from Mycobacterium fortuitum. Microbiology 1994, 140, 1989–1994. [Google Scholar] [CrossRef] [PubMed]
- Ariza, M.A.; Valero-Guillen, P.L. Delineation of molecular species of a family of diacyltrehaloses from Mycobacterium fortuitum by mass spectrometry. FEMS Microbiol. Lett. 1994, 119, 279–282. [Google Scholar] [CrossRef]
- Besra, G.S.; Bolton, R.C.; McNeil, M.R.; Ridell, M.; Simpson, K.E.; Glushka, J.; Van Halbeek, H.; Brennan, P.J.; Minnikin, D.E. Structural elucidation of a novel family of acyltrehaloses from Mycobacterium tuberculosis. Biochemistry 1992, 31, 9832–9837. [Google Scholar] [CrossRef]
- Gautier, N.; Marín, L.M.L.; Lanéelle, M.A.; Daffé, M. Structure of mycoside F, a family of trehalose-containing glycolipids of Mycobacterium fortuitum. FEMS Microbiol. Lett. 1992, 77, 81–87. [Google Scholar]
- Lee, K.-S.; Dubey, V.S.; Kolattukudy, P.E.; Song, C.-H.; Shin, A.R.; Jung, S.-B.; Yang, C.-S.; Kim, S.-Y.; Jo, E.-K.; Park, J.-K.; et al. Diacyltrehalose of Mycobacterium tuberculosis inhibits lipopolysaccharide- and mycobacteria-induced proinflammatory cytokine production in human monocytic cells. FEMS Microbiol. Lett. 2007, 267, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Saavedra, R.; Segura, E.; Leyva, R.; Esparza, L.A.; López-Marín, L.M. Mycobacterial Di-O-Acyl-Trehalose Inhibits Mitogen- and Antigen-Induced Proliferation of Murine T Cells In Vitro. Clin. Diagn. Lab. Immun. 2001, 8, 1081–1088. [Google Scholar] [CrossRef]
- Bailo, R.; Bhatt, A.; Ainsa, J.A. Lipid transport in Mycobacterium tuberculosis and its implications in virulence and drug development. Biochem. Pharmacol. 2015, 96, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Papa, F.; Cruaud, P.; David, H.L. Antigenicity and specificity of selected glycolipid fractions from Mycobacterium tuberculosis. Res. Microbiol. 1989, 140, 569–578. [Google Scholar] [CrossRef]
- Hamid, M.E.; Fraser, J.L.; Wallace, P.A.; Besra, G.; Goodfellow, M.; Minnikin, D.E. Antigenic glycolipids of Mycobacterium fortuitum based on trehalose acylated with 2-methyloctadec-2-enoic acid. Lett. Appl. Microbiol. Rev. 1993, 16, 132–135. [Google Scholar] [CrossRef]
- Ridell, M.; Wallerstr6m, G.; Minnikin, D.E.; Bolton, R.C.; Magnusson, M. A comparative serological study of antigenic glycolipids from Mycobacteriurn tuberculosis. Tubercle Lung Dis. 1992, 73, 71–75. [Google Scholar] [CrossRef]
- Tórtola, M.T.; Lanéelle, M.A.; Martín-Casabona, N. Comparison of two 2,3-diacyl trehalose antigens from Mycobacterium tuberculosis and Mycobacterium fortuitum for serology in tuberculosis patients. Clin. Diagn. Lab. Immun. 1996, 3, 563–566. [Google Scholar]
- Prabhakar, S.; Vivès, T.; Ferrières, V.; Benvegnu, T.; Legentil, L.; Lemiègre, L. A fully enzymatic esterification/transesterification sequence for the preparation of symmetrical and unsymmetrical trehalose diacyl conjugates. Green Chem. 2017, 19, 987–995. [Google Scholar] [CrossRef]
- Botté, C.Y.; Deligny, M.; Roccia, A.; Bonneau, A.-L.; Saïdani, N.; Hardré, H.; Aci, S.; Yamaryo-Botté, Y.; Jouhet, J.; Dubots, E.; et al. Chemical inhibitors of monogalactosyldiacylglycerol synthases in Arabidopsis thaliana. Nat. Chem. Biol. 2011, 7, 834–842. [Google Scholar] [CrossRef]
- Olsen, J.V.; Macek, B.; Lange, O.; Makarov, A.; Horning, S.; Mann, M. Higher-energy C-trap dissociation for peptide modification analysis. Nat. Meth. 2007, 4, 709–712. [Google Scholar] [CrossRef]
- Hsu, F.F. Mass spectrometry-based shotgun lipidomics—A critical review from the technical point of view. Anal. Bioanal. Chem. 2018, 410, 6387–6409. [Google Scholar] [CrossRef] [PubMed]
- Frankfater, C.; Jiang, X.; Hsu, F.F. Characterization of Long-Chain Fatty Acid as N-(4-Aminomethylphenyl) Pyridinium Derivative by MALDI LIFT-TOF/TOF Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2018, 29, 1688–1699. [Google Scholar] [CrossRef] [PubMed]
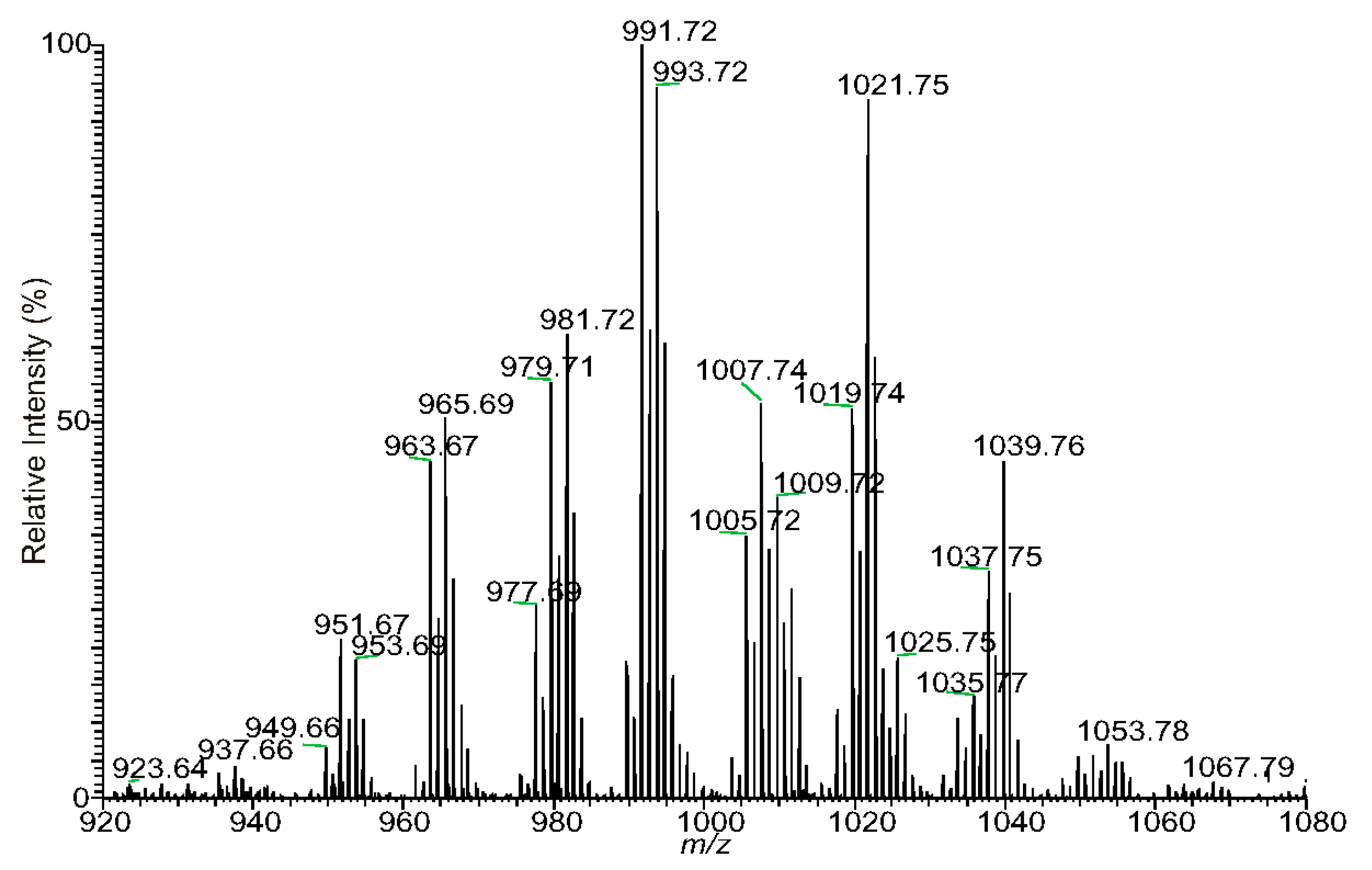
| Measured m/z | Theo. Mass m/z | Deviation mmu | Rel. Intensity % | Ele. Composition | Major structures | Minor structures |
|---|---|---|---|---|---|---|
| 935.6436 | 935.6430 | 0.55 | 3.42 | C51H92O13Na | 14:1/25:1 | 12:1/27:1 |
| 937.6594 | 937.6587 | 0.73 | 4.34 | C51H94O13Na | 14:0/25:1 | 12:0/27:1; 16:1/23:0; 15:1/24:0; 13:0/26:1 |
| 939.6747 | 939.6743 | 0.37 | 1.48 | C51H96O13Na | * | |
| 949.6585 | 949.6587 | −0.19 | 6.58 | C52H94O13Na | 16:1/24:1 | |
| 951.6742 | 951.6743 | −0.15 | 20.8 | C52H96O13Na | 16:1/24:0 | 16:0/24:1; 15:0/25:1; 17:1/23:0; 14:0/26:1; 13:0/27:1 |
| 953.6898 | 953.6900 | −0.12 | 18.49 | C52H98O13Na | 16:0/24:0 | |
| 961.6587 | 961.6587 | 0.01 | 4.31 | C53H94O13Na | * | |
| 963.6743 | 963.6743 | −0.05 | 44.16 | C53H96O13Na | 16:1/25:1; 14:1/27:1 | |
| 965.6898 | 965.6900 | −0.12 | 50.07 | C53H98O13Na | 16:0/25:1 | 14:0/27:1; 18:0/23:1 |
| 967.7054 | 967.7056 | −0.22 | 12.26 | C53H100O13Na | 18:0/23:0; 17:0/24:0 | 16:0/25:0 |
| 975.6740 | 975.6743 | −0.3 | 3.03 | C54H96O13Na | * | |
| 977.6899 | 977.6900 | −0.06 | 25.16 | C54H98O13Na | 16:1/26:1 | |
| 979.7056 | 979.7056 | −0.06 | 54.48 | C54H100O13Na | 18:0/24:1; 18:1/24:0 | 16:0/26:1; 17:0/25:1; 16:1/26:0; 15:0/27:1 |
| 981.7211 | 981.7213 | −0.15 | 62.62 | C54H102O13Na | 18:0/24:0 | 17:0/25:0; 16:0/26:0 |
| 989.6898 | 989.6900 | −0.17 | 18.03 | C55H98O13Na | 16:1/27:2 | |
| 991.7054 | 991.7056 | −0.2 | 100 | C55H100O13Na | 16:1/27:1 | 18:1/25:1; 16:0/27:2; 17:0/26:2 |
| 993.7210 | 993.7213 | −0.29 | 95.91 | C55H102O13Na | 16:0/27:1 | 18:0/25:1; 17:0/26:1 |
| 1005.7211 | 1005.7213 | −0.2 | 34.31 | C56H102O13Na | 16:1/28:1; 17:1/27:1 | 17:0/27:2; 18:1/26:1; 18:0/26:2 |
| 1007.7366 | 1007.7369 | −0.31 | 51.54 | C56H104O13Na | 17:0/27:1 | 16:0/28:1; 18:0/26:1; 19:0/25:1; 16:1/28:0 |
| 1009.7518 | 1009.7526 | −0.75 | 7.65 | C56H106O13Na | 18:0/26:0 | 20:0/24:0 |
| 1017.7209 | 1017.7213 | −0.38 | 11.79 | C57H102O13Na | 18:1/27:2 | |
| 1019.7367 | 1019.7369 | −0.25 | 52.39 | C57H104O13Na | 18:1/27:1 | 18:0/27:2; 17:1/28:1 |
| 1021.7522 | 1021.7526 | −0.37 | 92.1 | C57H106O13Na | 18:0/27:1 | 17:0/28:1 |
| 1033.7521 | 1033.7526 | −0.47 | 10.49 | C58H106O13Na | 18:1/28:1 | 19:0/27:2; 19:1/27:1; 18:0/28:2; 19:0/27:2 |
| 1035.7678 | 1035.7682 | −0.45 | 13.33 | C58H108O13Na | 19:0/27:1; 18:0/28:1 | 18:1/28:0; 17:0/29:1 |
| 1047.7677 | 1047.7682 | −0.5 | 2.56 | C59H108O13Na | 18:0/28:2 | |
| 1049.7835 | 1049.7839 | −0.31 | 5.47 | C59H110O13Na | 19:0/28:1 | 20:0/27:1; 18:0/29:1; 17:0/30:1 |
| 1061.7837 | 1061.7839 | −0.19 | 1.63 | C60H110O13Na | 18:1/30:1 | |
| 1063.7991 | 1063.7995 | −0.38 | 1.83 | C60H112O13Na | 18:0/30:1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frankfater, C.; Abramovitch, R.B.; Purdy, G.E.; Turk, J.; Legentil, L.; Lemiègre, L.; Hsu, F.-F. Multiple-stage Precursor Ion Separation and High Resolution Mass Spectrometry toward Structural Characterization of 2,3-Diacyltrehalose Family from Mycobacterium tuberculosis. Separations 2019, 6, 4. https://doi.org/10.3390/separations6010004
Frankfater C, Abramovitch RB, Purdy GE, Turk J, Legentil L, Lemiègre L, Hsu F-F. Multiple-stage Precursor Ion Separation and High Resolution Mass Spectrometry toward Structural Characterization of 2,3-Diacyltrehalose Family from Mycobacterium tuberculosis. Separations. 2019; 6(1):4. https://doi.org/10.3390/separations6010004
Chicago/Turabian StyleFrankfater, Cheryl, Robert B. Abramovitch, Georgiana E. Purdy, John Turk, Laurent Legentil, Loïc Lemiègre, and Fong-Fu Hsu. 2019. "Multiple-stage Precursor Ion Separation and High Resolution Mass Spectrometry toward Structural Characterization of 2,3-Diacyltrehalose Family from Mycobacterium tuberculosis" Separations 6, no. 1: 4. https://doi.org/10.3390/separations6010004
APA StyleFrankfater, C., Abramovitch, R. B., Purdy, G. E., Turk, J., Legentil, L., Lemiègre, L., & Hsu, F.-F. (2019). Multiple-stage Precursor Ion Separation and High Resolution Mass Spectrometry toward Structural Characterization of 2,3-Diacyltrehalose Family from Mycobacterium tuberculosis. Separations, 6(1), 4. https://doi.org/10.3390/separations6010004