Determination of 2-Thioxo-3-pyrrolidinecarbaldehyde in Salted Radish Root (Takuan-zuke) by High-Performance Liquid Chromatography with Fluorescence Detection after Pre-Column Derivatization Using 4-(N,N-dimethylaminosulfonyl)-7-hydrazino-2,1,3-benzoxadiazole
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
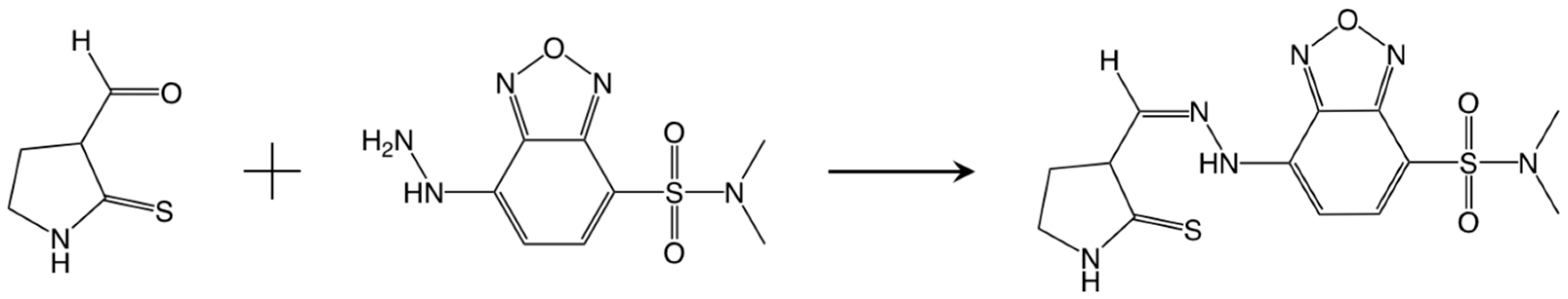
2.2. Fluorescent Labeling with DBD-H
2.2.1. TPC Standard Solution
2.2.2. Takuan-zuke Sample
2.3. Analysis of TPC and MTB-ITC Contained in Daikon-oroshi (Grated Radish)
2.3.1. Preparation of Grated Radish Juice
2.3.2. Fluorescent Labeling of TPC from Grated Radish Juice with DBD-H
2.3.3. Gas Chromatography Analysis of MTB-ITC in Grated Radish Juice
2.4. HPLC System and Conditions
2.5. GC System and Conditions
3. Results and Discussion
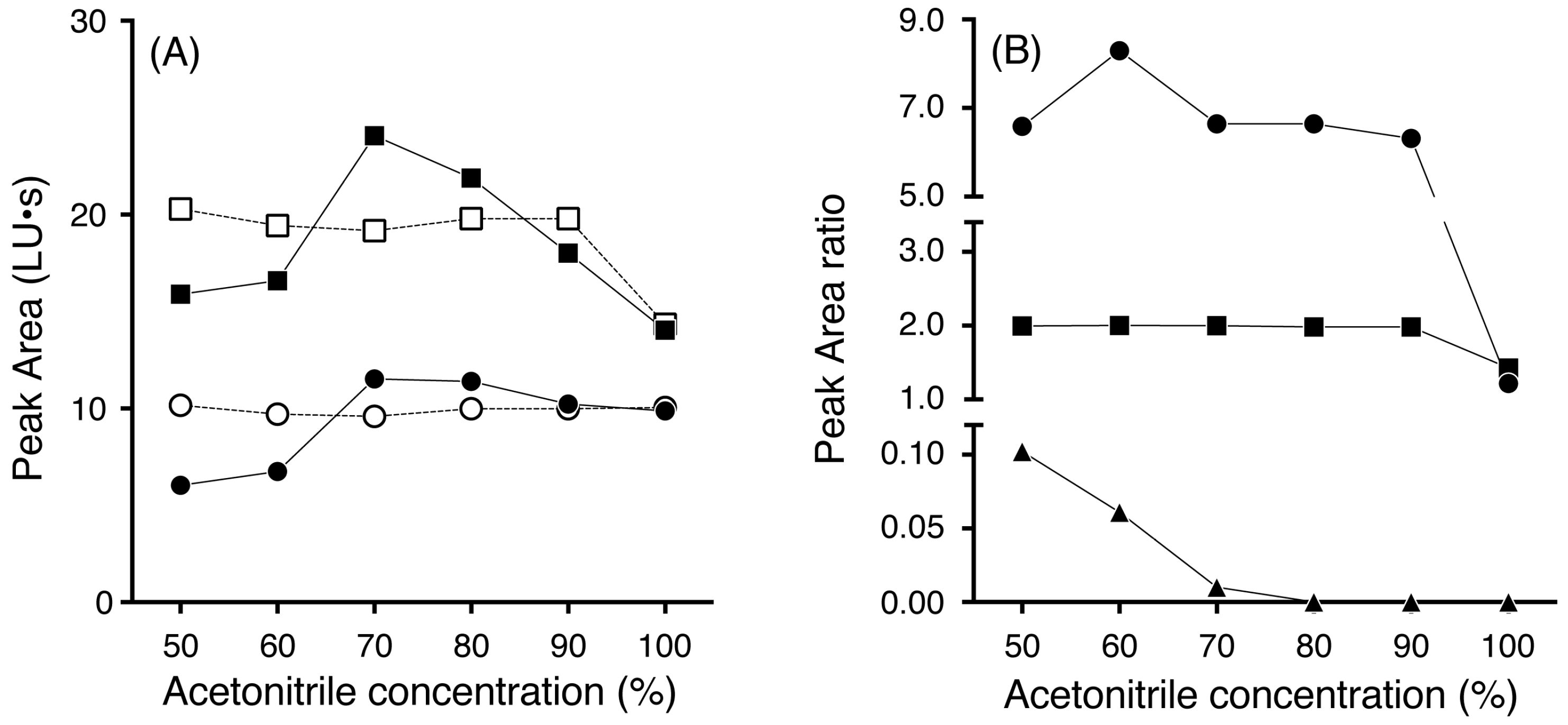
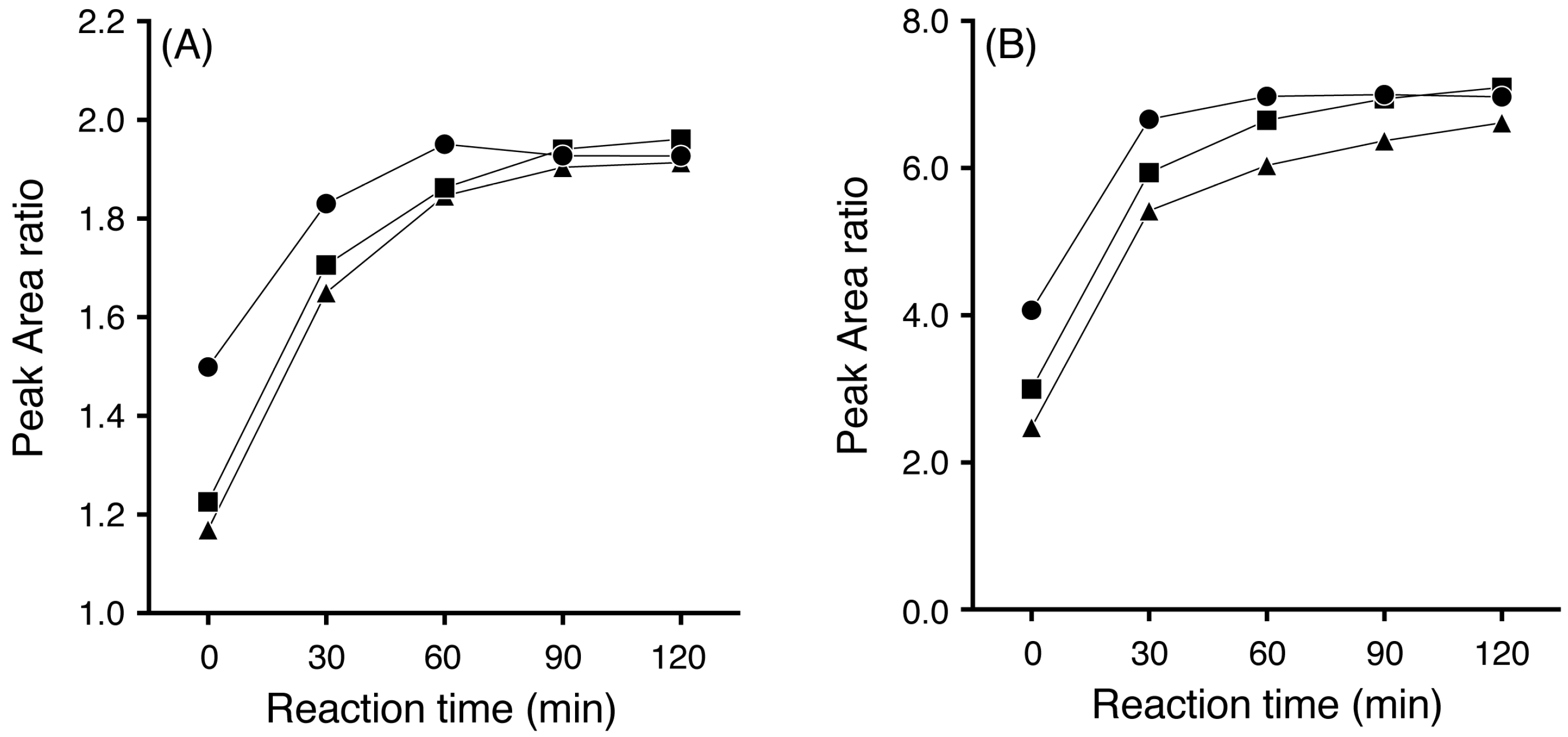
3.1. Effects of Acetonitrile Concentration and Reaction Time on Extraction and Reaction Efficiency
3.2. Effects of Trifluoroacetic Acid Concentration on Reaction Efficiency of DBD-H and Stability of DBD-TPC
3.3. Effect of pH on Stability of DBD-TPC
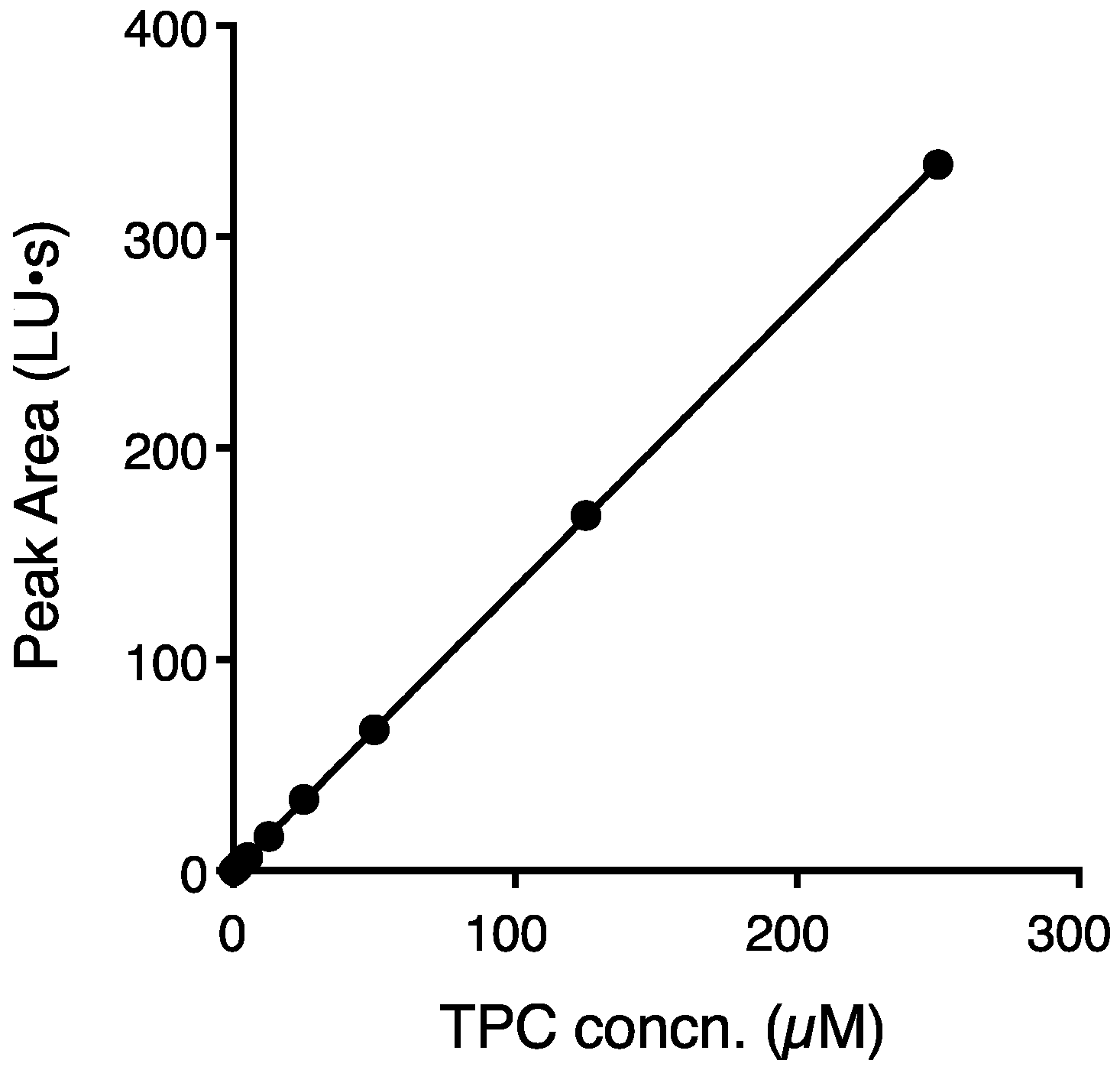
3.4. Verification of Sensitivity
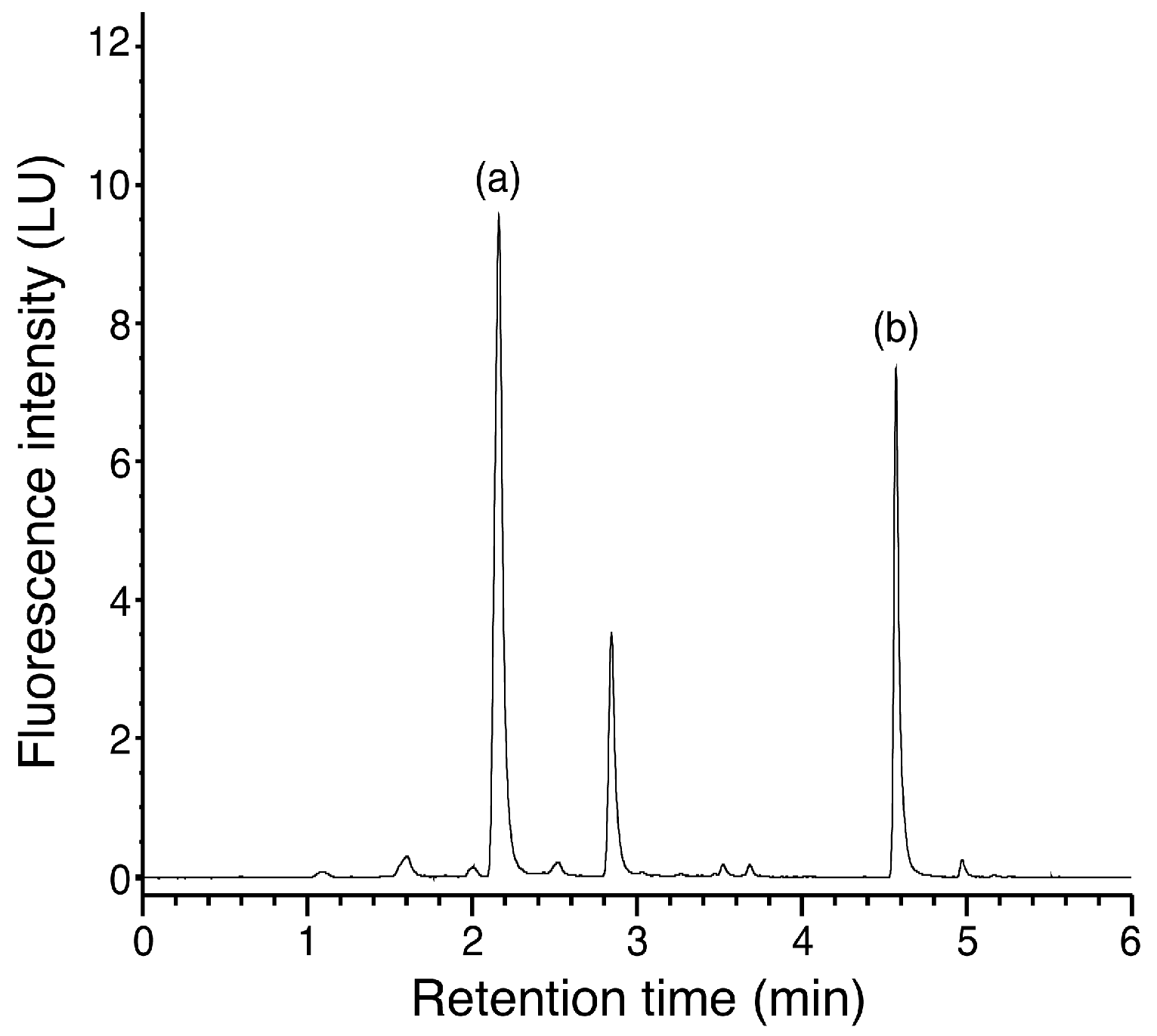
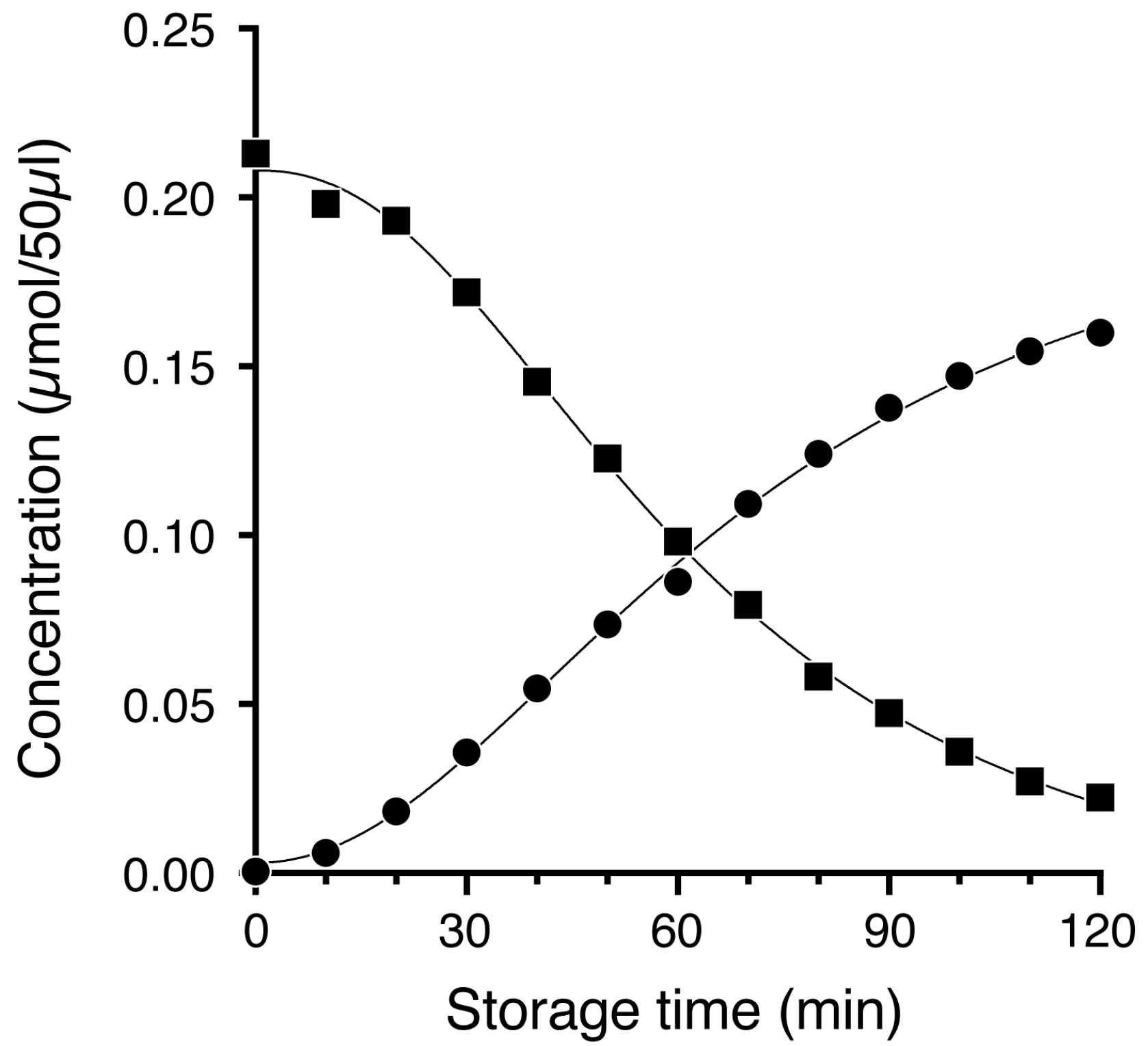
3.5. Quantification of TPC and MTB-ITC in Grated Radish Juice
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Friis, P.A.; Kjaer, A. 4-methylthio-3-butenyl isothiocyanate, the pungent principle of radish root. Acta Chem. Scand. 1966, 20, 698–705. [Google Scholar] [CrossRef]
- Kjr, A.; Madsen, J.O.; Maeda, Y.; Ozawa, Y.; Uda, Y. Volatiles in distillates of fresh radish of Japanese and Kenyan origin. Agric. Biol. Chem. 1978, 42, 1715–1721. [Google Scholar]
- Nakamura, Y.; Iwahashi, T.; Tanaka, A.; Koutani, J.; Matsuo, T.; Okamoto, S.; Sato, K.; Ohtsuki, K. 4-(methylthio)-3-butenyl isothiocyanate, a principal antimutagen in daikon (Raphanus sativus; Japanese white radish). J. Agric. Food Chem. 2001, 49, 5755–5760. [Google Scholar] [CrossRef] [PubMed]
- Okamura, T.; Umemura, T.; Inoue, T.; Tasaki, M.; Ishii, Y.; Nakamura, Y.; Park, E.Y.; Sato, K.; Matsuo, T.; Okamoto, S.; et al. Chemopreventive effects of 4-methylthio-3-butenyl isothiocyanate (Raphasatin) but not curcumin against pancreatic carcinogenesis in hamsters. J. Agric. Food Chem. 2013, 61, 2103–2108. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, M.; Omi, Y.; Fujii, N.; Ozaki, A.; Nakama, A.; Sakakibara, Y.; Suiko, M.; Nishiyama, K. Mustard oil in “Shibori Daikon” a variety of Japanese radish, selectively inhibits the proliferation of H-ras-transformed 3Y1 cells. Biosci. Biotechnol. Biochem. 2009, 73, 2217–2221. [Google Scholar] [CrossRef] [PubMed]
- Uda, Y.; Ozawa, Y.; Ohshima, T.; Kawakishi, S. Identification of enolated 2-thioxo-3-pyrrolidinecarbaldehyde, a new degradation product of 4-methylthio-3-butenyl isothiocyanate. Agric. Biol. Chem. 1990, 54, 613–617. [Google Scholar]
- Hashimoto, K.; Yanagi, K.; Fukushima, K.; Uda, Y. Effect of 3-hydroxymethylene-2-thioxopyrrolidine on growth of two species of mutans streptococci and their in vitro plaque formation. Int. J. Antimicrob. Agents 2001, 17, 97–102. [Google Scholar] [CrossRef]
- Uda, Y.; Matsuoka, H.; Shima, H.; Kumagami, H.; Maeda, Y. Antimicrobial activity of water-soluble products derived from radish mustard oil and identification of an active component therein. Nippon Shokuhin Kougyo Gakkaishi 1993, 40, 801–806. [Google Scholar] [CrossRef]
- Matsuoka, H.; Takahashi, A.; Yanagi, K.; Uda, Y. Antimicrobial action of 2-thioxo-3-pyrrolidinecarbardehyde, a major thiolactam compound generated from the pungent principle of radish in an aqueous medium. Food Sci. Technol. Int. Tokyo 1997, 3, 353–356. [Google Scholar] [CrossRef]
- Uda, Y.; Hayashi, H.; Takahashi, A.; Shimizu, A. Mutagenic and antimutagenic property of 3-hydroxymethylene-2-thioxopyrrolidine, a major product generating from pungent principle of radish. LWT-Food Sci. Technol. 2000, 33, 37–43. [Google Scholar] [CrossRef]
- Ozawa, Y.; Uda, Y.; Kawakishi, S. Generation of β-carboline derivative, the yellowish precursor of processed radish roots, from 4-methylthio-3-butenyl isothiocyanate and l-tryptophan. Agric. Biol. Chem. 1990, 54, 1849–1851. [Google Scholar]
- Matsuoka, H.; Takahashi, A.; Ozawa, Y.; Yamada, Y.; Uda, Y.; Kawakishi, S. 2-[3-(2-thioxopyrrolidin-3-ylidene)methyl]-tryptophan, a novel yellow pigment in salted radish roots. Biosci. Biotechnol. Biochem. 2002, 66, 1450–1454. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Yamada, T.; Uchiyama, Y.; Hayashi, S.; Kumakura, K.; Takahashi, H.; Kimura, N.; Matsuoka, H. Generation of the antioxidant yellow pigment derived from 4-methylthio-3-butenyl isothiocyanate in salted radish roots (takuan-zuke). Biosci. Biotechnol. Biochem. 2015, 79, 1512–1517. [Google Scholar] [CrossRef] [PubMed]
- Uzu, S.; Kanda, S.; Imai, K.; Nakashima, K.; Akiyama, S. Fluorogenic reagents: 4-aminosulphonyl-7-hydrazino-2,1,3-benzoxadiazole, 4-(N,N-dimethylaminosulphonyl)-7-hydrazino-2,1,3-benzoxadiazole and 4-hydrazino-7-nitro-2,1,3-benzoxadiazole hydrazine for aldehydes and ketones. Analyst 1990, 115, 1477–1482. [Google Scholar] [CrossRef]
- Matsuoka, H.; Uda, Y.; Mitani, K.; Yoneyama, K. Easy preparation method for 2-thioxopyrrolidine derivatives including 3-hydroxymethylene-2-thioxopyrrolidine, an antimicrobial degradation product of radish pungent principle, via (E,Z)-4-methoxy-3-butenyl isothiocyanate. Biosci. Biotechnol. Biochem. 1996, 60, 914–915. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobayashi, T.; Kumakura, K.; Kobayashi, W.; Takahashi, A.; Matsuoka, H. Determination of 2-Thioxo-3-pyrrolidinecarbaldehyde in Salted Radish Root (Takuan-zuke) by High-Performance Liquid Chromatography with Fluorescence Detection after Pre-Column Derivatization Using 4-(N,N-dimethylaminosulfonyl)-7-hydrazino-2,1,3-benzoxadiazole. Separations 2017, 4, 35. https://doi.org/10.3390/separations4040035
Kobayashi T, Kumakura K, Kobayashi W, Takahashi A, Matsuoka H. Determination of 2-Thioxo-3-pyrrolidinecarbaldehyde in Salted Radish Root (Takuan-zuke) by High-Performance Liquid Chromatography with Fluorescence Detection after Pre-Column Derivatization Using 4-(N,N-dimethylaminosulfonyl)-7-hydrazino-2,1,3-benzoxadiazole. Separations. 2017; 4(4):35. https://doi.org/10.3390/separations4040035
Chicago/Turabian StyleKobayashi, Taito, Kei Kumakura, Wataru Kobayashi, Asaka Takahashi, and Hiroki Matsuoka. 2017. "Determination of 2-Thioxo-3-pyrrolidinecarbaldehyde in Salted Radish Root (Takuan-zuke) by High-Performance Liquid Chromatography with Fluorescence Detection after Pre-Column Derivatization Using 4-(N,N-dimethylaminosulfonyl)-7-hydrazino-2,1,3-benzoxadiazole" Separations 4, no. 4: 35. https://doi.org/10.3390/separations4040035
APA StyleKobayashi, T., Kumakura, K., Kobayashi, W., Takahashi, A., & Matsuoka, H. (2017). Determination of 2-Thioxo-3-pyrrolidinecarbaldehyde in Salted Radish Root (Takuan-zuke) by High-Performance Liquid Chromatography with Fluorescence Detection after Pre-Column Derivatization Using 4-(N,N-dimethylaminosulfonyl)-7-hydrazino-2,1,3-benzoxadiazole. Separations, 4(4), 35. https://doi.org/10.3390/separations4040035




