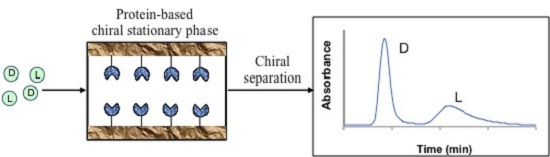
Chromatographic Studies of Protein-Based Chiral Separations
Abstract
:1. Introduction
2. Chromatographic Methods Used to Study Protein-Based Chiral Separations
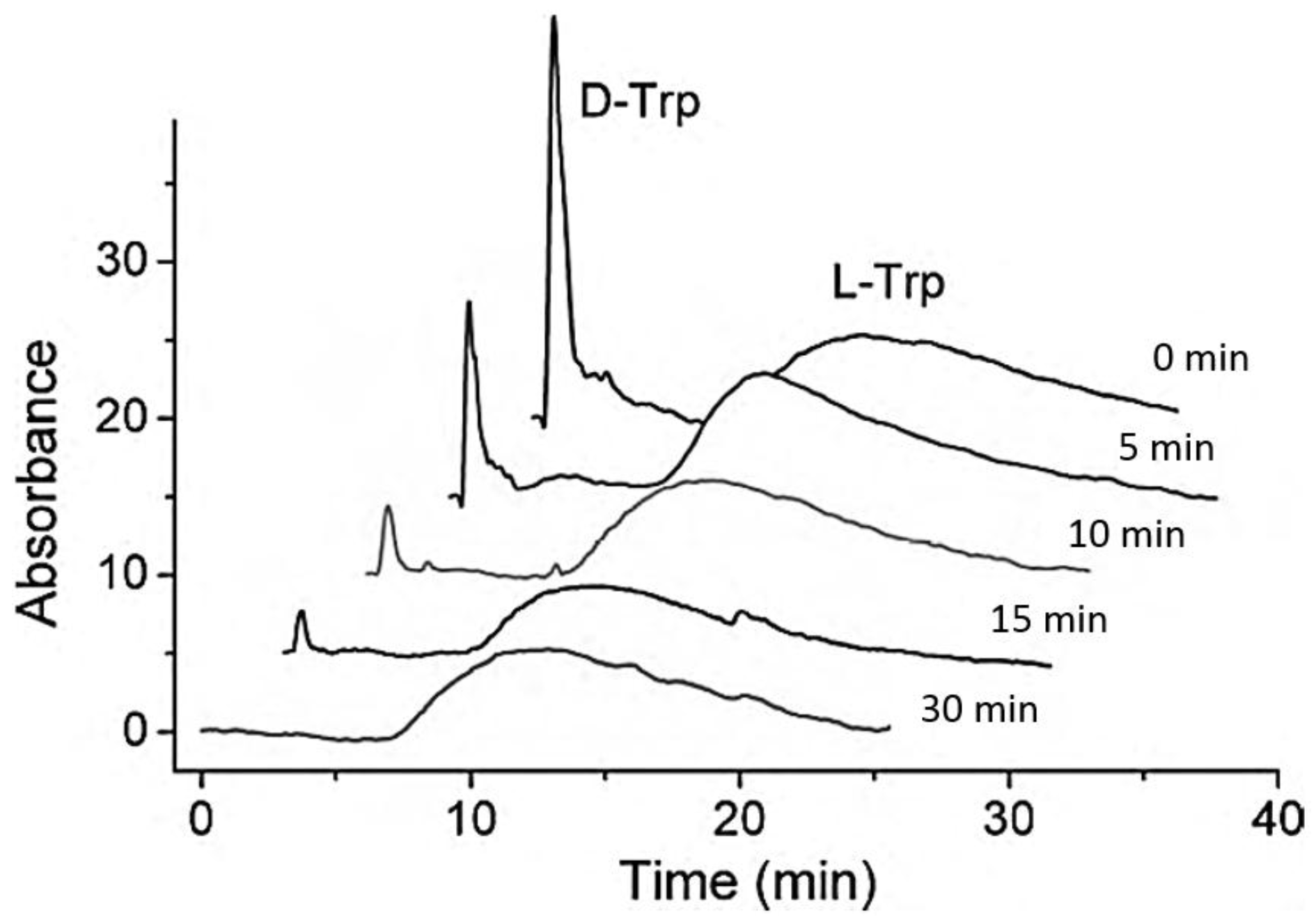
2.1. Zonal Elution
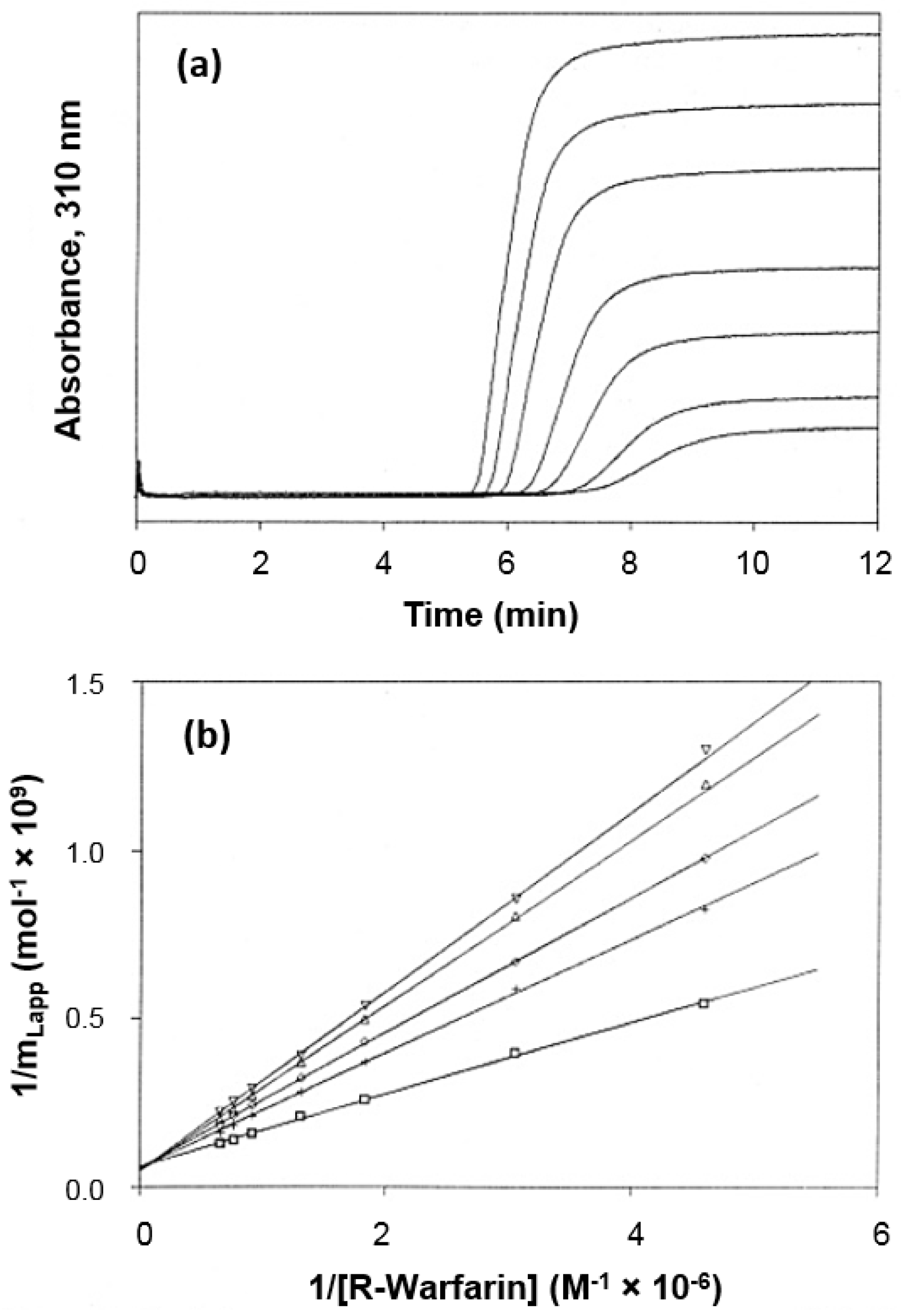
2.2. Frontal Analysis
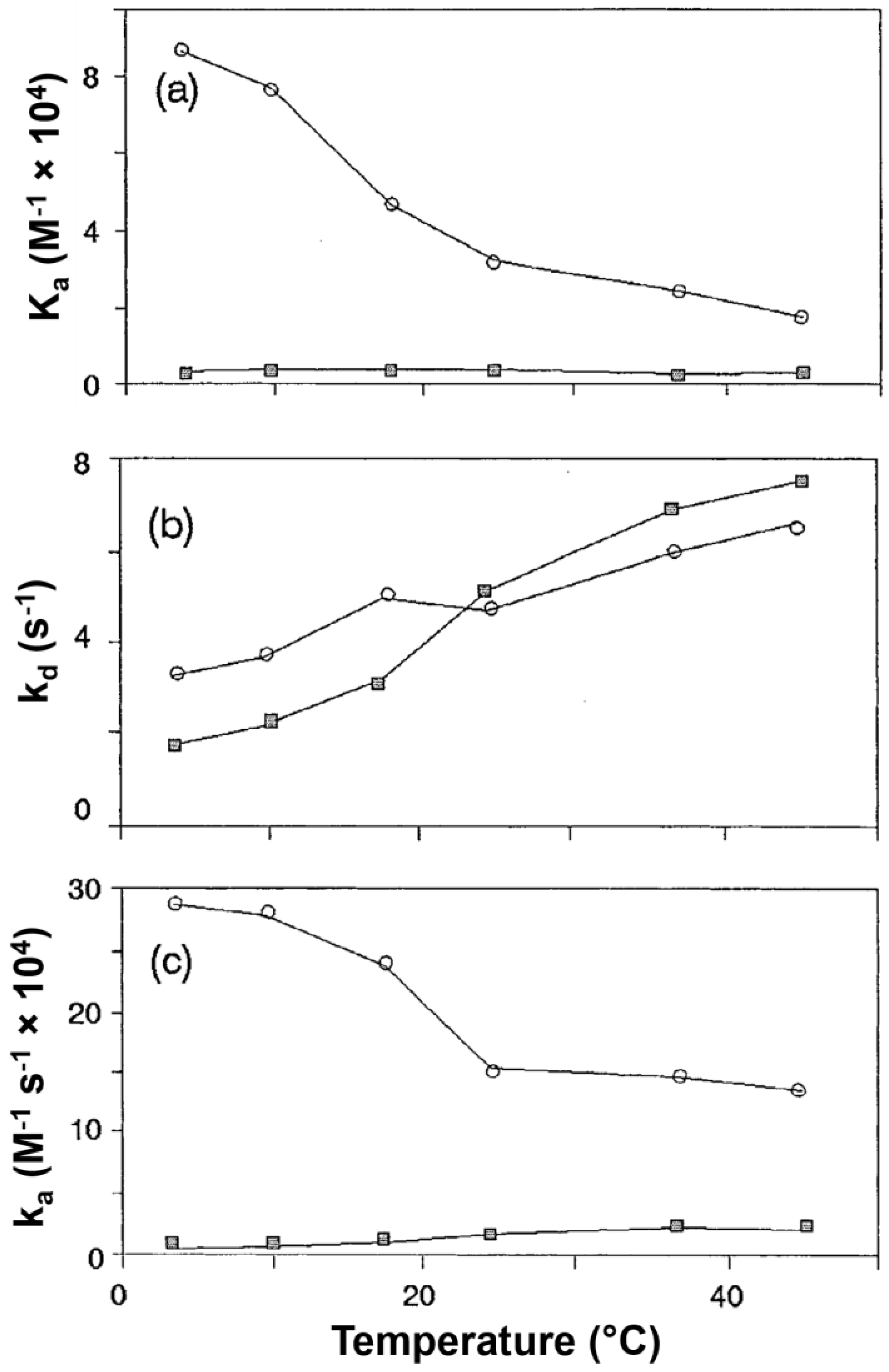
2.3. Kinetic Studies
3. Preparation of Protein-Based CSPs
3.1. Supports Used in Protein-Based CSPs
3.2. Development and Evaluation of Protein Immobilization Methods
3.2.1. Physical Adsorption
3.2.2. Covalent Immobilization
3.2.3. Encapsulation and Entrapment
4. Chromatographic Studies of Protein-Based CSPs
4.1. Serum Transport Proteins
4.1.1. Human Serum Albumin and Bovine Serum Albumin
4.1.2. Alpha1-Acid Glycoprotein
4.2. Enzymes
4.2.1. Penicillin G Acylase
4.2.2. Cellobiohydrolases
4.2.3. α-Chymotrypsin
4.3. Other Proteins
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pasteur, L. Mémoire sur la relation qui peut exister entre la forme cristalline et la composition chimique, et sur la cause de la polarisation rotatoire. C. R. Séances Acad. Sci. 1848, 26, 535–538. (In French) [Google Scholar]
- Drayer, D. The early history of stereochemistry. In Drug Stereochemistry, Analytical Methods and Pharmacology, 2nd ed.; Wainer, I.W., Ed.; Marcel Dekker: New York, NY, USA, 1993; pp. 5–14. [Google Scholar]
- Patel, S.; Wainer, I.W.; Lough, W.J. Affinity-based chiral stationary phases. In Handbook of Affinity Chromatography, 2nd ed.; Hage, D.S., Ed.; CRC Press: Boca Raton, FL, USA, 2006; pp. 571–592. [Google Scholar]
- Okamoto, Y. Helical polymers for efficient enantiomer separation. Adv. Polym. Sci. 2013, 261, 391–414. [Google Scholar]
- Hyun, M.H.; Cho, Y.J. Chiral Separation by HPLC with Pirkle-Type Chiral Stationary Phases. In Chiral Separations: Methods and Protocols; Gübitz, G., Schmid, M.G., Eds.; Humana Press: Totowa, NJ, USA, 2004; pp. 197–205. [Google Scholar]
- Subramanian, G. Chiral Separation Techniques: A Practical Approach; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar]
- He, Y. Chiral analysis in drug discovery and development. Innov. Pharm. Technol. 2010, 35, 18–23. [Google Scholar]
- Lammerhofer, M.; Lindner, W. Recent developments in liquid chromatographic enantioseparation. In Handbook of Analytical Separations, 2nd ed.; Valko, K., Ed.; Marcel Dekker: New York, NY, USA, 2000; Volume 1, pp. 337–437. [Google Scholar]
- Nie, Y.; Liu, X.; Yang, X.; Zhao, Z. Review: Recent application of chiral liquid chromatography-tandem mass spectrometric methods for enantiomeric pharmaceutical and biomedical determinations. J. Chromatogr. Sci. 2013, 51, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Kasprzyk-Hordern, B. Pharmacologically active compounds in the environment and their chirality. Chem. Soc. Rev. 2010, 39, 4466–4503. [Google Scholar] [CrossRef] [PubMed]
- Nagori, B.P.; Deora, M.S.; Saraswat, P. Chiral drug analysis and their application. Int. J. Pharm. Sci. Rev. Res. 2011, 6, 106–113. [Google Scholar]
- Nguyen, L.A.; He, H.; Pham-Huy, C. Chiral drugs: An overview. Int. J. Biomed. Sci. 2006, 2, 85–100. [Google Scholar] [PubMed]
- Ilisz, I.; Berkecz, R.; Peter, A. Application of chiral derivatizing agents in the high-performance liquid chromatographic separation of amino acid enantiomers: A review. J. Pharm. Biomed. Anal. 2008, 47, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B. Nature of chiral drugs and their occurrence in environment. J. Xenobiotics 2014, 4, 14–19. [Google Scholar] [CrossRef]
- Evans, S.E.; Kasprzyk-Hordern, B. Applications of chiral chromatography coupled with mass spectrometry in the analysis of chiral pharmaceuticals in the environment. Trends Environ. Anal. Chem. 2014, 1, e34–e51. [Google Scholar] [CrossRef]
- Anderson, J.; Berthod, A.; Pino, V.; Stalcup, A.M. Analytical Separation Science; Wiley: Hoboken, NJ, USA, 2016. [Google Scholar]
- Haginaka, J. Protein-based chiral stationary phases for high-performance liquid chromatography enantioseparations. J. Chromatogr. A 2001, 906, 253–273. [Google Scholar] [CrossRef]
- Millot, M.C. Separation of drug enantiomers by liquid chromatography and capillary electrophoresis, using immobilized proteins as chiral selectors. J. Chromatogr. B 2003, 797, 131–159. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, D.R.; Wang-Iverson, D.B.; Tymiak, A.A. Enantioselective chromatography in drug discovery. Drug Discov. Today 2005, 10, 571–577. [Google Scholar] [CrossRef]
- Haginaka, J. Recent progresses in protein-based chiral stationary phases for enantioseparations in liquid chromatography. J. Chromatogr. B 2008, 875, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Welch, C.J. Microscale chiral HPLC in support of pharmaceutical process research. Chirality 2009, 21, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Allenmark, S. Chromatographic Enantioseparation: Methods and Applications, 2nd ed.; Ellis Horwood: New York, NY, USA, 1991. [Google Scholar]
- Calleri, E.; Temporini, C.; Massolini, G.; Caccialanza, G. Penicillin G acylase-based stationary phases: Analytical applications. J. Pharm. Biomed. Anal. 2004, 35, 243–258. [Google Scholar] [CrossRef]
- Montes, T.; Grazu, V.; Lopez-Gallego, F.; Hermoso, J.A.; Garcia, J.L.; Manso, I.; Galan, B.; Gonzalez, R.; Fernandez-Lafuente, R.; Guisan, J.M. Genetic modification of the penicillin G acylase surface to improve its reversible immobilization on ionic exchangers. Appl. Environ. Microbiol. 2007, 73, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Prin, C.; Bene, M.C.; Gobert, B.; Montagne, P.; Faure, G.C. Isoelectric Restriction of Human-Immunoglobulin Isotypes. BBA Gen. Subj. 1995, 1243, 287–289. [Google Scholar] [CrossRef]
- Weber, P.C.; Ohlendorf, D.H.; Wendoloski, J.J.; Salemme, F.R. Structural origins of high-affinity biotin binding to streptavidin. Science 1989, 243, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Berthod, A. Chiral Recognition in Separation Methods. Mechanisms and Applications; Springer-Verlag: Berlin, Germany, 2010. [Google Scholar]
- Haginaka, J.; Seyama, C.; Yasuda, H.; Takahashi, K. Investigation of enantioselectivity and enantiomeric elution order of propranolol and its ester derivatives on an ovomucoid-bonded column. J. Chromatogr. A 1992, 598, 67–72. [Google Scholar] [CrossRef]
- Zhai, Z.; Chen, Y.; Wang, Y.J.; Luo, G.S. Chiral separation performance of micrometer-sized monodispersed silica spheres with high protein loading. Chirality 2009, 21, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Chaiken, I.M. Analytical Affinity Chromatography; CRC Press: Boca Raton, FL, USA, 1987. [Google Scholar]
- Hage, D.S. Chromatographic and electrophoretic studies of protein binding to chiral solutes. J. Chromatogr. A 2001, 906, 459–481. [Google Scholar] [CrossRef]
- Hage, D.S.; Chen, J. Quantitative affinity chromatography: Practical aspects. In Handbook of Affinity Chromatography, 2nd ed.; Hage, D.S., Ed.; CRC Press: Boca Raton, FL, USA, 2006; pp. 595–628. [Google Scholar]
- Kim, H.S.; Mallik, R.; Hage, D.S. Chromatographic analysis of carbamazepine binding to human serum albumin: II. Comparison of the Schiff base and N-hydroxysuccinimide immobilization methods. J. Chromatogr. B 2006, 837, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Xuan, H.; Hage, D.S. Immobilization of α1-acid glycoprotein for chromatographic studies of drug-protein binding. Anal. Biochem. 2005, 346, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Mallik, R.; Wa, C.; Hage, D.S. Development of sulfhydryl-reactive silica for protein immobilization in high-performance affinity chromatography. Anal. Chem. 2007, 79, 1411–1424. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, R.; Li, Z.; Zheng, X.; Hage, D.S. Analysis of multi-site drug-protein interactions by high-performance affinity chromatography: Binding by glimepiride to normal or glycated human serum albumin. J. Chromatogr. A 2015, 1408, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, R.; Li, Z.; Zheng, X.; Hage, D.S. Analysis of glipizide binding to normal and glycated human serum albumin by high-performance affinity chromatography. Anal. Bioanal. Chem. 2015, 407, 5309–5321. [Google Scholar] [CrossRef] [PubMed]
- Arnold, F.H.; Schofield, S.A.; Blanch, H.W. Analytical Affinity-Chromatography: I. Local Equilibrium-Theory and the Measurement of Association and Inhibition Constants. J. Chromatogr. A 1986, 355, 1–12. [Google Scholar] [CrossRef]
- Vidalmadjar, C.; Jaulmes, A.; Racine, M.; Sebille, B. Determination of Binding Equilibrium-Constants by Numerical-Simulation in Zonal High-Performance Affinity-Chromatography. J. Chromatogr. A 1988, 458, 13–25. [Google Scholar] [CrossRef]
- Hage, D.S.; Tweed, S.A. Recent advances in chromatographic and electrophoretic methods for the study of drug-protein interactions. J. Chromatogr. B 1997, 699, 499–525. [Google Scholar] [CrossRef]
- Haginaka, J.; Kanasugi, N. Enantioselectivity of bovine serum albumin-bonded columns produced with isolated protein fragments. II. Characterization of protein fragments and chiral binding sites. J. Chromatogr. A 1997, 769, 215–223. [Google Scholar] [CrossRef]
- Kaliszan, R. QSRR: Quantitative structure-(chromatographic) retention relationships. Chem. Rev. 2007, 107, 3212–3246. [Google Scholar] [CrossRef] [PubMed]
- Kaliszan, R.; Noctor, T.A.G.; Wainer, I.W. Stereochemical aspects of benzodiazepine binding to human serum albumin. II. Quantitative relationships between structure and enantioselective retention in high performance liquid affinity chromatography. Mol. Pharmacol. 1992, 42, 512–517. [Google Scholar] [PubMed]
- Hage, D.S.; Noctor, T.A.G.; Wainer, I.W. Characterization of the protein-binding of chiral drugs by high-performance affinity chromatography. Interactions of R-ibuprofen and S-ibuprofen with human serum-albumin. J. Chromatogr. A 1995, 693, 23–32. [Google Scholar] [CrossRef]
- Yang, J.; Hage, D.S. Characterization of the binding and chiral separation of d- and l-tryptophan on a high-performance immobilized human serum albumin column. J. Chromatogr. A 1993, 645, 241–250. [Google Scholar] [CrossRef]
- Hage, D.S. High-performance affinity chromatography: A powerful tool for studying serum protein binding. J. Chromatogr. B 2002, 768, 3–30. [Google Scholar] [CrossRef]
- Sengupta, A.; Hage, D.S. Characterization of minor site probes for human serum albumin by high-performance affinity chromatography. Anal. Chem. 1999, 71, 3821–3827. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Z.; Fitos, I.; Hage, D.S. Chromatographic analysis of allosteric effects between ibuprofen and benzodiazepines on human serum albumin. Chirality 2006, 18, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Z.; Hage, D.S. Quantitative analysis of allosteric drug-protein binding by biointeraction chromatography. Nat. Biotechnol. 2004, 22, 1445–1448. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Z.; Hage, D.S. Quantitative studies of allosteric effects by biointeraction chromatography: Analysis of protein binding for low-solubility drugs. Anal. Chem. 2006, 78, 2672–2683. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Z.; Ohnmacht, C.; Hage, D.S. Studies of phenytoin binding to human serum albumin by high-performance affinity chromatography. J. Chromatogr. B 2004, 809, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Noctor, T.A.; Wainer, I.W.; Hage, D.S. Allosteric and competitive displacement of drugs from human serum albumin by octanoic acid, as revealed by high-performance liquid affinity chromatography, on a human serum albumin-based stationary phase. J. Chromatogr. A 1992, 577, 305–315. [Google Scholar] [CrossRef]
- Yang, J.; Hage, D.S. Effect of mobile phase composition on the binding kinetics of chiral solutes on a protein-based high-performance liquid chromatography column: Interactions of d- and l-tryptophan with immobilized human serum albumin. J. Chromatogr. A 1997, 766, 15–25. [Google Scholar] [CrossRef]
- Mallik, R.; Xuan, H.; Hage, D.S. Development of an affinity silica monolith containing alpha1-acid glycoprotein for chiral separations. J. Chromatogr. A 2007, 1149, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Yoo, M.J.; Hage, D.S. Analysis of free fractions for chiral drugs using ultrafast extraction and multi-dimensional high-performance affinity chromatography. Analyst 2013, 138, 6262–6265. [Google Scholar] [CrossRef] [PubMed]
- Pfaunmiller, E.L.; Hartmann, M.; Dupper, C.M.; Soman, S.; Hage, D.S. Optimization of human serum albumin monoliths for chiral separations and high-performance affinity chromatography. J. Chromatogr. A 2012, 1269, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Hage, D.S. Role of binding capacity versus binding strength in the separation of chiral compounds on protein-based high-performance liquid chromatography columns. Interactions of d- and l-tryptophan with human serum albumin. J. Chromatogr. A 1996, 725, 273–285. [Google Scholar] [CrossRef]
- Xuan, H.; Hage, D.S. Evaluation of a hydrazide-linked α1-acid glycoprotein chiral stationary phase: Separation of R- and S-propranolol. J. Sep. Sci. 2006, 29, 1412–1422. [Google Scholar] [CrossRef] [PubMed]
- Mallik, R.; Hage, D.S. Development of an affinity silica monolith containing human serum albumin for chiral separations. J. Pharm. Biomed. Anal. 2008, 46, 820–830. [Google Scholar] [CrossRef] [PubMed]
- Hage, D.S.; Anguizola, J.; Barnaby, O.; Jackson, A.; Yoo, M.J.; Papastavros, E.; Pfaunmiller, E.; Sobansky, M.; Tong, Z. Characterization of drug interactions with serum proteins by using high-performance affinity chromatography. Curr. Drug Metab. 2011, 12, 313–328. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Li, Z.; Beeram, S.; Podariu, M.; Matsuda, R.; Pfaunmiller, E.L.; White, C.I.; Carter, N.; Hage, D.S. Analysis of biomolecular interactions using affinity microcolumns: A review. J. Chromatogr. B 2014, 968, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, S.C.; Andersson, S.; Allenmark, S.G.; Guiochon, G. Estimation of the Number of Enantioselective Sites of Bovine Serum-Albumin Using Frontal Chromatography. Chirality 1993, 5, 513–515. [Google Scholar] [CrossRef] [PubMed]
- Tweed, S.A.; Loun, B.; Hage, D.S. Effects of ligand heterogeneity in the characterization of affinity columns by frontal analysis. Anal. Chem. 1997, 69, 4790–4798. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, A.; Tian, T.; Kortum, L.; Hage, D.S. Development of tryptophan-modified human serum albumin columns for site-specific studies of drug-protein interactions by high-performance affinity chromatography. J. Chromatogr. B 1998, 715, 183–190. [Google Scholar] [CrossRef]
- Loun, B.; Hage, D.S. Chiral separation mechanisms in protein-based HPLC columns. 1. Thermodynamic studies of (R)- and (S)-warfarin binding to immobilized human serum albumin. Anal. Chem. 1994, 66, 3814–3822. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Sobansky, M.R.; Hage, D.S. Analysis of drug interactions with high-density lipoprotein by high-performance affinity chromatography. Anal. Biochem. 2010, 397, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Sobansky, M.R.; Hage, D.S. Identification and analysis of stereoselective drug interactions with low-density lipoprotein by high-performance affinity chromatography. Anal. Bioanal. Chem. 2012, 403, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Sobansky, M.R.; Hage, D.S. Analysis of drug interactions with very low density lipoprotein by high-performance affinity chromatography. Anal. Bioanal. Chem. 2014, 406, 6203–6211. [Google Scholar] [CrossRef] [PubMed]
- Sobansky, M.R.; Hage, D.S. Analysis of drug interactions with lipoproteins by high-performance affinity chromatography. Adv. Med. Biol. 2012, 53, 199–216. [Google Scholar] [PubMed]
- Chen, J.; Schiel, J.E.; Hage, D.S. Noncompetitive peak decay analysis of drug-protein dissociation by high-performance affinity chromatography. J. Sep. Sci. 2009, 32, 1632–1641. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.; Hage, D.S. Characterization of interaction kinetics between chiral solutes and human serum albumin by using high-performance affinity chromatography and peak profiling. J. Chromatogr. A 2011, 1218, 6892–6897. [Google Scholar] [CrossRef] [PubMed]
- Loun, B.; Hage, D.S. Chiral separation mechanisms in protein-based HPLC columns. 2. Kinetic studies of (R)- and (S)-warfarin binding to immobilized human serum albumin. Anal. Chem. 1996, 68, 1218–1225. [Google Scholar] [CrossRef] [PubMed]
- Bi, C.; Beeram, S.; Li, Z.; Zheng, X.; Hage, D.S. Kinetic analysis of drug-protein interactions by affinity chromatography. Drug Discov. Today Technol. 2015, 17, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Schiel, J.E.; Ohnmacht, C.M.; Hage, D.S. Measurement of drug-protein dissociation rates by high-performance affinity chromatography and peak profiling. Anal. Chem. 2009, 81, 4320–4333. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.; Schiel, J.E.; Papastavros, E.; Ohnmacht, C.M.; Smith, Q.R.; Hage, D.S. Kinetic studies of drug-protein interactions by using peak profiling and high-performance affinity chromatography: Examination of multi-site interactions of drugs with human serum albumin columns. J. Chromatogr. A 2011, 1218, 2065–2071. [Google Scholar] [CrossRef] [PubMed]
- Schiel, J.E.; Hage, D.S. Kinetic studies of biological interactions by affinity chromatography. J. Sep. Sci. 2009, 32, 1507–1522. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Bi, C.; Li, Z.; Podariu, M.; Hage, D.S. Analytical methods for kinetic studies of biological interactions: A review. J. Pharm. Biomed. Anal. 2015, 113, 163–180. [Google Scholar] [CrossRef] [PubMed]
- Yoo, M.J.; Hage, D.S. High-throughput analysis of drug dissociation from serum proteins using affinity silica monoliths. J. Sep. Sci. 2011, 34, 2255–2263. [Google Scholar] [CrossRef] [PubMed]
- Yoo, M.J.; Hage, D.S. Use of peak decay analysis and affinity microcolumns containing silica monoliths for rapid determination of drug-protein dissociation rates. J. Chromatogr. A 2011, 1218, 2072–2078. [Google Scholar] [CrossRef] [PubMed]
- Stewart, K.K.; Doherty, R.F. Resolution of dl-tryptophan by affinity chromatography on bovine-serum albumin-agarose columns. Proc. Natl. Acad. Sci. USA 1973, 70, 2850–2852. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, X.; Ji, Y.B. Monoliths with proteins as chiral selectors for enantiomer separation. Talanta 2012, 91, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.B.; Wu, R.A.; Wu, M.H.; Zou, H.F. Recent progress of chiral monolithic stationary phases in CEC and capillary LC. Electrophoresis 2010, 31, 1457–1466. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Matsumoto, N.; Sakai-Kato, K.; Toyo’oka, T. Investigation of chromatographic performances and binding characteristics of BSA-encapsulated capillary column prepared by the sol-gel method. J. Pharm. Biomed. Anal. 2003, 30, 1845–1850. [Google Scholar] [CrossRef]
- Kato, M.; Sakai-Kato, K.; Matsumoto, N.; Toyo’oka, T. A protein-encapsulation technique by the sol-gel method for the preparation of monolithic columns for capillary electrochromatography. Anal. Chem. 2002, 74, 1915–1921. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Saruwatari, H.; Sakai-Kato, K.; Toyo’oka, T. Silica sol-gel/organic hybrid material for protein encapsulated column of capillary electrochromatography. J. Chromatogr. A 2004, 1044, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Sakai-Kato, K.; Kato, M.; Nakakuki, H.; Toyo’oka, T. Investigation of structure and enantioselectivity of BSA-encapsulated sol-gel columns prepared for capillary electrochromatography. J. Pharm. Biomed. Anal. 2003, 31, 299–309. [Google Scholar] [CrossRef]
- Yao, C.; Qi, L.; Qiao, J.; Zhang, H.; Wang, F.; Chen, Y.; Yang, G. High-performance affinity monolith chromatography for chiral separation and determination of enzyme kinetic constants. Talanta 2010, 82, 1332–1337. [Google Scholar] [CrossRef] [PubMed]
- Sinner, F.M.; Buchmeiser, M.R. Ring-opening metathesis polymerization: Access to a new class of functionalized, monolithic stationary phases for liquid chromatography. Angew. Chem. Int. Ed. Engl. 2000, 39, 1433–1436. [Google Scholar] [CrossRef]
- Mohammad, J.; Li, Y.-M.; El-Ahmad, M.; Nakazato, K.; Pettersson, G.; Hjertéean, S. Chiral-recognition chromatography of β-blockers on continuous polymer beds with immobilized cellulase as enantioselective protein. Chirality 1993, 5, 464–470. [Google Scholar] [CrossRef]
- Mallik, R.; Hage, D.S. Affinity monolith chromatography. J. Sep. Sci. 2006, 29, 1686–1704. [Google Scholar] [CrossRef] [PubMed]
- Yoo, M.J.; Hage, D.S. Affinity monolith chromatography: Principles and recent developments. In Monolithic Chromatography and Its Modern Applications; Wang, P.G., Ed.; ILM Publications: St. Albans, UK, 2010; pp. 3–25. [Google Scholar]
- Pfaunmiller, E.L.; Paulemond, M.L.; Dupper, C.M.; Hage, D.S. Affinity monolith chromatography: A review of principles and recent analytical applications. Anal. Bioanal. Chem. 2013, 405, 2133–2145. [Google Scholar] [CrossRef] [PubMed]
- Yoo, M.J.; Hage, D.S. Evaluation of silica monoliths in affinity microcolumns for high-throughput analysis of drug-protein interactions. J. Sep. Sci. 2009, 32, 2776–2785. [Google Scholar] [CrossRef] [PubMed]
- Mallik, R.; Jiang, T.; Hage, D.S. High-performance affinity monolith chromatography: Development and evaluation of human serum albumin columns. Anal. Chem. 2004, 76, 7013–7022. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yu, Q.W.; Lin, B.; Feng, Y.Q. Synthesis and chromatographic properties of a chiral stationary phase derived from bovine serum albumin immobilized on magnesia-zirconia using phosphonate spacers. J. Sep. Sci. 2007, 30, 804–812. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Ryu, J.K.; Park, J.K.; McNeff, C.V.; Carr, P.W. Separation of enantiomers on bovine serum albumin coated zirconia in reversed-phase liquid chromatography. Chromatographia 2001, 53, 405–408. [Google Scholar] [CrossRef]
- Lammerhofer, M. Chiral recognition by enantioselective liquid chromatography: Mechanisms and modern chiral stationary phases. J. Chromatogr. A 2010, 1217, 814–856. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zou, H.F.; Ni, J.Y.; Zhang, Y.K. Open tubular capillary electrochromatography with adsorbed stationary phase. Anal. Chim. Acta 1999, 378, 73–76. [Google Scholar] [CrossRef]
- Liu, Z.; Otsuka, K.; Terabe, S. Chiral separation by open tubular capillary electrochromatography with adsorbed avidin as a stationary phase. J. Sep. Sci. 2001, 24, 17–26. [Google Scholar] [CrossRef]
- Liu, Z.; Otsuka, K.; Terabe, S.; Motokawa, M.; Tanaka, N. Physically adsorbed chiral stationary phase of avidin on monolithic silica column for capillary electrochromatography and capillary liquid chromatography. Electrophoresis 2002, 23, 2973–2981. [Google Scholar] [CrossRef]
- Nakamura, M.; Kiyohara, S.; Saito, K.; Sugita, K.; Sugo, T. Chiral separation of dl-tryptophan using porous membranes containing multilayered bovine serum albumin crosslinked with glutaraldehyde. J. Chromatogr. A 1998, 822, 53–58. [Google Scholar] [CrossRef]
- Taleb, N.L.; Millot, M.C.; Sebille, B. Enantioselectivity properties of human serum albumin immobilized on anion-exchangers based on polyvinylimidazole-coated silica—Effect of protein loading on separation properties. J. Chromatogr. A 1997, 776, 45–53. [Google Scholar] [CrossRef]
- Millot, M.C.; Servagent-Noinville, S.; Taleb, N.L.; Baron, M.H.; Revault, M.; Sebille, B. Structural changes of human serum albumin immobilized on chromatographic supports: A high-performance liquid chromatography and Fourier-transform infrared spectroscopy study. J. Chromatogr. B 2001, 753, 101–113. [Google Scholar] [CrossRef]
- Harada, K.; Yuan, Q.; Nakayama, M.; Sugii, A. Effects of organic modifiers on the chiral recognition by different types of silica-immobilized bovine serum albumin. J. Chromatogr. A 1996, 740, 207–213. [Google Scholar] [CrossRef]
- Marle, I.; Karlsson, A.; Pettersson, C. Separation of enantiomers using α-chymotrypsin-silica as a chiral stationary phase. J. Chromatogr. A 1992, 604, 185–196. [Google Scholar] [CrossRef]
- Han, N.Y.; Hautala, J.T.; Bo, T.; Wiedmer, S.K.; Riekkola, M.L. Immobilization of phospholipid-avidin on fused-silica capillaries for chiral separation in open-tubular capillary electrochromatography. Electrophoresis 2006, 27, 1502–1509. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Hage, D.S. Immobilization methods for affinity chromatography. In Handbook of Affinity Chromatography, 2nd ed.; Hage, D.S., Ed.; CRC Press: Boca Raton, FL, USA, 2006; pp. 35–78. [Google Scholar]
- Henriksson, H.; Munoz, I.G.; Isaksson, R.; Pettersson, G.; Johansson, G. Cellobiohydrolase 58 (P.c. Cel 7D) is complementary to the homologous CBH I (T.r. Cel 7A) in enantioseparations. J. Chromatogr. A 2000, 898, 63–74. [Google Scholar] [CrossRef]
- Kim, H.S.; Kye, Y.S.; Hage, D.S. Development and evaluation of N-hydroxysuccinimide-activated silica for immobilizing human serum albumin in liquid chromatography columns. J. Chromatogr. A 2004, 1049, 51–61. [Google Scholar] [CrossRef]
- Nystrom, A.; Strandberg, A.; Aspegren, A.; Behr, S.; Karlsson, A. Use of immobilized amyloglucosidase as chiral selector in chromatography. Immobilization and performance in liquid chromatography. Chromatographia 1999, 50, 209–214. [Google Scholar] [CrossRef]
- Hage, D.S.; Austin, J. High-performance affinity chromatography and immobilized serum albumin as probes for drug- and hormone-protein binding. J. Chromatogr. B 2000, 739, 39–54. [Google Scholar] [CrossRef]
- Tittelbach, V.; Gilpin, R.K. Species Dependency of the Liquid-Chromatographic Properties of Silica-Immobilized Serum Albumins. Anal. Chem. 1995, 67, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Koidl, J.; Hodl, H.; Schmid, M.G.; Konrad, M.; Petschauer, S.; Kostner, G.M.; Gubitz, G. Chiral separation of T3 enantiomers using stereoselective antibodies as a selector in micro-HPLC. J. Biochem. Biophys. Methods 2006, 69, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Lua, A.C.; Chou, T.Y. Preparation of immunoaffinity columns for direct enantiomeric separation of amphetamine and/or methamphetamine. J. Chromatogr. A 2002, 967, 191–199. [Google Scholar] [CrossRef]
- Oda, Y.; Asakawa, N.; Abe, S.; Yoshida, Y.; Sato, T. Avidin protein-conjugated column for direct injection analysis of drug enantiomers in plasma by high-performance liquid chromatography. J. Chromatogr. B 1991, 572, 133–141. [Google Scholar] [CrossRef]
- Oda, Y.; Ohe, H.; Asakawa, N.; Yoshida, Y.; Sato, T.; Nakagawa, T. Resolution of 1-benzyl-4-[(5,6-dimethoxy-1-indanon)-2-yl] methylpiperidine hydrochloride enantiomers in plasma by high-performance liquid-chromatography with direct injection into avidin-conjugated column. J. Liq. Chromatogr. 1992, 15, 2997–3012. [Google Scholar] [CrossRef]
- Haginaka, J.; Seyama, C.; Murashima, T.; Fujima, H.; Wada, H. Retention and Enantioselective Properties of Ovomucoid-Bonded Silica Columns—Influence of Physical-Properties of Base Materials and Spacer Length. J. Chromatogr. A 1994, 660, 275–281. [Google Scholar] [CrossRef]
- Pinkerton, T.C.; Howe, W.J.; Ulrich, E.L.; Comiskey, J.P.; Haginaka, J.; Murashima, T.; Walkenhorst, W.F.; Westler, W.M.; Markley, J.L. Protein binding chiral discrimination of HPLC stationary phases made with whole, fragmented, and third domain turkey ovomucoid. Anal. Chem. 1995, 67, 2354–2367. [Google Scholar] [CrossRef] [PubMed]
- Domenici, E.; Bertucci, C.; Salvadori, P.; Felix, G.; Cahagne, I.; Motellier, S.; Wainer, I.W. Synthesis and Chromatographic Properties of an HPLC Chiral Stationary Phase Based Upon Human Serum-Albumin. Chromatographia 1990, 29, 170–176. [Google Scholar] [CrossRef]
- Felix, G.; Liu, M. New method for grafting proteins on silica gel. Biol. Sci. 1989, 8, 2–6. [Google Scholar]
- Felix, G.; Descorps, V. Stereochemical resolution of racemates, in HPLC, using a chiral stationary phase based upon immobilized α-chymotrypsin. I. Structural chiral separations. Chromatographia 1999, 49, 595–605. [Google Scholar] [CrossRef]
- Marle, I.; Jonsson, S.; Isaksson, R.; Pettersson, C.; Pettersson, G. Chiral stationary phases based on intact and fragmented cellobiohydrolase-I immobilized on silica. J. Chromatogr. A 1993, 648, 333–347. [Google Scholar] [CrossRef]
- Massolini, G.; Calleri, E.; De Lorenzi, E.; Pregnolato, M.; Terreni, M.; Felix, G.; Gandini, C. Immobilized penicillin G acylase as reactor and chiral selector in liquid chromatography. J. Chromatogr. A 2001, 921, 147–160. [Google Scholar] [CrossRef]
- Bi, C.; Jackson, A.; Vargas-Badilla, J.; Li, R.; Rada, G.; Anguizola, J.; Pfaunmiller, E.; Hage, D.S. Entrapment of alpha1-acid glycoprotein in high-performance affinity columns for drug-protein binding studies. J. Chromatogr. B 2016, 1021, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.J.; Anguizola, J.; Pfaunmiller, E.L.; Hage, D.S. Use of entrapment and high-performance affinity chromatography to compare the binding of drugs and site-specific probes with normal and glycated human serum albumin. Anal. Bioanal. Chem. 2013, 405, 5833–5841. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.J.; Xuan, H.; Hage, D.S. Entrapment of proteins in glycogen-capped and hydrazide-activated supports. Anal. Biochem. 2010, 404, 106–108. [Google Scholar] [CrossRef] [PubMed]
- Peters, T., Jr. All About Albumin: Biochemistry, Genetics, and Medical Applications; Academic Press: San Diego, CA, USA, 1996. [Google Scholar]
- Wainer, I.W. Drug Stereochemistry: Analytical Methods and Pharmacology, 2nd ed.; Marcel Dekker: New York, NY, USA, 1993. [Google Scholar]
- Losso, J.N.; Vanderstoep, J.; Nakai, S. Removal of bovine serum albumin from cow’s milk using chicken egg-yolk antibodies immobilized on chitosan gel. Food Agric. Immunol. 1998, 10, 47–56. [Google Scholar] [CrossRef]
- Ascoli, G.A.; Domenici, E.; Bertucci, C. Drug binding to human serum albumin: Abridged review of results obtained with high-performance liquid chromatography and circular dichroism. Chirality 2006, 18, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Sudlow, G.; Birkett, D.J.; Wade, D.N. Further Characterization of Specific Drug Binding-Sites on Human-Serum Albumin. Mol. Pharmacol. 1976, 12, 1052–1061. [Google Scholar] [PubMed]
- Petitpas, I.; Bhattacharya, A.A.; Twine, S.; East, M.; Curry, S. Crystal structure analysis of warfarin binding to human serum albumin—Anatomy of drug site I. J. Biol. Chem. 2001, 276, 22804–22809. [Google Scholar] [CrossRef] [PubMed]
- Kragh-Hansen, U.; Chuang, V.T.G.; Otagiri, M. Practical aspects of the ligand-binding and enzymatic properties of human serum albumin. Biol. Pharm. Bull. 2002, 25, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Noctor, T.A.G.; Pham, C.D.; Kaliszan, R.; Wainer, I.W. Stereochemical Aspects of Benzodiazepine Binding to Human Serum-Albumin. I. Enantioselective High-Performance Liquid Affinity Chromatographic Examination of Chiral and Achiral Binding Interactions between 1,4-Benzodiazepines and Human Serum-Albumin. Mol. Pharmacol. 1992, 42, 506–511. [Google Scholar] [PubMed]
- Allenmark, S.; Andersson, S. Optical Resolution of Some Biologically-Active Compounds by Chiral Liquid-Chromatography on BSA-Silica (Resolvosil) Columns. Chirality 1989, 1, 154–160. [Google Scholar] [CrossRef]
- Loun, B.; Hage, D.S. Characterization of thyroxine-albumin binding using high-performance affinity chromatography. I. Interactions at the warfarin and indole sites of albumin. J. Chromatogr. B 1992, 579, 225–235. [Google Scholar] [CrossRef]
- Wang, Q.Y.; Xiong, Y.J.; Lu, B.Z.; Fan, J.; Zheng, S.R.; Zhang, W.G. Effect of chromatographic conditions on enantioseparation of bovine serum albumin chiral stationary phase in HPLC and thermodynamic studies. Chirality 2013, 25, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Loun, B.; Hage, D.S. Characterization of thyroxine-albumin binding using high-performance affinity-chromatography II. Comparison of the binding of thyroxine, triiodothyronines and related compounds at the warfarin and indole sites of human serum albumin. J. Chromatogr. B 1995, 665, 303–314. [Google Scholar] [CrossRef]
- Allenmark, S. Optical resolution by liquid-chromatography on immobilized bovine serum-albumin. J. Liq. Chromatogr. 1986, 9, 425–442. [Google Scholar] [CrossRef]
- Fitos, I.; Visy, J.; Simonyi, M.; Hermansson, J. Chiral high-performance liquid chromatographic separations of vinca alkaloid analogues on α1-acid glycoprotein and human serum albumin columns. J. Chromatogr. A 1992, 609, 163–171. [Google Scholar] [CrossRef]
- Lu, J.; Ye, F.; Zhang, A.; Wei, Z.; Peng, Y.; Zhao, S. Preparation and characterization of silica monolith modified with bovine serum albumin-gold nanoparticles conjugates and its use as chiral stationary phases for capillary electrochromatography. J. Sep. Sci. 2011, 34, 2329–2336. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.; Zheng, Y.; Hu, W.; Ji, Y. Preparation and evaluation of bovine serum albumin immobilized chiral monolithic column for affinity capillary electrochromatography. Anal. Biochem. 2014, 464, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Li, J.; Zhao, J.; Liao, H.; Xu, L.; Shi, Z.G. Penetrable silica microspheres for immobilization of bovine serum albumin and their application to the study of the interaction between imatinib mesylate and protein by frontal affinity chromatography. Anal. Bioanal. Chem. 2016, 408, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.P.; Wang, X.N.; Liu, C.M.; Meng, X.Y.; Qiu, J.D. Facile preparation of protein stationary phase based on polydopamine/graphene oxide platform for chip-based open tubular capillary electrochromatography enantioseparation. J. Chromatogr. A 2014, 1323, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Fournier, T.; Medjoubi-N, N.; Porquet, D. Alpha-1-acid glycoprotein. Biochim. Biophys. Acta 2000, 1482, 157–171. [Google Scholar] [CrossRef]
- Zsila, F.; Iwao, Y. The drug binding site of human α1-acid glycoprotein: Insight from induced circular dichroism and electronic absorption spectra. Biochim. Biophys. Acta 2007, 1770, 797–809. [Google Scholar] [CrossRef] [PubMed]
- Ceciliani, F.; Pocacqua, V. The acute phase protein α1-acid glycoprotein: A model for altered glycosylation during diseases. Curr. Protein Pept. Sci. 2007, 8, 91–108. [Google Scholar] [CrossRef] [PubMed]
- Schmid, K.; Nimerg, R.B.; Kimura, A.; Yamaguchi, H.; Binette, J.P. The carbohydrate units of human plasma α1-acid glycoprotein. Biochim. Biophys. Acta 1977, 492, 291–302. [Google Scholar] [CrossRef]
- Filip, Z.; Jan, K.; Vendula, S.; Jana, K.Z.; Kamil, M.; Kamil, K. Albumin and α1-acid glycoprotein: Old acquaintances. Expert Opin. Drug Metab. Toxicol. 2013, 9, 943–954. [Google Scholar] [CrossRef] [PubMed]
- Israili, Z.H.; Dayton, P.G. Human alpha-1-glycoprotein and its interactions with drugs. Drug Metab. Rev. 2001, 33, 161–235. [Google Scholar] [CrossRef] [PubMed]
- Burton, M.E.; Shaw, L.M.; Schentag, J.J.; Evans, W.E. Applied Pharmacokinetics and Pharmacodynamics: Principles of Therapeutic Drug Monitoring; Lippincott: Baltimore, MD, USA, 2006. [Google Scholar]
- Schonfeld, D.L.; Ravelli, R.B.; Mueller, U.; Skerra, A. The 1.8-A crystal structure of alpha1-acid glycoprotein (Orosomucoid) solved by UV RIP reveals the broad drug-binding activity of this human plasma lipocalin. J. Mol. Biol. 2008, 384, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, K.; Nishi, K.; Chuang, V.T.G.; Maruyama, T.; Otagiri, M. Molecular Aspects of Human Alpha-1 Acid Glycoprotein—Structure and Function. In Acute Phase Proteins; Janciauskiene, S., Ed.; InTech: Rijeka, Croatia, 2013; pp. 139–162. [Google Scholar]
- Mallik, R.; Xuan, H.; Guiochon, G.; Hage, D.S. Immobilization of alpha1-acid glycoprotein for chromatographic studies of drug-protein binding II. correction for errors in association constant measurements. Anal. Biochem. 2008, 376, 154–156. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Karlsson, A.; Aspegren, A. The use of mobile phase pH and column temperature to reverse the retention order of enantiomers on chiral-AGP®. Chromatographia 1998, 47, 189–196. [Google Scholar] [CrossRef]
- Loeser, E.; Yowell, G.; Drumm, P. Effect of tertiary alcohol additives on enantioselectivity of the chiral-AGP column. J. Liq. Chromatogr. Relat. Technol. 2006, 29, 2625–2640. [Google Scholar] [CrossRef]
- Breton, D.; Buret, D.; Clair, P.; Lafosse, A. Chiral separation of atropine by high-performance liquid chromatography. J. Chromatogr. A 2005, 1088, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Enquist, M.; Hermansson, J. Separation and quantitation of (R)- and (S)-atenolol in human plasma and urine using an α1-AGP column. Chirality 1989, 1, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, I.; Kmetec, V.; Mrhar, A.; Grabnar, I. Determination of warfarin enantiomers and hydroxylated metabolites in human blood plasma by liquid chromatography with achiral and chiral separation. J. Chromatogr. B 2005, 818, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Sandstrom, E.; Lennernas, H.; Ohlen, K.; Karlsson, A. Enantiomeric separation of verapamil and norverapamil using Chiral-AGP® as the stationary phase. J. Pharm. Biomed. Anal. 1999, 21, 43–49. [Google Scholar] [CrossRef]
- Soares, R.; Singh, A.K.; Kedor-Hackmann, E.R.; Santoro, M.I. Determination of atropine enantiomers in ophthalmic solutions by liquid chromatography using a Chiral AGP column. J. AOAC Int. 2009, 92, 1663–1672. [Google Scholar] [PubMed]
- Etter, M.L.; George, S.; Graybiel, K.; Eichhorst, J.; Lehotay, D.C. Determination of free and protein-bound methadone and its major metabolite EDDP: Enantiomeric separation and quantitation by LC/MS/MS. Clin. Biochem. 2005, 38, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Du, J.B.; Ma, Z.Y.; Zhang, Y.F.; Wang, T.; Chen, X.Y.; Zhong, D.F. Enantioselective determination of ornidazole in human plasma by liquid chromatography-tandem mass spectrometry on a Chiral-AGP column. J. Pharm. Biomed. Anal. 2013, 86, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, K.; Samata, N.; Urasaki, Y.; Fukazawa, I.; Uchida, N.; Uchida, E.; Yasuhara, H. Enantioselective determination of azelnidipine in human plasma using liquid chromatography-tandem mass spectrometry. J. Chromatogr. B 2007, 852, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Massolini, G.; Temporini, C.; Calleri, E. Penicillin G acylase as chiral selector in LC and CE: Exploring the origins of enantioselectivity. J. Chromatogr. B 2008, 875, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Temporini, C.; Calleri, E.; Fracchiolla, G.; Carbonara, G.; Loiodice, F.; Lavecchia, A.; Tortorella, P.; Brusotti, G.; Massolini, G. Enantiomeric separation of 2-aryloxyalkyl- and 2-arylalkyl-2-aryloxyacetic acids on a penicillin G acylase-based chiral stationary phase: Influence of the chemical structure on retention and enantioselectivity. J. Pharm. Biomed. Anal. 2007, 45, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Lavecchia, A.; Cosconati, S.; Novellino, E.; Calleri, E.; Temporini, C.; Massolini, G.; Carbonara, G.; Fracchiolla, G.; Loiodice, F. Exploring the molecular basis of the enantioselective binding of penicillin G acylase towards a series of 2-aryloxyalkanoic acids: A docking and molecular dynamics study. J. Mol. Graph. Model. 2007, 25, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Gotti, R.; Fiori, J.; Calleri, E.; Temporini, C.; Lubda, D.; Massolini, G. Chiral capillary liquid chromatography based on penicillin G acylase immobilized on monolithic epoxy silica column. J. Chromatogr. A 2012, 1234, 45–49. [Google Scholar] [CrossRef] [PubMed]
- den Haan, R.; Kroukamp, H.; van Zyl, J.-H.D.; van Zyl, W.H. Cellobiohydrolase secretion by yeast: Current state and prospects for improvement. Process Biochem. 2013, 48, 1–12. [Google Scholar] [CrossRef]
- Isaksson, R.; Pettersson, C.; Pettersson, G.; Jonsson, S.; Stalberg, J.; Hermansson, J.; Marle, I. Cellulases as chiral selectors. Trends Anal. Chem. 1994, 13, 431–439. [Google Scholar] [CrossRef]
- Divne, C.; Stahlberg, J.; Reinikainen, T.; Ruohonen, L.; Pettersson, G.; Knowles, J.K.; Teeri, T.T.; Jones, T.A. The three-dimensional crystal structure of the catalytic core of cellobiohydrolase I from Trichoderma reesei. Science 1994, 265, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Marle, I.; Erlandsson, P.; Hansson, L.; Isaksson, R.; Pettersson, C.; Pettersson, G. Separation of enantiomers using cellulase (CBH-I) silica as a chiral stationary phase. J. Chromatogr. A 1991, 586, 233–248. [Google Scholar] [CrossRef]
- Fornstedt, T.; Sajonz, P.; Guiochon, G. Thermodynamic study of an unusual chiral separation. Propranolol enantiomers on an immobilized cellulase. J. Am. Chem. Soc. 1997, 119, 1254–1264. [Google Scholar] [CrossRef]
- Henriksson, H.; Stahlberg, J.; Isaksson, R.; Pettersson, G. The active sites of cellulases are involved in chiral recognition: A comparison of cellobiohydrolase 1 and endoglucanase 1. FEBS Lett. 1996, 390, 339–344. [Google Scholar] [CrossRef]
- Bagnall, J.P.; Evans, S.E.; Wort, M.T.; Lubben, A.T.; Kasprzyk-Hordern, B. Using chiral liquid chromatography quadrupole time-of-flight mass spectrometry for the analysis of pharmaceuticals and illicit drugs in surface and wastewater at the enantiomeric level. J. Chromatogr. A 2012, 1249, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Castrignano, E.; Lubben, A.; Kasprzyk-Hordern, B. Enantiomeric profiling of chiral drug biomarkers in wastewater with the usage of chiral liquid chromatography coupled with tandem mass spectrometry. J. Chromatogr. A 2016, 1438, 84–99. [Google Scholar] [CrossRef]
- Boyer, P.D. The Enzymes III Hydrolysis: Peptide Bonds, 3rd ed.; Academic Press: New York, NY, USA, 1971. [Google Scholar]
- Ma, W.Z.; Tang, C.; Lai, L.H. Specificity of trypsin and chymotrypsin: Loop-motion-controlled dynamic correlation as a determinant. Biophys. J. 2005, 89, 1183–1193. [Google Scholar] [CrossRef] [PubMed]
- Wainer, I.W.; Jadaud, P.; Schombaum, G.R.; Kadodkar, S.V.; Henry, M.P. Enzymes as HPLC stationary phases for chiral resolutions—Initial investigations with α-chymotrypsin. Chromatographia 1988, 25, 903–907. [Google Scholar] [CrossRef]
- Jadaud, P.; Wainer, I.W. Stereochemical recognition of enantiomeric and diastereomeric dipeptides by high-performance liquid-chromatography on a chiral stationary phase based upon immobilized α-chymotrypsin. J. Chromatogr. A 1989, 476, 165–174. [Google Scholar] [CrossRef]
- Iredale, J.; Aubry, A.F.; Wainer, I. The effects of pH and alcoholic organic modifiers on the direct separation of some acidic, basic and neutral compounds on a commercially available ovomucoid column. Chromatographia 1991, 31, 329–334. [Google Scholar] [CrossRef]
- Oda, Y.; Asakawa, N.; Kajima, T.; Yoshida, Y.; Sato, T. On-line determination and resolution of verapamil enantiomers by high-performance liquid chromatography with column switching. J. Chromatogr. A 1991, 541, 411–418. [Google Scholar] [CrossRef]
- Haginaka, J.; Seyama, C.; Murashima, T. Retentive and Enantioselective Properties of Ovomucoid-Bonded Silica Columns—Influence of Protein Purity and Isolation Method. J. Chromatogr. A 1995, 704, 279–287. [Google Scholar] [CrossRef]
- Miwa, T.; Ichikawa, M.; Tsuno, M.; Hattori, T.; Miyakawa, T.; Kayano, M.; Miyake, Y. Direct liquid-chromatographic resolution of racemic compounds—Use of ovomucoid as a column ligand. Chem. Pharm. Bull. 1987, 35, 682–686. [Google Scholar] [CrossRef]
- Liu, K.; Dai, X.; Zhong, D.; Chen, X. Quantitative determination of ondansetron in human plasma by enantioselective liquid chromatography-tandem mass spectrometry. J. Chromatogr. B 2008, 864, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhong, D.; Chen, X. Enantioselective determination of doxazosin in human plasma by liquid chromatography-tandem mass spectrometry using ovomucoid chiral stationary phase. J. Chromatogr. B 2010, 878, 2415–2420. [Google Scholar] [CrossRef] [PubMed]
- Landsteiner, K. The Specificity of Serological Reactions; Charles C Thomas: Springfield, IL, USA, 1936. [Google Scholar]
- Hofstetter, H.; Hofstetter, O. Antibodies as tailor-made chiral selectors for detection and separation of stereoisomers. TrAC Trends Anal. Chem. 2005, 24, 869–879. [Google Scholar] [CrossRef]
- Hofstetter, H.; Cary, J.R.; Eleniste, P.P.; Hertweck, J.K.; Lindstrom, H.J.; Ranieri, D.I.; Smith, G.B.; Undesser, L.P.; Zeleke, J.M.; Zeleke, T.K. New developments in the production and use of stereoselective antibodies. Chirality 2005, 17, S9–S18. [Google Scholar] [CrossRef] [PubMed]
- Franco, E.J.; Hofstetter, H.; Hofstetter, O. Determination of lactic acid enantiomers in human urine by high-performance immunoaffinity LC-MS. J. Pharm. Biomed. Anal. 2009, 49, 1088–1091. [Google Scholar] [CrossRef] [PubMed]
- Hofstetter, O.; Lindstrom, H.; Hofstetter, H. Effect of the mobile phase on antibody-based enantiomer separations of amino acids in high-performance liquid chromatography. J. Chromatogr. A 2004, 1049, 85–95. [Google Scholar] [CrossRef]
- Zeleke, J.M.; Smith, G.B.; Hofstetter, H.; Hofstetter, O. Enantiomer separation of amino acids in immunoaffinity micro LC-MS. Chirality 2006, 18, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Zeleke, T.K.; Zeleke, J.M.; Hofstetter, H.; Hofstetter, O. Stereoselective antibodies to free α-hydroxy acids. J. Mol. Recognit. 2005, 18, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Livnah, O.; Bayer, E.A.; Wilchek, M.; Sussman, J.L. Three-dimensional structures of avidin and the avidin-biotin complex. Proc. Natl. Acad. Sci. USA 1993, 90, 5076–5080. [Google Scholar] [CrossRef] [PubMed]
- Haque, A.; Stewart, J.T. Chiral separations of selected pharmaceuticals on avidin column. J. Liq. Chromatogr. Relat. Technol. 1998, 21, 2675–2687. [Google Scholar] [CrossRef]
- Kitagawa, F.; Inoue, K.; Hasegawa, T.; Kamiya, M.; Okamoto, Y.; Kawase, M.; Otsuka, K. Chiral separation of acidic drug components by open tubular electrochromatography using avidin immobilized capillaries. J. Chromatogr. A 2006, 1130, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Ikawa, Y.; Kitagawa, F.; Otsuka, K. Preparation of fritless capillary using avidin immobilized magnetic particles for electrochromatographic chiral separation. J. Chromatogr. A 2007, 1143, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Olsson, J.; Blomberg, L.G. Enantioseparation of omeprazole and its metabolite 5-hydroxyomeprazole using open tubular capillary electrochromatography with immobilized avidin as chiral selector. J. Chromatogr. B 2008, 875, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Ravelet, C.; Michaud, M.; Ravel, A.; Grosset, C.; Villet, A.; Peyrin, E. Streptavidin chiral stationary phase for the separation of adenosine enantiomers. J. Chromatogr. A 2004, 1036, 155–160. [Google Scholar] [CrossRef] [PubMed]



| Type of Protein | Molecular Mass (kDa) | Isoelectric Point, pI |
|---|---|---|
| Serum transport protein | ||
| α1-Acid glycoprotein (AGP) | 41 | 2.7 |
| Human serum albumin (HSA) | 66 | 4.7 |
| Bovine serum albumin (BSA) | 66.5 | 4.7–4.9 |
| Enzymes | ||
| Cellobiohydrolases | ||
| CBH I | 60–70 | 3.9 |
| CBH II | ||
| α-Chymotrypsin | 25 | 8.1–8.3 |
| Lysozyme | 14 | 11 |
| Penicillin G acylase | 90 | 6.4 |
| Other proteins | ||
| Antibodies (e.g., immunoglobulin G) | ~150 | 4.4–10 |
| Avidin | 68 | 10 |
| Ovomucoid | 28.8 | 3.9–4.5 |
| Streptavidin | 60 | 5 |
| Type of Support | Silica Monolith | Silica Particles | GMA/EDMA Monolith |
|---|---|---|---|
| Protein content (nmol/g support) | 1800 (± 40) | 490 (± 20) | 250 (± 10) |
| Protein coverage (nmol/m2) | 5.8 (± 0.2) | 4.9 (± 0.2) | 3.6 (± 0.1) |
| Retention factor, k | 31.1 (± 0.4) (R) | 11.6 (± 0.2) (R) | 4.53 (± 0.06) (R) |
| 37.2 (± 0.4) (S) | 13.7 (± 0.3) (S) | 6.86 (± 0.05) (S) | |
| Separation factor, α | 1.20 (± 0.01) | 1.18 (± 0.05) | 1.51 (± 0.06) |
| Resolution, Rs | 1.11 (± 0.03) | 0.55 (± 0.03) | 0.79 (± 0.03) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bi, C.; Zheng, X.; Azaria, S.; Beeram, S.; Li, Z.; Hage, D.S. Chromatographic Studies of Protein-Based Chiral Separations. Separations 2016, 3, 27. https://doi.org/10.3390/separations3030027
Bi C, Zheng X, Azaria S, Beeram S, Li Z, Hage DS. Chromatographic Studies of Protein-Based Chiral Separations. Separations. 2016; 3(3):27. https://doi.org/10.3390/separations3030027
Chicago/Turabian StyleBi, Cong, Xiwei Zheng, Shiden Azaria, Sandya Beeram, Zhao Li, and David S. Hage. 2016. "Chromatographic Studies of Protein-Based Chiral Separations" Separations 3, no. 3: 27. https://doi.org/10.3390/separations3030027
APA StyleBi, C., Zheng, X., Azaria, S., Beeram, S., Li, Z., & Hage, D. S. (2016). Chromatographic Studies of Protein-Based Chiral Separations. Separations, 3(3), 27. https://doi.org/10.3390/separations3030027