Electrochemical Characterization of a Polymer Inclusion Membrane Made of Cellulose Triacetate and Aliquat 336 and Its Application to Sulfonamides Separation
Abstract
:1. Introduction
2. Results and Discussion
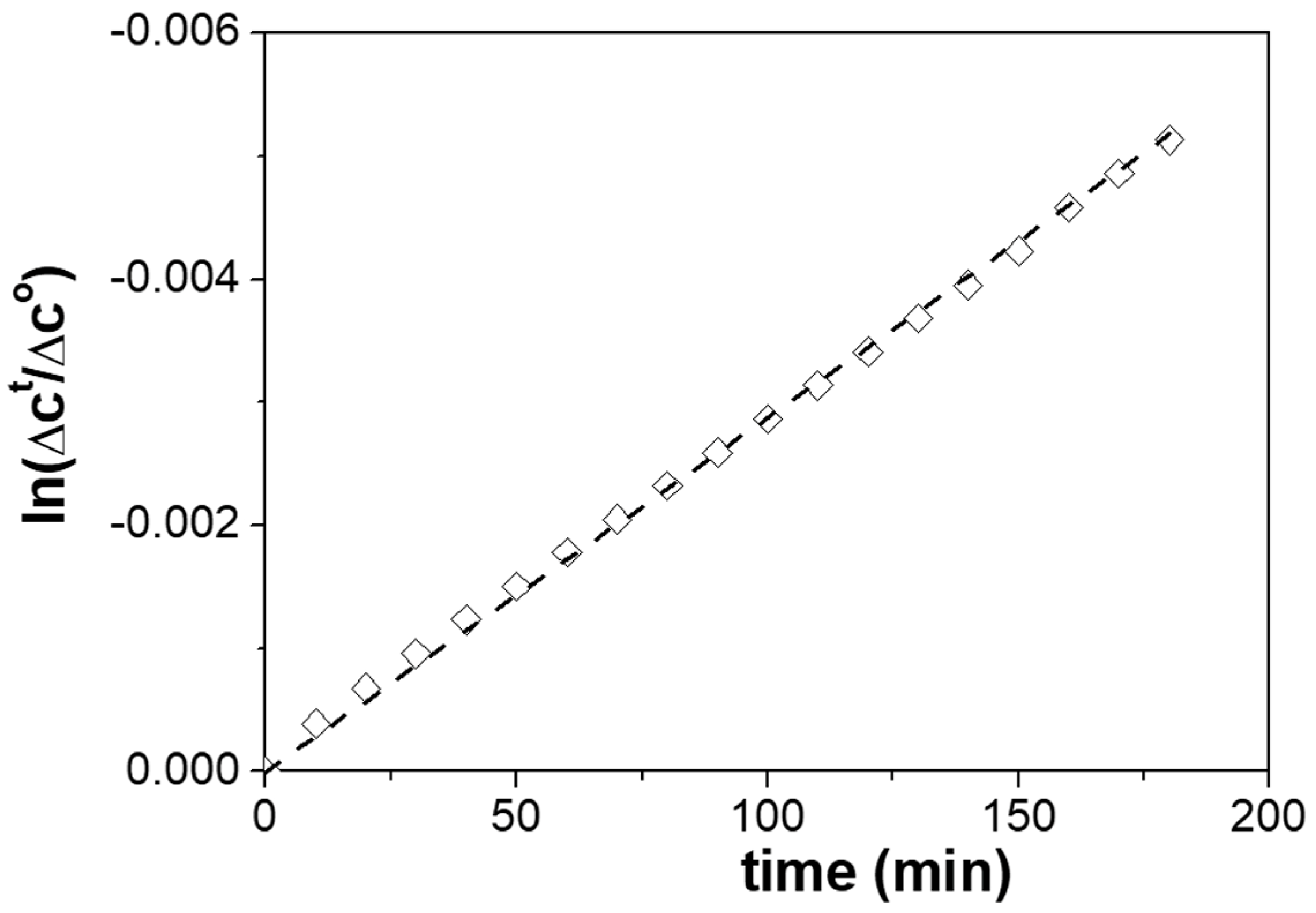
Application of the PIM to the Separation of Sulfonamides
3. Materials and Methods
3.1. Membrane Preparation
XPS Measurements
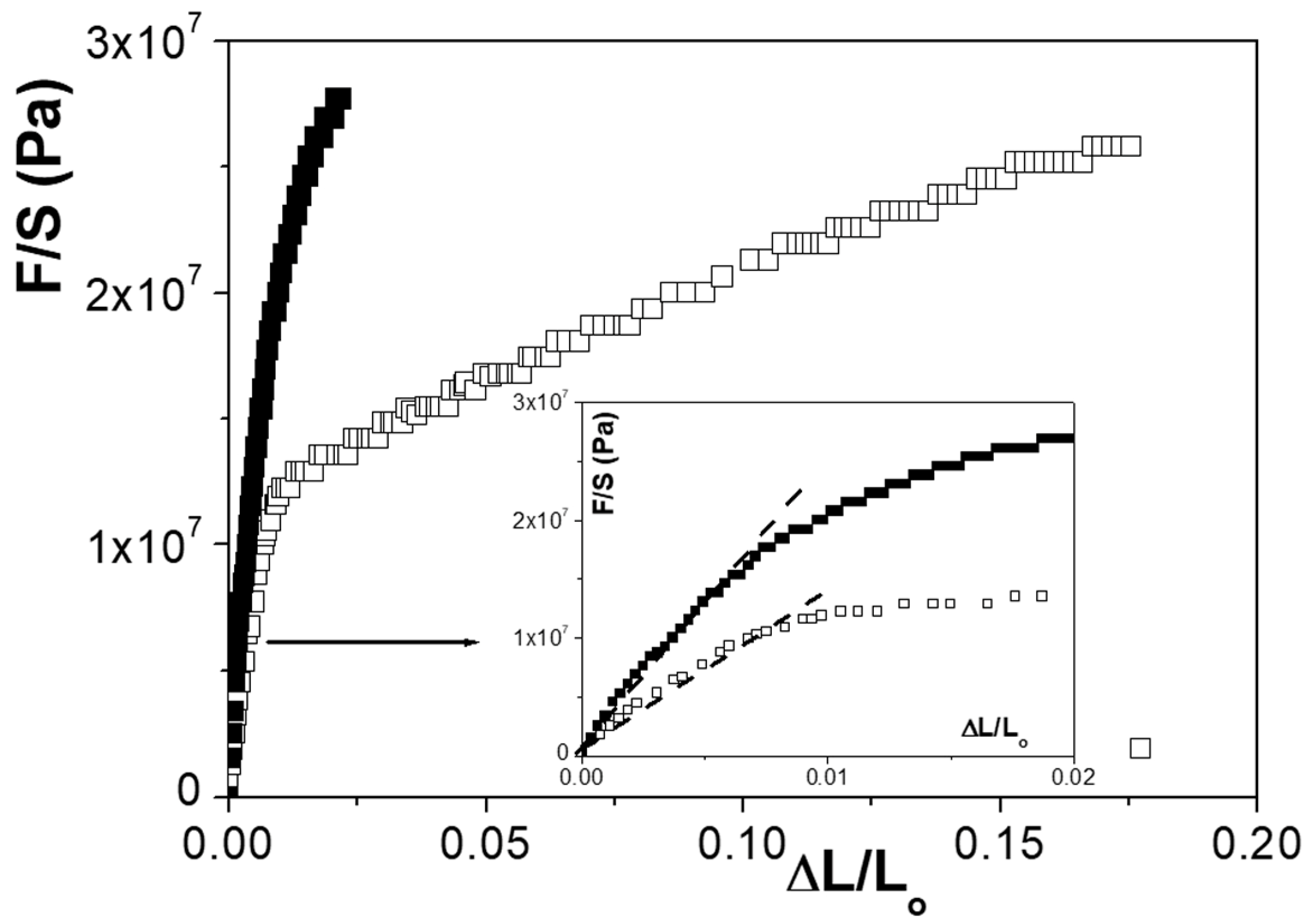
3.2. Elastic Measurements
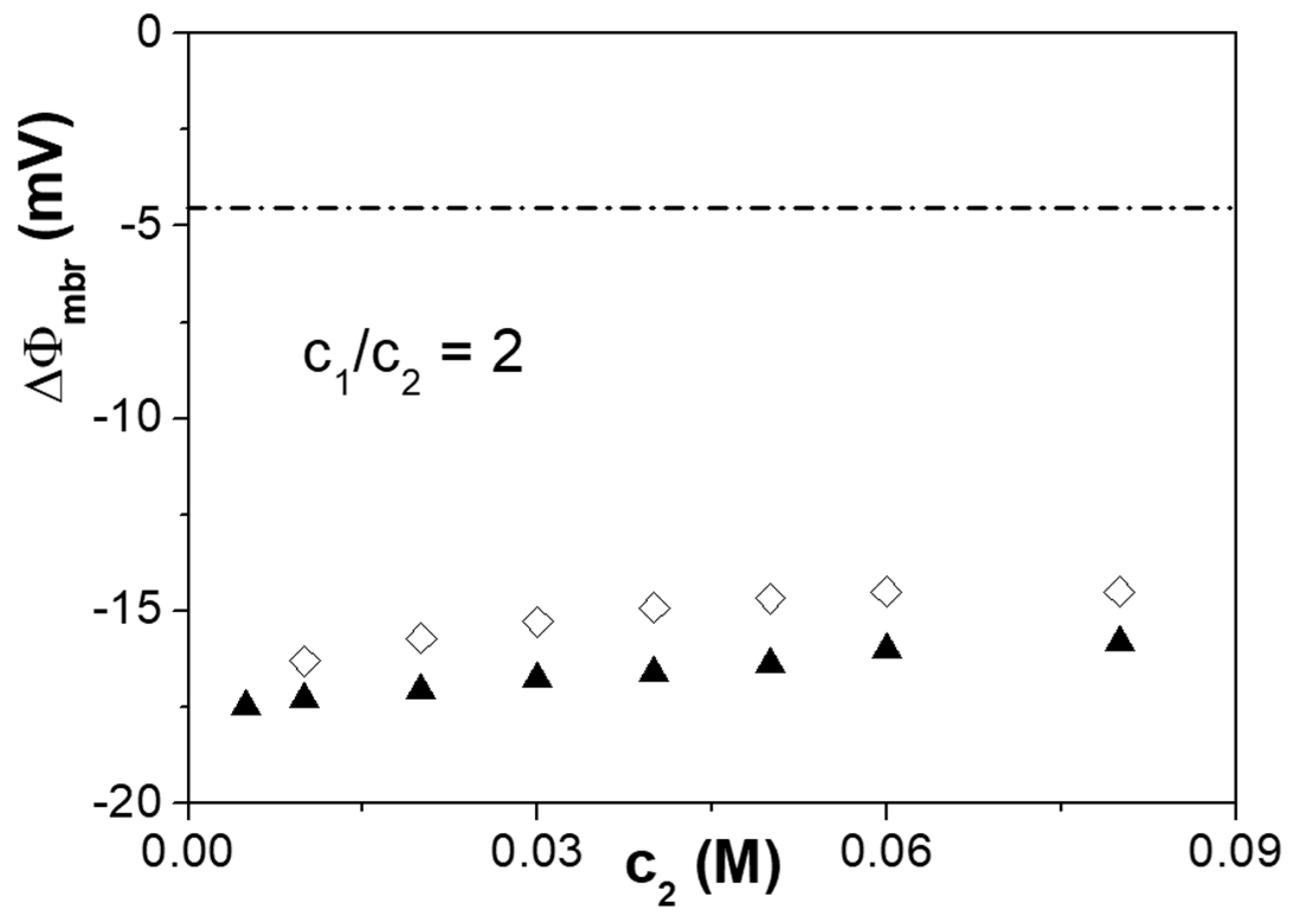
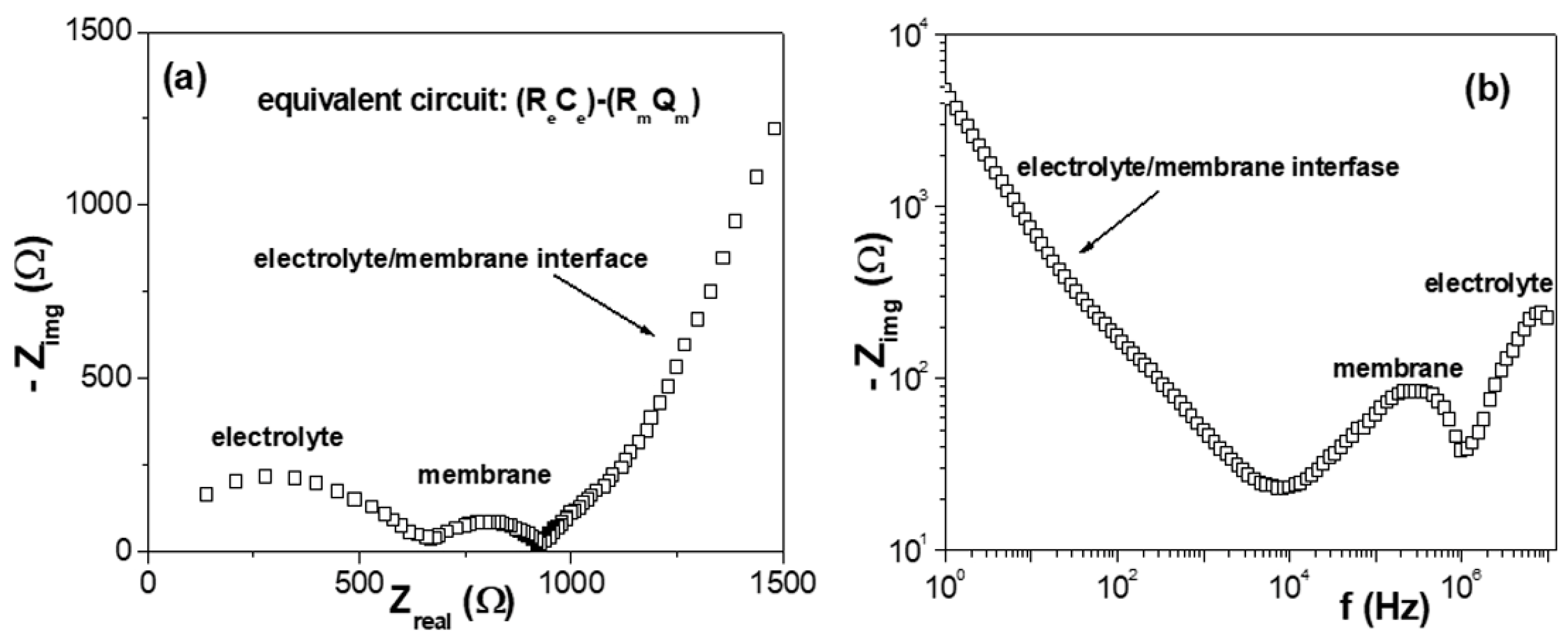
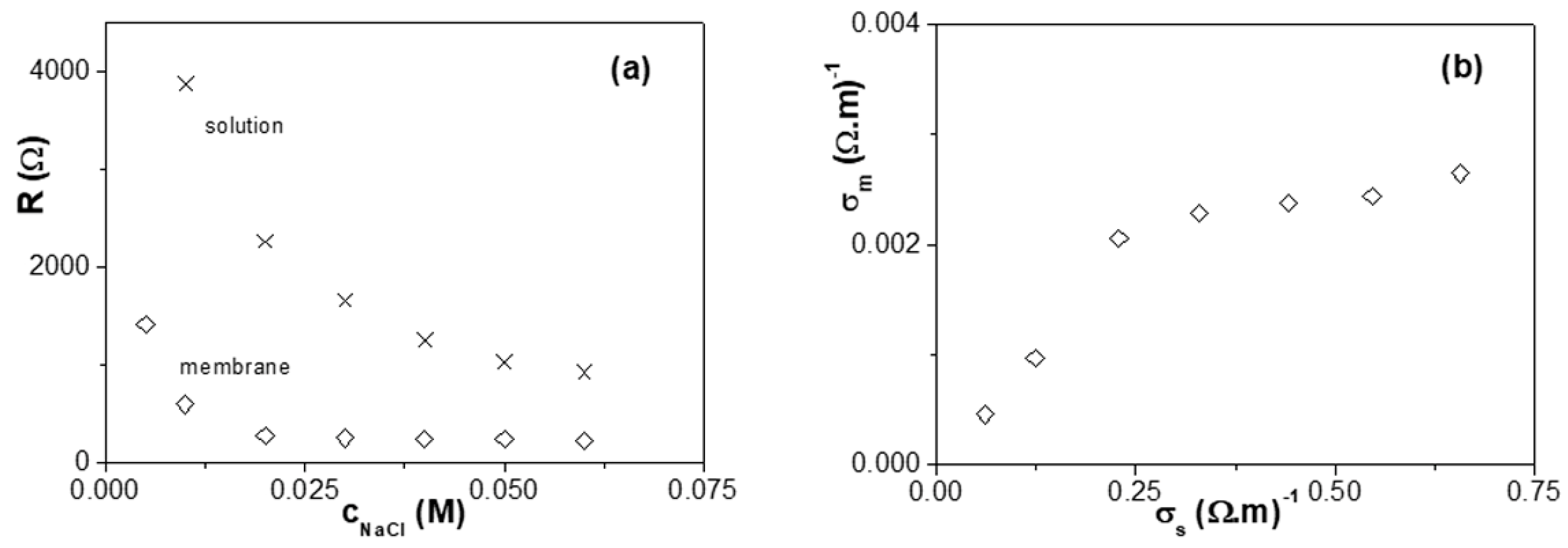
3.3. Electrochemical Characterization: Cell Potentials, Electrochemical Impedance Spectroscopy, and Salt Diffusion Measurements
3.4. Transport Experiments
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bennet, M.D.; Leo, D.J. Ionic Liquids as stable solvents for ionic polymer transducers. Sens. Actuators A 2004, 115, 79–90. [Google Scholar] [CrossRef]
- Welton, T. Room-Temperature Ionic Liquids. Solvents for synthesis and catalysis. Chem. Rev. 1999, 99, 2071–2083. [Google Scholar] [PubMed]
- Armand, M.; Endres, F.; MacFarlane, D.R.; Ohno, H.; Scrosati, B. Ionic-liquid Materials for the Electrochemical Challenges of the Future. Nat. Mater. 2009, 8, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Fortunato, R.; Branco, L.; Afonso, C.A.M.; Benavente, J.; Crespo, J.G. Electrical impedance spectroscopy characterization of supported ionic liquid membranes. J. Membr. Sci. 2006, 270, 42–49. [Google Scholar] [CrossRef]
- Regel-Rosocka, M.; Matema, K. Ionic Liquids in Separation of Metal Ions from Aqueous Solutions. In Application of Ionic Liquids in Science and Technology; Handy, S., Ed.; InTech: Rijeka, Croatia, 2011; pp. 375–798. ISBN 978-953-307-605-8. [Google Scholar]
- Ejigu, A.; Walsh, D.A. Electrocatalysis in Room Temperature Ionic Liquids. In Electrochemistry in Ionic Liquids: Application; Torriero, A.A.J., Ed.; Springer: New York, NY, USA, 2015; pp. 483–506. ISBN 978-3-319-15131-1. [Google Scholar]
- Albo, J.; Luis, P.; Irabien, A. Carbon dioxide capture from flue gases using a cross-flow membrane contactor and the ionic liquid 1-ethyl-3-methylimidazolium ethylsulfate. Ind. Eng. Chem. Res. 2010, 49, 11045–11051. [Google Scholar] [CrossRef]
- Jogelnig, D.; Stojanovic, A.; Galanski, M.; Groessl, M.; Jirsa, F.; Krachler, R.; Keppler, B.K. Greener synthesis of new ammonium ionic liquids and their potential as extracting agents. Tetrahedron Lett. 2008, 49, 2782–2785. [Google Scholar] [CrossRef]
- Rubio, A.M.; Tomás-Alonso, F.; Hernández Fernández, J.; Pérez de los Ríos, A.; Hernández Fernández, F.J. Green Aspect of Ionic Liquids. In Ionic Liquids in Separation Technology; Pérez de los Ríos, A., Hernández Fernández, F.J., Eds.; Elsevier B.V.: Amsterdam, The Netherlands, 2014; pp. 82–92. ISBN 978-0-444-63257-9. [Google Scholar]
- Almeida, M.I.S.G.; Cattrall, R.W.; Kolev, S.D. Recent trends in transport and extraction of metal ions using polymer inclusion membranes (PIMs). J. Membr. Sci. 2012, 415–416, 9–23. [Google Scholar] [CrossRef]
- Nghiem, L.D.; Mornane, P.; Potter, I.D.; Perera, J.M.; Cattrall, R.W.; Kolev, S.D. Extraction and transport of metal ions and small organic compounds using polymer inclusion membranes (PIMs). J. Membr. Sci. 2006, 281, 7–41. [Google Scholar] [CrossRef]
- Rodriguez de San Miguel, E.; Aguilar, J.C.; de Gyes, J. Structural effects on metal ion migration across polymer inclusion membranes: Dependence of transport profiles on nature of active plasticizer. J. Membr. Sci. 2008, 307, 105–116. [Google Scholar] [CrossRef]
- Pont, N.; Salvadó, V.; Fontàs, C. Selective transport and removal of Cd from chloride solutions by polymer inclusion membranes. J. Membr. Sci. 2008, 318, 340–345. [Google Scholar] [CrossRef]
- Pospiech, B. Application of Phosphonium Ionic Liquids as Ion Carriers in Polymer Inclusion Membranes (PIMs) for Separation of Cadmium(II) and Copper(II) from Aqueous Solutions. J. Solut. Chem. 2015, 44, 2431–2447. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Rodríguez, A.; Matamoros, V.; Kolev, S.D.; Fontàs, C. Development of a polymer inclusion membrane (PIM) for the preconcentration of antibiotics in environmental water samples. J. Membr. Sci. 2015, 492, 32–39. [Google Scholar] [CrossRef]
- Güell, R.; Anticó, E.; Kolev, S.D.; Benavente, J.; Salvadó, V.; Fontás, C. Development and characterization of polymer inclusion membranes for the separation and speciation of inorganic as species. J. Membr. Sci. 2011, 383, 88–95. [Google Scholar] [CrossRef]
- Sakai, Y.; Kadota, K.; Hayashita, T.; Cattrall, R.W.; Kolev, S.D. The effect of the counter anion on the transport of thiourea in a PVC-based polymer inclusion membrane using Capriquat as carrier. J. Membr. Sci. 2010, 346, 250–255. [Google Scholar] [CrossRef]
- Vázquez, M.I.; Romero, V.; Fontàs, C.; Anticó, E.; Benavente, J. Polymer inclusion membranes (PIMs) with the ionic liquid (IL) Aliquat 336 as extractant: Effect of base polymer and IL concentration on their physical-chemical and elastic characteristics. J. Membr. Sci. 2014, 455, 312–319. [Google Scholar] [CrossRef]
- Vera, R.; Gelde, L.; Anticó, E.; Martínez de Yuso, M.V.; Benavente, J.; Fontàs, C. Tuning physicochemical, electrochemical and transport characteristics of polymer inclusion membrane by varying the counter-anion of the ionic liquid Aliquat 336. J. Membr. Sci. 2017, 529, 87–94. [Google Scholar] [CrossRef]
- Gizli, N.; Çinarli, S.; Demircioglu, M. Characterization of poly(vinylchloride) (PVC) based cation exchange membrane prepared with ionic liquids. Sep. Purif. Technol. 2012, 97, 96–107. [Google Scholar] [CrossRef]
- Kumar, R.; Pandey, A.K.; Sharma, M.K.; Panicker, L.V.; Sodaye, S.; Suresh, G.; Ramagiri, S.V.; Bellare, J.R.; Goswami, A. Diffusional transport of ions in plasticized anaion-exchange membranes. J. Phys. Chem. B 2011, 115, 5856–5867. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Alonso, F.; Rubio, A.M.; Giménez, M.; de los Ríos, A.P.; Salar-García, M.J.; Ortiz-Martínez, V.M.; Hernández-Fernández, F.J. Influence of ionic liquid composition on the stability of polyvinyl chloride-based ionic liquid inclusion membranes in aqueous solution. AICHE J. 2016, 63, 770–780. [Google Scholar] [CrossRef]
- Kagaya, S.; Ryokan, Y.; Cattrall, R.W.; Kolev, S.D. Stability studies of poly(vinyl chloride)-based polymer inclusion membranes containing Aliquat 336 as a carrier. Sep. Purif. Technol. 2012, 101, 69–75. [Google Scholar] [CrossRef]
- Ariza, M.J.; Benavente, J.; Rodríguez-Castellón, E. The Capability of X-ray Photoelectron Spectroscopy in the Characterization of Membranes: Correlation between Surface Chemical and Transport Properties in Polymeric Membranes. In Handbook of Membrane Research: Properties, Performance and Applications; Gorley, S.V., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2009; pp. 257–290. ISBN 978-1-60741-638-8. [Google Scholar]
- Martínez de Yuso, M.V.; Benavente, J.; Rodríguez-Castellón, E. Use of XPS Technique for Studying Chemical Surface Changes in Modified Membranes. Procedia Eng. 2012, 44, 1204–1206. [Google Scholar] [CrossRef]
- Ramos, J.D.; Milano, C.; Romero, V.; Escalera, S.; Alba, M.C.; Vázquez, M.I.; Benavente, J. Water effect on physical-chemical and elastic parameters for a dense cellulose regenerated membrane. Transport of different aqueous electrolyte solutions. J. Membr. Sci. 2010, 352, 153–159. [Google Scholar]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-Ray Photoelectron Spectroscopy; Perkin-Elmer Corporation: Minneapolis, MN, USA, 1992; ISBN 9780962702624. [Google Scholar]
- Vázquez, M.I.; de Lara, R.; Benavente, J. Chemical surface, diffusional, electrical and elastic characterizations of two different dense regenerated cellulose membranes. J. Colloid Interface Sci. 2008, 328, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Robison, R.A.; Stokes, R.H. Electrolyte Solutions, 2nd ed.; Butterworths: London, UK, 1959; ISBN 0486422259. [Google Scholar]
- Porras, B.; Romero, V.; Benavente, J. Effect of acid/basic solutions contact on ion transport numbers and conductivity for an anion-exchange membrane. Desalination Water Treat. 2014, 57, 127–134. [Google Scholar] [CrossRef]
- Benavente, J.; García, J.M.; Riley, R.; Lozano, A.E.; de Abajo, J. Sulfonated poly(ether ether sulfone). Characterization and study of dielectrical properties by impedance spectroscopy. J. Membr. Sci. 2000, 175, 43–52. [Google Scholar] [CrossRef]
- Benavente, J.; Jonsson, G. Transport of Na2SO4, and MgSO4 solutions through a composite membrane. J. Membr. Sci. 1993, 80, 275–283. [Google Scholar] [CrossRef]
- Cañas, A.; Ariza, M.J.; Benavente, J. Characterization of active and porous sublayers of a composite reverse osmosis membrane by impedance spectroscopy, streaming and membrane potentials, salt diffusion and X-ray photoelectron spectroscopy measurements. J. Membr. Sci. 2001, 183, 135–146. [Google Scholar] [CrossRef]
- Benavente, J.; Silva, V.; Pradanos, P.; Hernandez, A.; Palacio, L.; Jonsson, G. A comparison of the volume charge density of nanofiltration membranes obtained from retention and conductivity experiments. Langmuir 2010, 26, 11841–11849. [Google Scholar] [CrossRef] [PubMed]
- Ross Macdonald, J.; Kenan, W.R. Impedance Spectroscopy: Emphasizing Solid Materials and Systems; Wiley & Sons, Inc.: New York, NY, USA, 1987; ISBN 0-471-83122-0. [Google Scholar]
- Benavente, J. Use of impedance spectroscopy for characterization of membranes and the effect of different modifications. In Membrane Modification: Technology and Applications; Hilal, N., Khayet, M., Wright, C.J., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 21–40. ISBN 781439866351. [Google Scholar]
- Mulder, M. Basic Principles of Membrane Technology; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1992; ISBN 0-7923-0978-2. [Google Scholar]
- Martínez de Yuso, M.V.; Neves, L.A.; Coelhoso, I.M.; Crespo, J.G.; Benavente, J.; Rodríguez-Castellón, E. A study of chemical modifications of a Nafion membrane by incorporation of different room temperature ionic liquids. Fuel Cells 2012, 12, 606–613. [Google Scholar] [CrossRef]
- Romero, V.; Vázquez, M.I.; Benavente, J. Study of ionic and diffusive transport through a regenerated cellulose nanoporous membrane. J. Membr. Sci. 2013, 433, 152–159. [Google Scholar] [CrossRef]

| PIM | C (%) | O (%) | N (%) | Cl (%) | Si (%) |
|---|---|---|---|---|---|
| 26% AlqCl/74% CTA-w | 72.6 | 23.7 | 1.6 (0.38) | 0.31 | 1.6 |
| 26% AlqCl/74% CTA-d a | 66.7 | 29.6 | 0.89 (0.50) | 0.44 | 0.3 |
| CTA-w | 73.7 | 24.1 | 0.65 | n.d. | 1.6 |
| CTA-d | 66.6 | 32.3 | n.d. | n.d. | 0.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benavente, J.; Romero, V.; Vázquez, M.I.; Anticó, E.; Fontàs, C. Electrochemical Characterization of a Polymer Inclusion Membrane Made of Cellulose Triacetate and Aliquat 336 and Its Application to Sulfonamides Separation. Separations 2018, 5, 5. https://doi.org/10.3390/separations5010005
Benavente J, Romero V, Vázquez MI, Anticó E, Fontàs C. Electrochemical Characterization of a Polymer Inclusion Membrane Made of Cellulose Triacetate and Aliquat 336 and Its Application to Sulfonamides Separation. Separations. 2018; 5(1):5. https://doi.org/10.3390/separations5010005
Chicago/Turabian StyleBenavente, Juana, Virginia Romero, María Isabel Vázquez, Enriqueta Anticó, and Clàudia Fontàs. 2018. "Electrochemical Characterization of a Polymer Inclusion Membrane Made of Cellulose Triacetate and Aliquat 336 and Its Application to Sulfonamides Separation" Separations 5, no. 1: 5. https://doi.org/10.3390/separations5010005
APA StyleBenavente, J., Romero, V., Vázquez, M. I., Anticó, E., & Fontàs, C. (2018). Electrochemical Characterization of a Polymer Inclusion Membrane Made of Cellulose Triacetate and Aliquat 336 and Its Application to Sulfonamides Separation. Separations, 5(1), 5. https://doi.org/10.3390/separations5010005







