Effect of Solvents and Extraction Methods on Recovery of Bioactive Compounds from Defatted Gac (Momordica cochinchinensis Spreng.) Seeds
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Solvents, Reagents, and Chemicals
2.1.2. Gac Seeds
Preparation of Defatted Gac Seed Powder
2.2. Methods
2.2.1. Extraction Methods
2.2.2. Determination of Trypsin Inhibitor Activity (TIA)
- Substrate solution: A substrate solution of 92 mmol L−1 BAPNA was made in 0.05 mol L−1 Tris-buffer (pH 8.2) containing 0.02 mol L−1 CaCl2. The BAPNA was first dissolved in DMSO and then diluted with the buffer solution pre-warmed to 37 °C. This solution was prepared daily and kept at 37 °C while in use.
- Trypsin solution: 20 mg of trypsin (type I) from bovine pancreas was dissolved in 0.001 mol L−1 HCl to make 1 L, stored at 4 °C for use within 2–3 weeks. When subjected to the analytical procedure for the standard, 2 mL of this solution gave an absorbance value in the range of 0.576 ± 0.026 after subtracting the reagent blank at 385 nm.
Determination of TIA
Calculation
- AI: Change in absorbance due to inhibition per 1 mL of diluted extract
- V: Original volume of solvent (mL)
- D: Dilution factor for the filtered extract
- S: Weight of defatted Gac seed powder sample extracted (g)
- 19: Constant figure based on the absorbance given by 1 mg of pure trypsin
- m%: moisture content of defatted Gac seed powder
2.2.3. Determination of Total Saponin Content (TSC)
2.2.4. Determination of Total Phenolic Content (TPC)
2.2.5. Determination of Antioxidant Capacity
2.2.6. Determination of Total Solids
2.2.7. Statistical Analyses
3. Results and Discussion
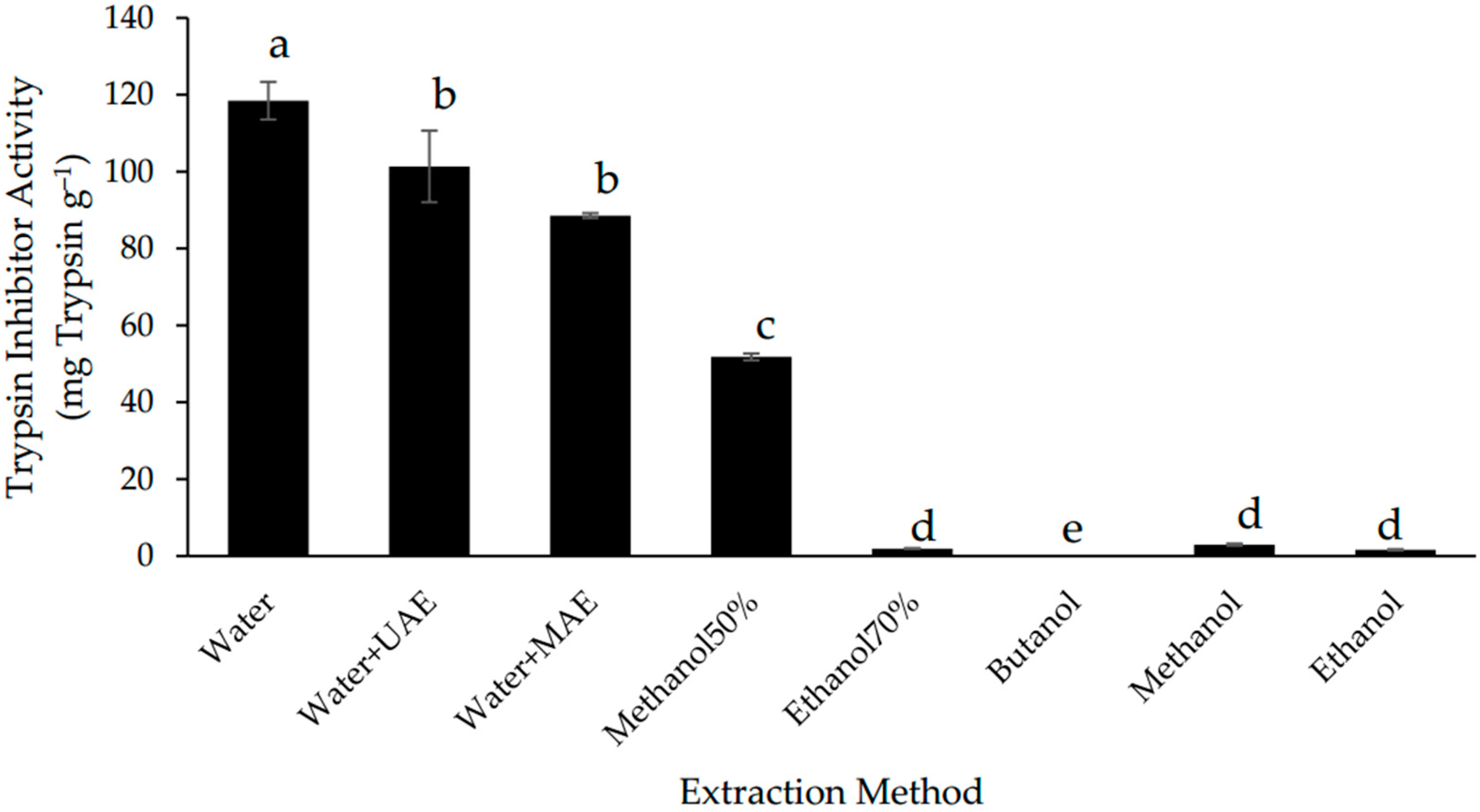
3.1. Effect of Extraction Methods on the Trypsin Inhibitor Yield
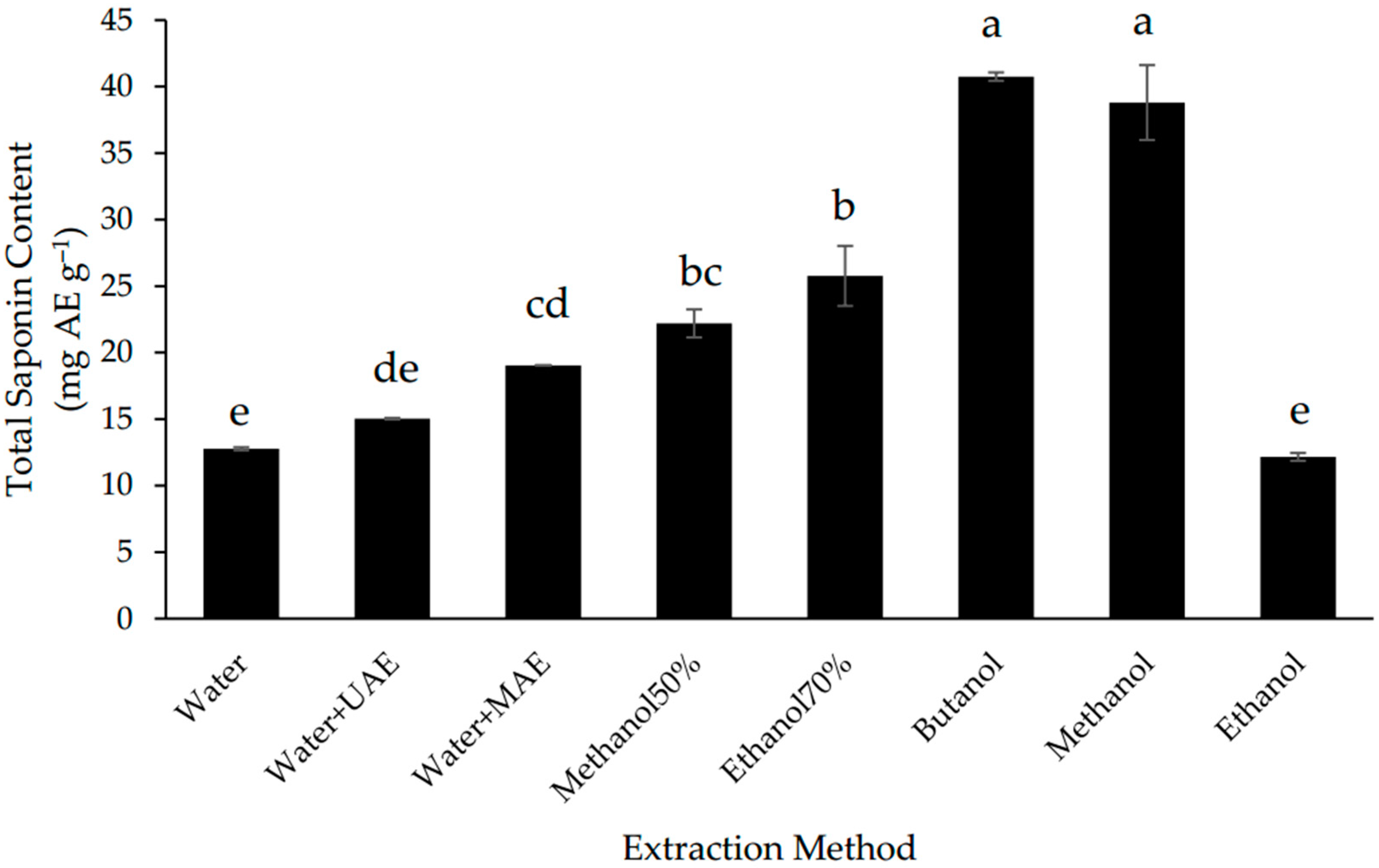
3.2. Effect of Extraction Methods on the Total Saponin Content (TSC)
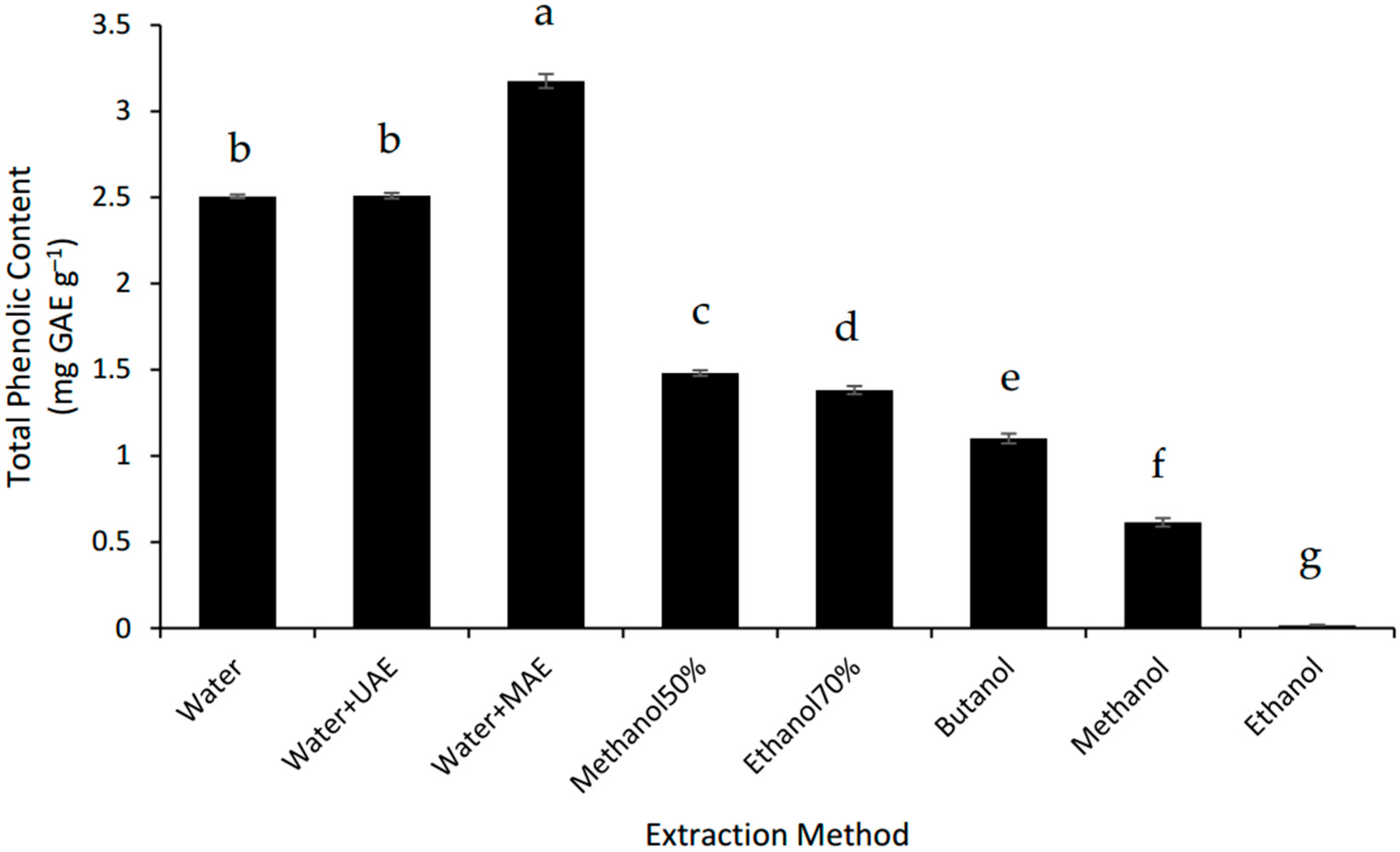
3.3. Effect of Extraction Methods on Total Phenolic Content (TPC)
3.4. Effect of Extraction Methods on Total Solids and Antioxidant Capacity
3.5. Correlations between Bioactive Compounds and Total Solids and Antioxidant Activity in the Extracts
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wimalasiri, D.; Piva, T.; Urban, S.; Huynh, T. Morphological and genetic diversity of Momordica cochinchinenesis (Cucurbitaceae) in Vietnam and Thailand. Genet. Resour. Crop Evol. 2016, 63, 19–33. [Google Scholar] [CrossRef]
- Behera, T.; John, K.J.; Bharathi, L.; Karuppaiyan, R.; Momordica. Wild Crop Relatives: Genomic and Breeding Resources; Kole, C., Ed.; Springer: Heidelberg, NY, USA, 2011; pp. 217–246. ISBN 978-3-642-20449-4. [Google Scholar]
- Chuyen, H.V.; Nguyen, M.H.; Roach, P.D.; Golding, J.B.; Parks, S.E. Gac fruit (Momordica cochinchinensis Spreng.): A rich source of bioactive compounds and its potential health benefits. Int. J. Food Sci. Technol. 2015, 50, 567–577. [Google Scholar] [CrossRef]
- Masayo, I.; Hikaru, O.; Tatsuo, Y.; Masako, T.; Yoshie, R.; Shuji, H.; Kunihide, M.; Ryuichi, H. Studies on the constituents of Momordica cochinchinensis Spreng. I. Isolation and characterization of the seed saponins, Momordica saponins I and II. Chem. Pharm. Bull. 1985, 33, 464–478. [Google Scholar]
- Chan, L.Y.; Wang, C.K.L.; Major, J.M.; Greenwood, K.P.; Lewis, R.J.; Craik, D.J.; Daly, N.L. Isolation and characterization of peptides from Momordica cochinchinensis seeds. J. Nat. Prod. 2009, 72, 1453–1458. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.C.; Fong, W.; Ng, T. Multiple trypsin inhibitors from Momordica cochinchinensis seeds, the Chinese drug mubiezhi. Peptides 2004, 25, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.Y.; He, W.; Tan, N.; Zeng, G.; Craik, D.J.; Daly, N.L. A new family of cystine knot peptides from the seeds of Momordica cochinchinensis. Peptides 2013, 39, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.M.; Mu, B.Z. (Eds.) Semen momordicae. In Chinese Materia Medicia; Traditional Chinese Materia Medica Press: Beijing, China, 2005; pp. 601–602. [Google Scholar]
- Lin, Z.Y.; Liu, X.; Yang, F.; Yu, Y.Q. Structural characterization and identification of five triterpenoid saponins isolated from Momordica cochinchinensis extracts by liquid chromatography/tandem mass spectrometry. Int. J. Mass Spectrom. 2012, 328, 43–66. [Google Scholar] [CrossRef]
- Kubola, J.; Siriamornpun, S. Phytochemicals and antioxidant activity of different fruit fractions (peel, pulp, aril and seed) of Thai gac (Momordica cochinchinensis Spreng). Food Chem. 2011, 127, 1138–1145. [Google Scholar] [CrossRef] [PubMed]
- Samuelsson, G.; Kyerematen, G.; Farah, M.H. Preliminary chemical characterization of pharmacologically active compounds in aqueous plant extracts. J. Ethnopharmacol. 1985, 14, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K.; Latha, L.Y. Extraction, isolation and characterization of bioactive compounds from plants’ extracts. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cheok, C.Y.; Salman, H.A.K.; Sulaiman, R. Extraction and quantification of saponins: A review. Food Res. Int. 2014, 59, 16–40. [Google Scholar] [CrossRef]
- Mahatmanto, T.; Poth, A.G.; Mylne, J.S.; Craik, D.J. A comparative study of extraction methods reveals preferred solvents for cystine knot peptide isolation from Momordica cochinchinensis seeds. Fitoterapia 2014, 95, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Tatke, P.; Jaiswal, Y. An overview of microwave assisted extraction and its applications in herbal drug research. Res. J. Med. Plant 2011, 5, 21–31. [Google Scholar] [CrossRef]
- Pingret, D.; Fabiano-Tixier, A.S.; Chemat, F. Ultrasound-assisted extraction. In Natural Product Extraction: Principles and Applications; Mauricio, R., Juliana, P., Eds.; RSC Pub: Cambridge, UK, 2013; pp. 89–111, ISBN 1849736065, 9781849736060. [Google Scholar]
- Chuyen, H.V.; Nguyen, M.H.; Roach, P.D.; Golding, J.B.; Parks, S.E. Microwave-assisted extraction and ultrasound-assisted extraction for recovering carotenoids from Gac peel and their effects on antioxidant capacity of the extracts. Food Sci. Nutr. 2018, 6, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Weller, C.L. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol. 2006, 17, 300–312. [Google Scholar] [CrossRef]
- Bhuyan, D.J.; Vuong, Q.V.; Chalmers, A.C.; van Altena, I.A.; Bowyer, M.C.; Scarlett, C.J. Development of the ultrasonic conditions as an advanced technique for extraction of phenolic compounds from Eucalyptus robusta. Sep. Sci. Technol. 2017, 52, 100–112. [Google Scholar] [CrossRef]
- Wu, J.; Lin, L.; Chau, F.T. Ultrasound-assisted extraction of ginseng saponins from ginseng roots and cultured ginseng cells. Ultrason. Sonochem. 2001, 8, 347–352. [Google Scholar] [CrossRef]
- Bhuyan, D.J.; Vuong, Q.V.; Chalmers, A.C.; van Altena, I.A.; Bowyer, M.C.; Scarlett, C.J. Microwave-assisted extraction of Eucalyptus robusta leaf for the optimal yield of total phenolic compounds. Ind. Crops Prod. 2015, 69, 290–299. [Google Scholar] [CrossRef]
- Anh, V.L.; Sophie, E.P.; Minh, H.N.; Paul, D.R. Optimisation of the microwave-assisted ethanol extraction of saponins from Gac (Momordica cochinchinensis Spreng.) seeds. Medicines 2018, 5, 70. [Google Scholar] [CrossRef]
- Makkar, H.P.; Siddhuraju, P.; Becker, K. Trypsin Inhibitor. In Plant Secondary Metabolites; Humana Press: New York, NY, USA, 2007; pp. 1–6. ISBN 1588299937. [Google Scholar]
- Stauffer, C.E. Measuring trypsin inhibitor in soy meal: Suggested improvements in the standard method. Cereal Chem. 1990, 67, 296–302. [Google Scholar]
- Tan, S.P.; Kha, T.C.; Parks, S.E.; Stathopoulos, C.E.; Roach, P.D. Effects of the spray-drying temperatures on the physiochemical properties of an encapsulated bitter melon aqueous extract powder. Powder Technol. 2015, 281, 65–75. [Google Scholar] [CrossRef]
- Tan, S.P.; Vuong, Q.V.; Stathopoulos, C.E.; Parks, S.E.; Roach, P.D. Optimized aqueous extraction of saponins from bitter melon for production of a saponin-enriched bitter melon powder. J. Food Sci. 2014, 79, E1372–E1381. [Google Scholar] [CrossRef] [PubMed]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Byrne, D.H. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compost. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Boye, J.I.; Ma, Z. Impact of Processing on Bioactive Compounds of Field Peas. In Processing and Impact on Active Components in Food; Preedy, V., Ed.; Elsevier: Boston, MA, USA, 2015; pp. 63–70. ISBN 978-0-12-404699-3. [Google Scholar]
- Serrano, M.; Rebollar, P.; Sueiro, S.; Hermida, M.; Mateos, G. Influence of duration of storage on protein quality traits of soybean meals. J. Appl. Poult. Res. 2013, 22, 423–429. [Google Scholar] [CrossRef]
- Smith, C.; van Megen, W.; Twaalfhoven, L.; Hitchcock, C. The determination of trypsin inhibitor levels in foodstuffs. J. Sci. Food Agric. 1980, 31, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Kwok, K.C.; Liang, H.H. Inhibitory activity and conformation changes of soybean trypsin inhibitors induced by ultrasound. Ultrason. Sonochem. 2008, 15, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Vagadia, B.H.; Vanga, S.K.; Raghavan, V. Inactivation methods of soybean trypsin inhibitor—A review. Trends Food Sci. Technol. 2017, 64, 115–125. [Google Scholar] [CrossRef]
- Pysz, M.; Polaszczyk, S.; Leszczyńska, T.; Piątkowska, E. Effect of microwave field on trypsin inhibitors activity and protein quality of broad bean seeds (Vicia faba var. major). Acta. Sci. Pol. Technol. Aliment. 2012, 11, 193–198. [Google Scholar] [PubMed]
- Nicholls, A.; Sharp, K.A.; Honig, B. Protein folding and association: Insights from the interfacial and thermodynamic properties of hydrocarbons. Proteins Struct. Funct. Bioinform. 1991, 11, 281–296. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, W.; Stark, C.; Ferket, P.; Brake, J. Effects of trypsin inhibitor and particle size of expeller-extracted soybean meal on broiler live performance and weight of gizzard and pancreas. Poult. Sci. 2014, 93, 2245–2252. [Google Scholar] [CrossRef] [PubMed]
- Jirgensons, B. Effects of n-propyl alcohol and detergents on the optical rotatory dispersion of α-chymotrypsinogen, β-casein, histone fraction F1, and soybean trypsin inhibitor. J. Biol. Chem. 1967, 242, 912–918. [Google Scholar] [PubMed]
- Pesoti, A.R.; Oliveira, B.M.; Oliveira, A.C.; Pompeu, D.G.; Gonçalves, D.B.; Marangoni, S.; Silva, J.A.; Granjeiro, P.A. Extraction, purification and characterization of inhibitor of trypsin from Chenopodium quinoa seeds. Food Sci. Technol. 2015, 35, 588–597. [Google Scholar] [CrossRef]
- Güçlü-Üstündağ, Ö.; Mazza, G. Saponins: Properties, applications and processing. Crit. Rev. Food Sci. Nutr. 2007, 47, 231–258. [Google Scholar] [CrossRef] [PubMed]
- Madl, T.; Sterk, H.; Mittelbach, M.; Rechberger, G.N. Tandem mass spectrometric analysis of a complex triterpene saponin mixture of Chenopodium quinoa. J. Am. Soc. Mass Spectrom. 2006, 17, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, J.F.; Gagnon, J.; Chiche, L.; Nguyen, T.M.; Andrieu, J.P.; Heitz, A.; Trinh, H.T.; Pham, T.T.; Le, N.D. Squash trypsin inhibitors from Momordica cochinchinensis exhibit an atypical macrocyclic structure. Biochemistry 2000, 39, 5722–5730. [Google Scholar] [CrossRef] [PubMed]
- Sastry, M.; Murray, D.R. The contribution of trypsin inhibitors to the nutritional value of chick pea seed protein. J. Sci. Food Agric. 1987, 40, 253–261. [Google Scholar] [CrossRef]
- Shamsi, T.N.; Parveen, R.; Afreen, S.; Azam, M.; Sen, P.; Sharma, Y.; Fatima, S. Trypsin inhibitors from Cajanus cajan and Phaseolus limensis possess antioxidant, anti-inflammatory, and antibacterial activity. J. Diet. Suppl. 2018, 15, 939–950. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.B.; He, T.P.; Li, H.B.; Tang, H.W.; Xia, E.Q. The structure-activity relationship of the antioxidant peptides from natural proteins. Molecules 2016, 21, 72. [Google Scholar] [CrossRef] [PubMed]
- Kähkönen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef] [PubMed]
- Pham, H.N.T.; Nguyen, V.T.; Vuong, V.Q.; Bowyer, M.C.; Scarlett, C.J. Bioactive compound yield and antioxidant capacity of Helicteres hirsuta Lour. stem as affected by various solvents and drying methods. J. Food Process. Preserv. 2017, 41, e12879. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [PubMed]
| Component (mL) | Reagent Blank (a) | Standard (b) | Sample Blank (c) | Sample (d) |
|---|---|---|---|---|
| Deionised water | 2 | 2 | 1 | 1 |
| Trypsin solution | - | 2 | - | 2 |
| Diluted extract | - | - | 1 | 1 |
| Trypsin solution after reaction inactivation | 2 | - | 2 | - |
| Extraction Method | Total Solids (g kg−1) | ABTS (μmol TE g−1) | FRAP (μmol TE g−1) |
|---|---|---|---|
| Water | 120.50 ± 2.16 b | 21.74 ± 0.84 a,b | 3.73 ± 0.40 b |
| Water + UAE | 116.31 ± 0.40 c | 19.86 ± 0.91 b,c | 3.13 ± 0.10 b |
| Water + MAE | 141.46 ± 1.08 a | 23.56 ± 0.82 a | 3.76 ± 0.12 b |
| 50% methanol | 83.13 ± 1.00 d | 18.55 ± 1.09 c | 3.76 ± 0.26 b |
| 70% ethanol | 79.37 ± 0.68 e | 18.61 ± 0.96 c | 4.71 ± 0.39 a |
| Butanol | 43.79 ± 0.58 g | 17.36 ± 1.49 c | 5.25 ± 0.04 a |
| Methanol | 48.76 ± 0.55 f | 10.87 ± 0.57 d | 3.36 ± 0.21 b |
| Ethanol | 28.90 ± 0.19 h | 6.05 ± 0.35 e | 1.97 ± 0.08 c |
| Bioactive Compounds | R-Squared Value | ||
|---|---|---|---|
| TS | ABTS | FRAP | |
| TIA | 0.80 † | 0.50 | 0.02 |
| TSC | 0.24 | 0.02 | 0.38 |
| TPC | 0.96 † | 0.85 † | 0.05 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
V. Le, A.; E. Parks, S.; H. Nguyen, M.; D. Roach, P. Effect of Solvents and Extraction Methods on Recovery of Bioactive Compounds from Defatted Gac (Momordica cochinchinensis Spreng.) Seeds. Separations 2018, 5, 39. https://doi.org/10.3390/separations5030039
V. Le A, E. Parks S, H. Nguyen M, D. Roach P. Effect of Solvents and Extraction Methods on Recovery of Bioactive Compounds from Defatted Gac (Momordica cochinchinensis Spreng.) Seeds. Separations. 2018; 5(3):39. https://doi.org/10.3390/separations5030039
Chicago/Turabian StyleV. Le, Anh, Sophie E. Parks, Minh H. Nguyen, and Paul D. Roach. 2018. "Effect of Solvents and Extraction Methods on Recovery of Bioactive Compounds from Defatted Gac (Momordica cochinchinensis Spreng.) Seeds" Separations 5, no. 3: 39. https://doi.org/10.3390/separations5030039
APA StyleV. Le, A., E. Parks, S., H. Nguyen, M., & D. Roach, P. (2018). Effect of Solvents and Extraction Methods on Recovery of Bioactive Compounds from Defatted Gac (Momordica cochinchinensis Spreng.) Seeds. Separations, 5(3), 39. https://doi.org/10.3390/separations5030039






