Detecting Adulterated Commercial Sweet Sorghum Syrups with Ion Chromatography Oligosaccharide Fingerprint Profiles
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals and Reagents
2.2. IC-IPAD Saccharide Analysis
2.3. Brix (% Refractometric Dry Substance) and pH
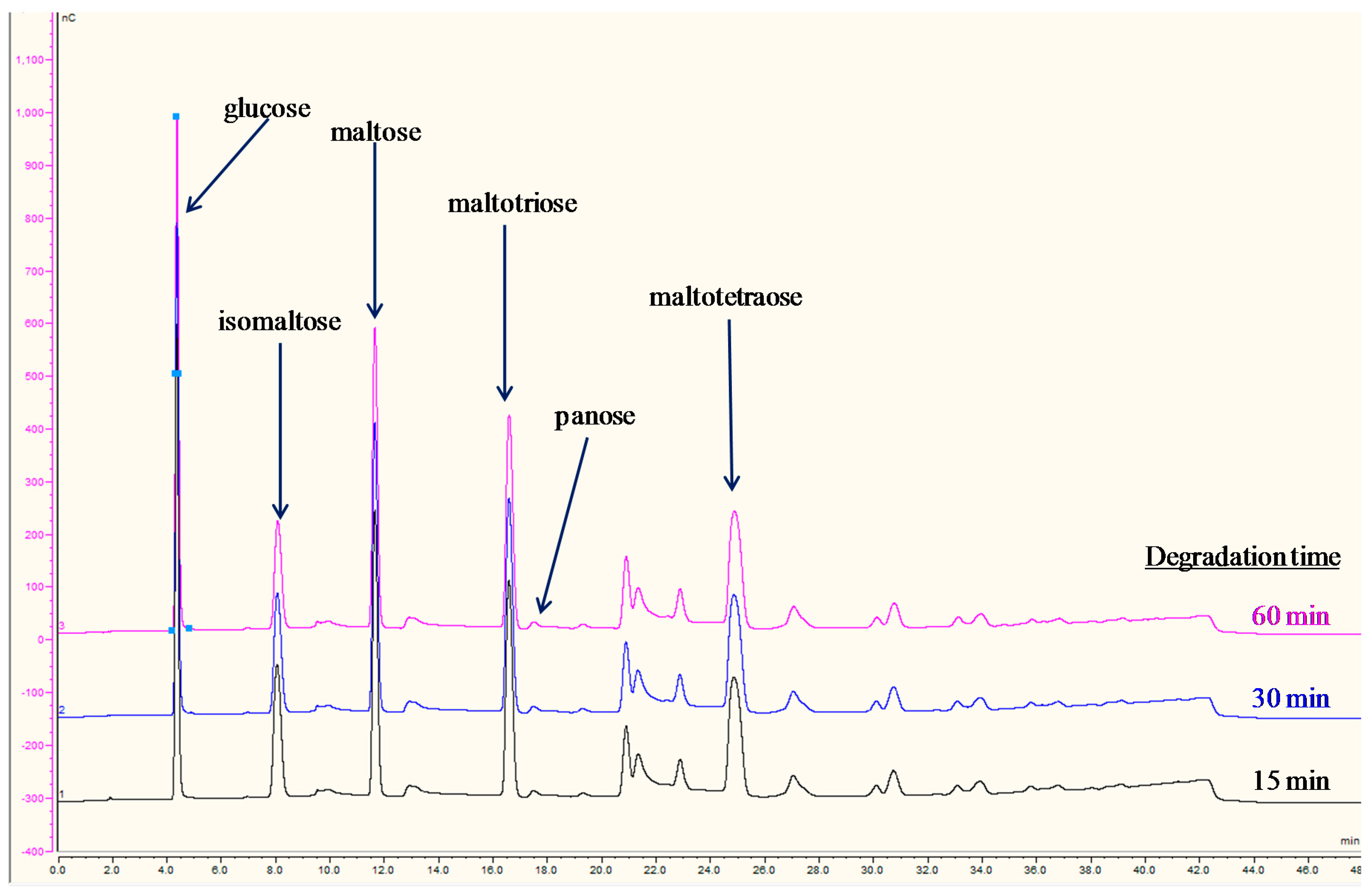
2.4. Amylase Activity and Action on Starch
3. Results and Discussion
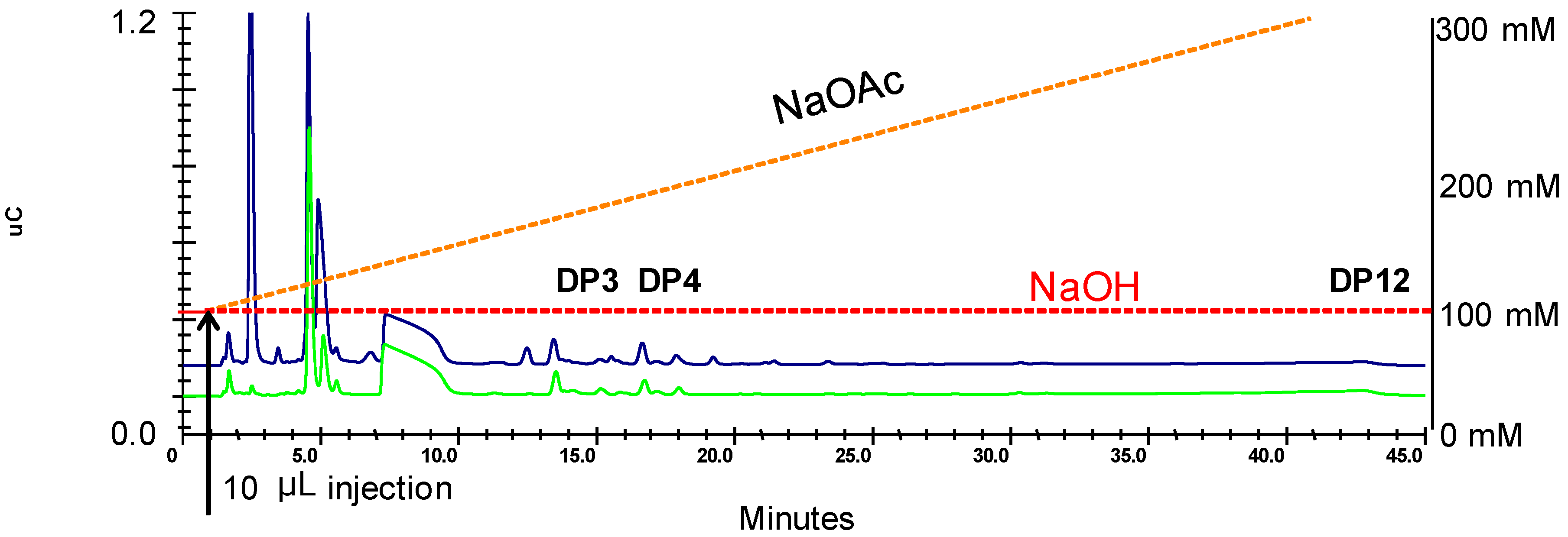
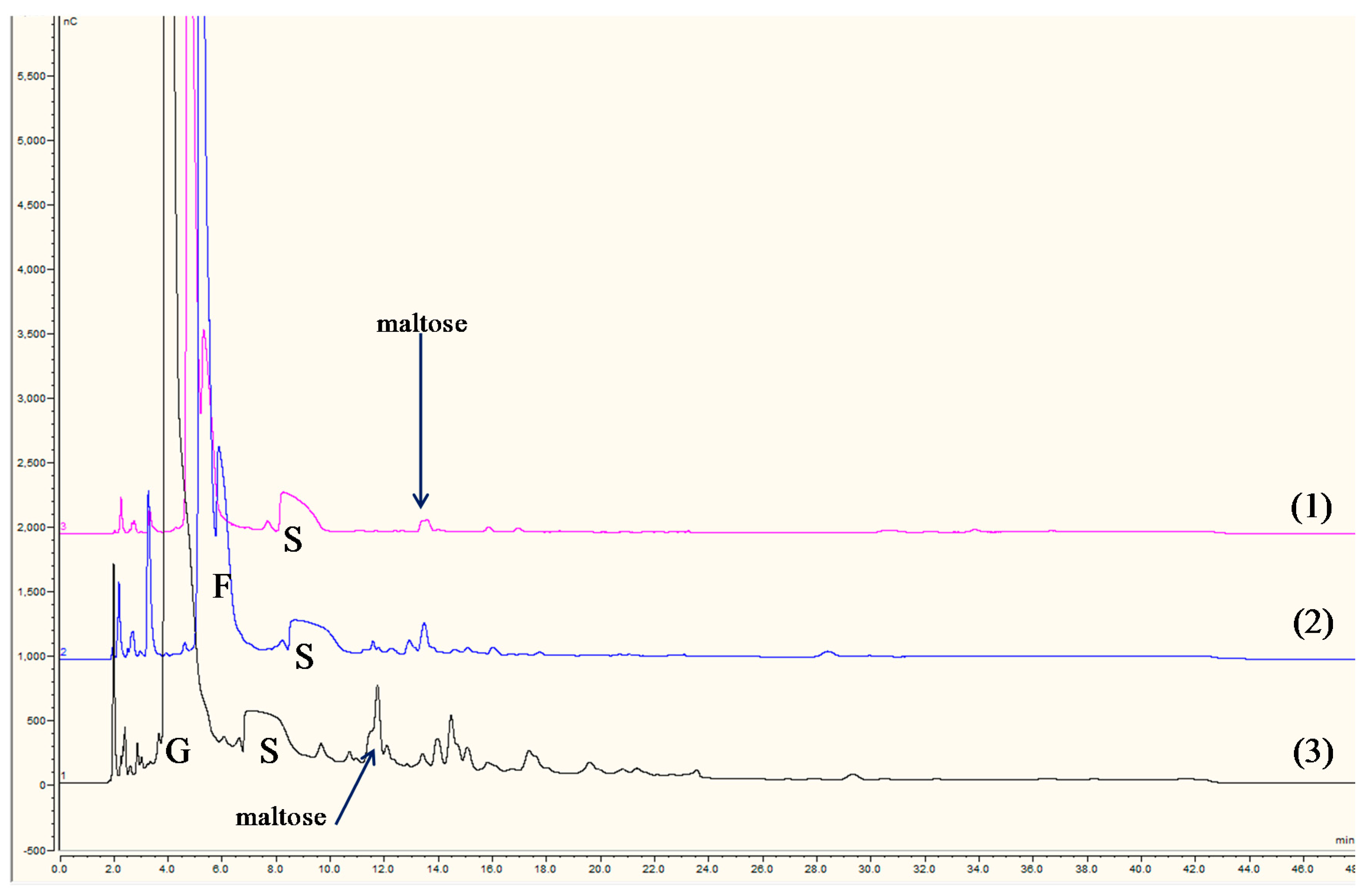
3.1. IC-IPAD Fingerprint Chromatograms of Known Sweet Sorghum Syrups
3.2. IC-IPAD Fingerprint Chromatograms of Starch Hydrolysis Products
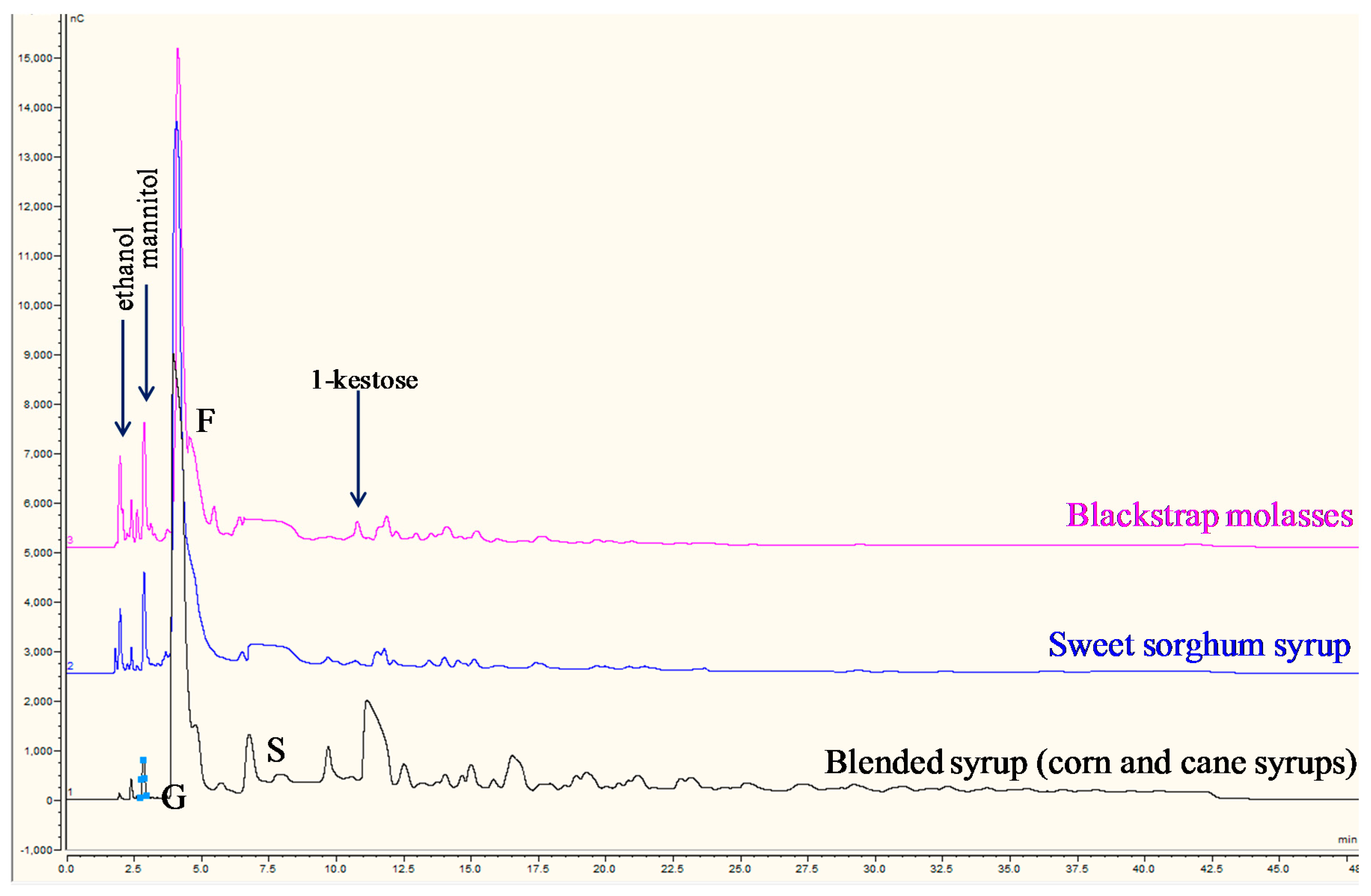
3.3. Comparison of Commercial Sugar Syrups
3.3.1. Mannitol Marker
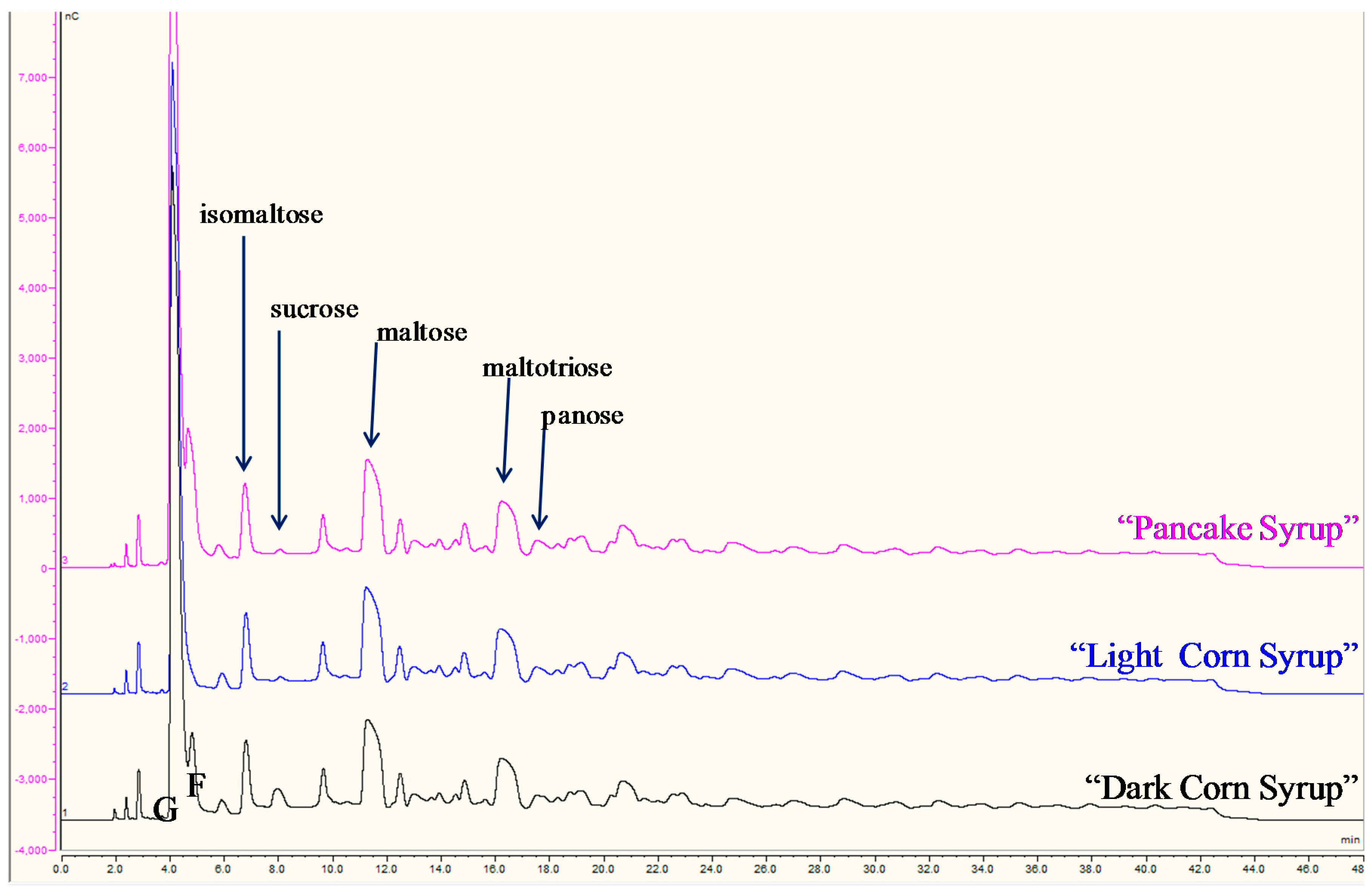
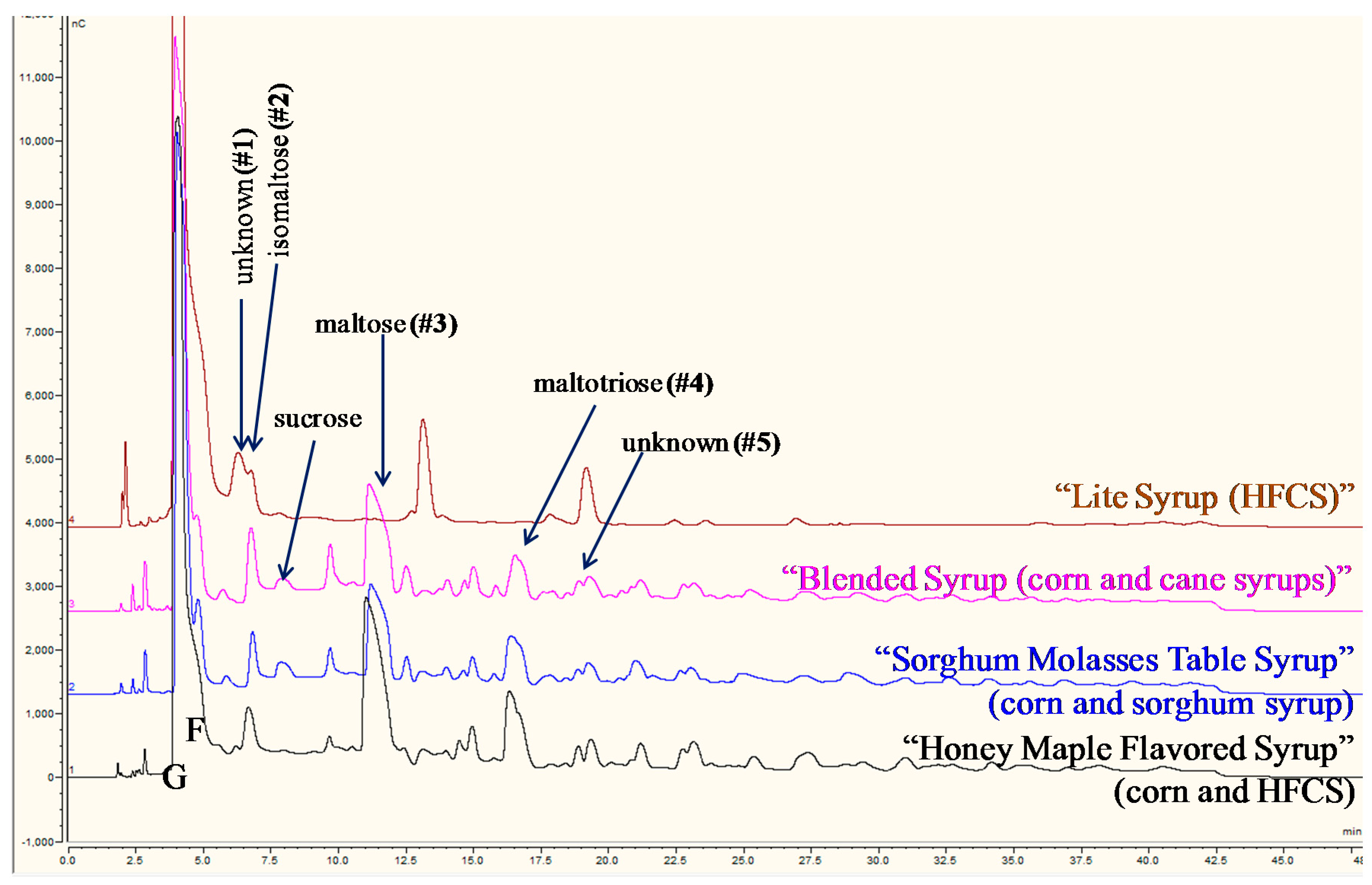
3.3.2. Corn, High Fructose Corn (HFCS), and Cane Syrups
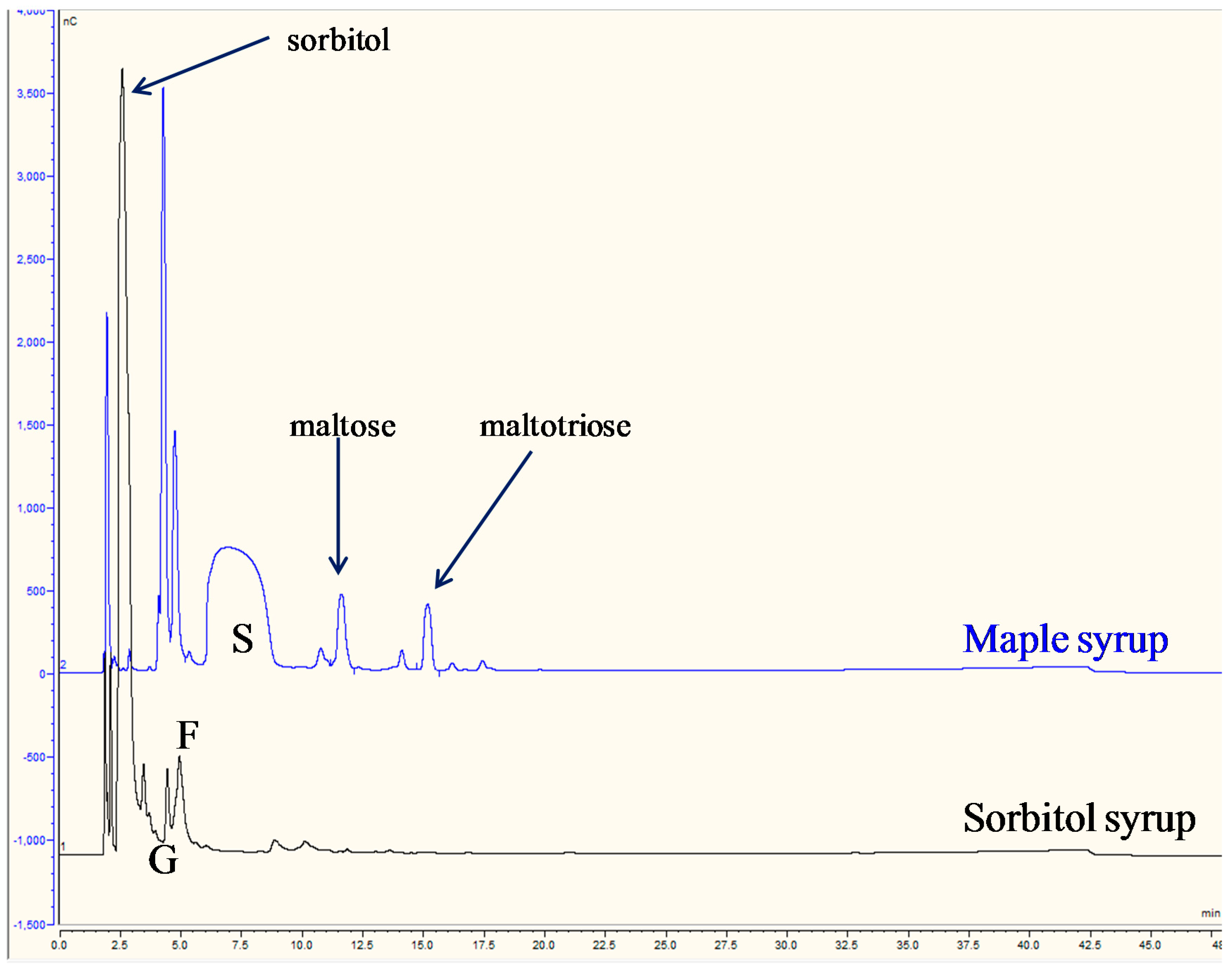
3.3.3. Maple and Sorbitol Syrups
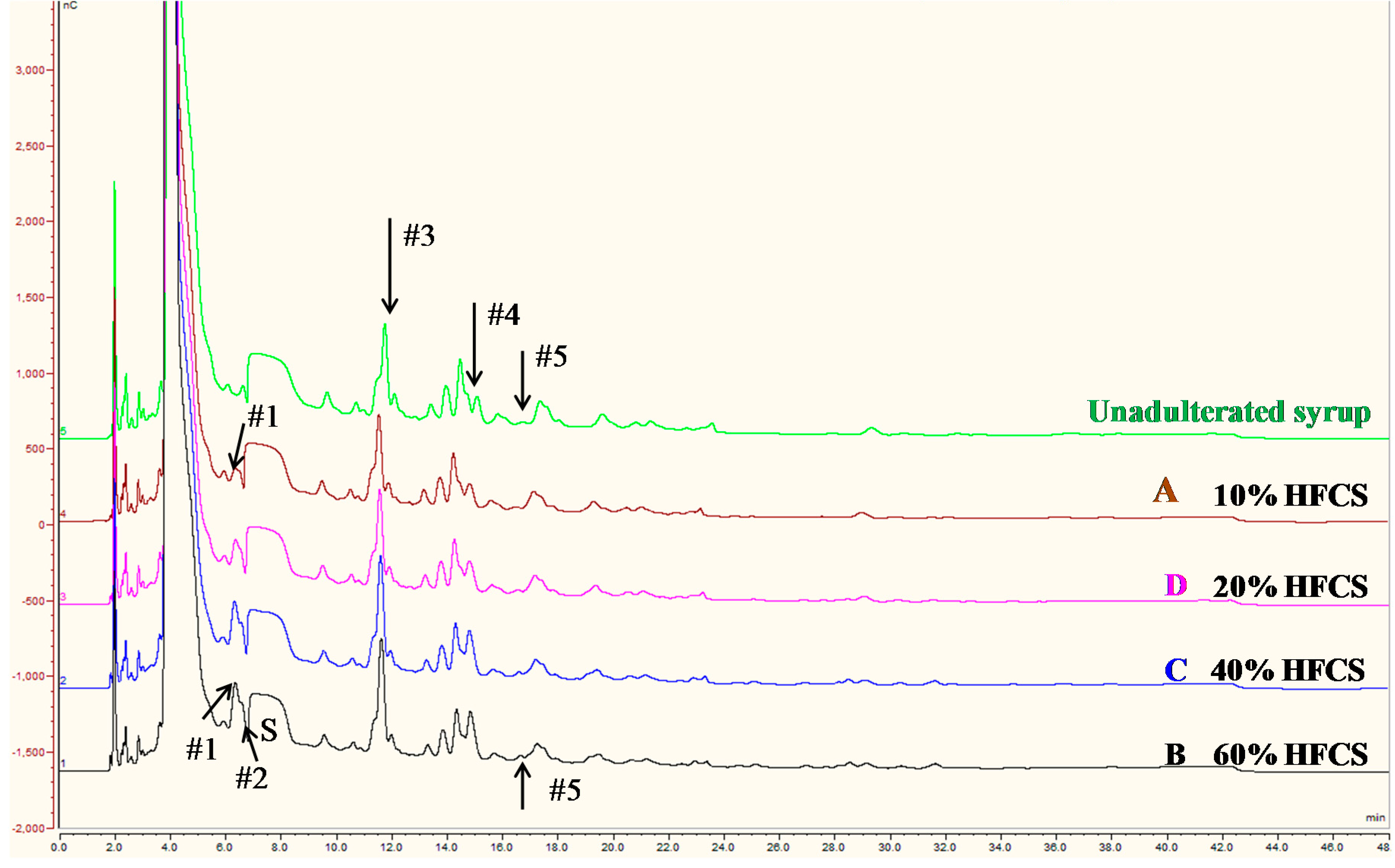
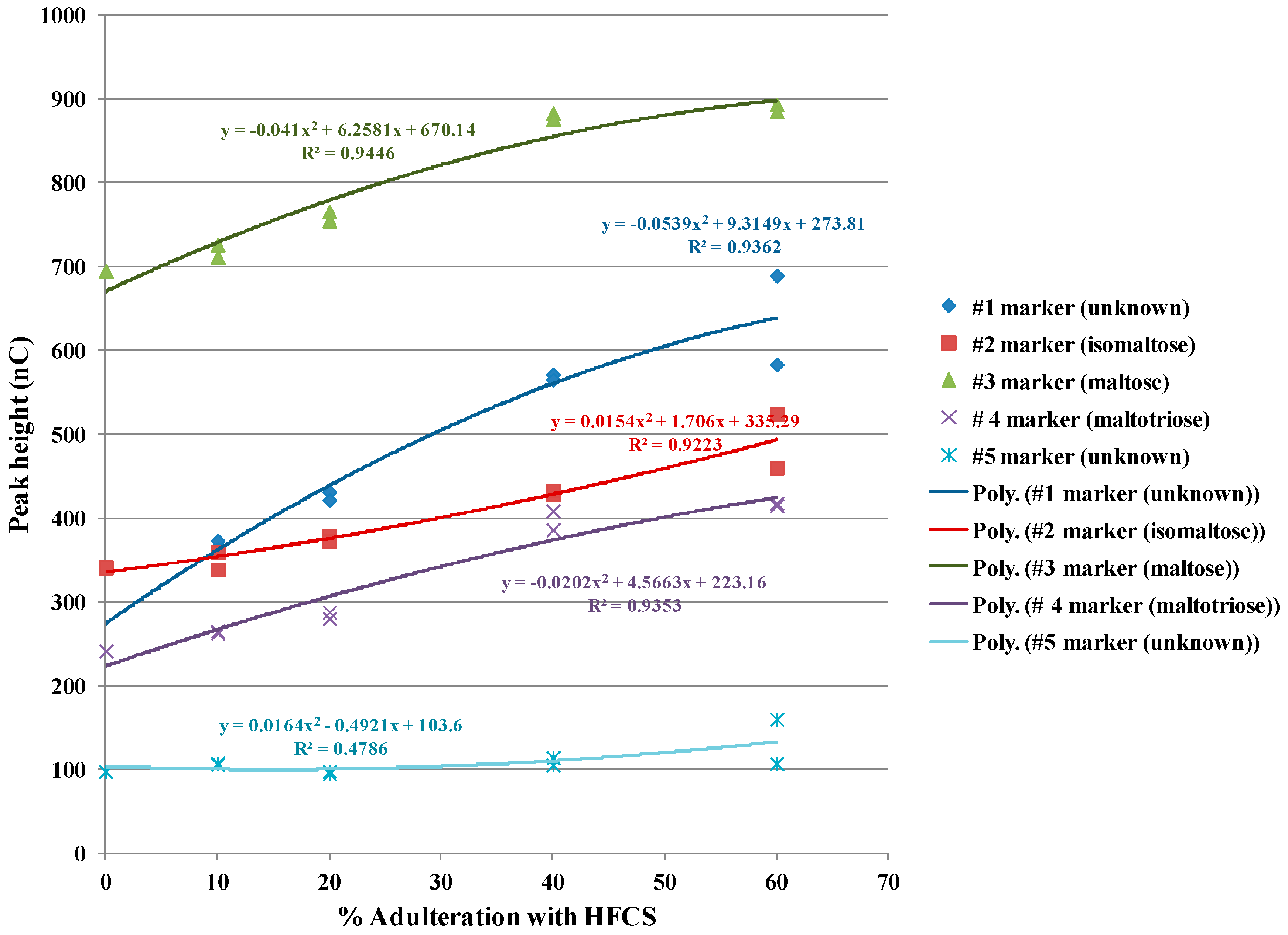
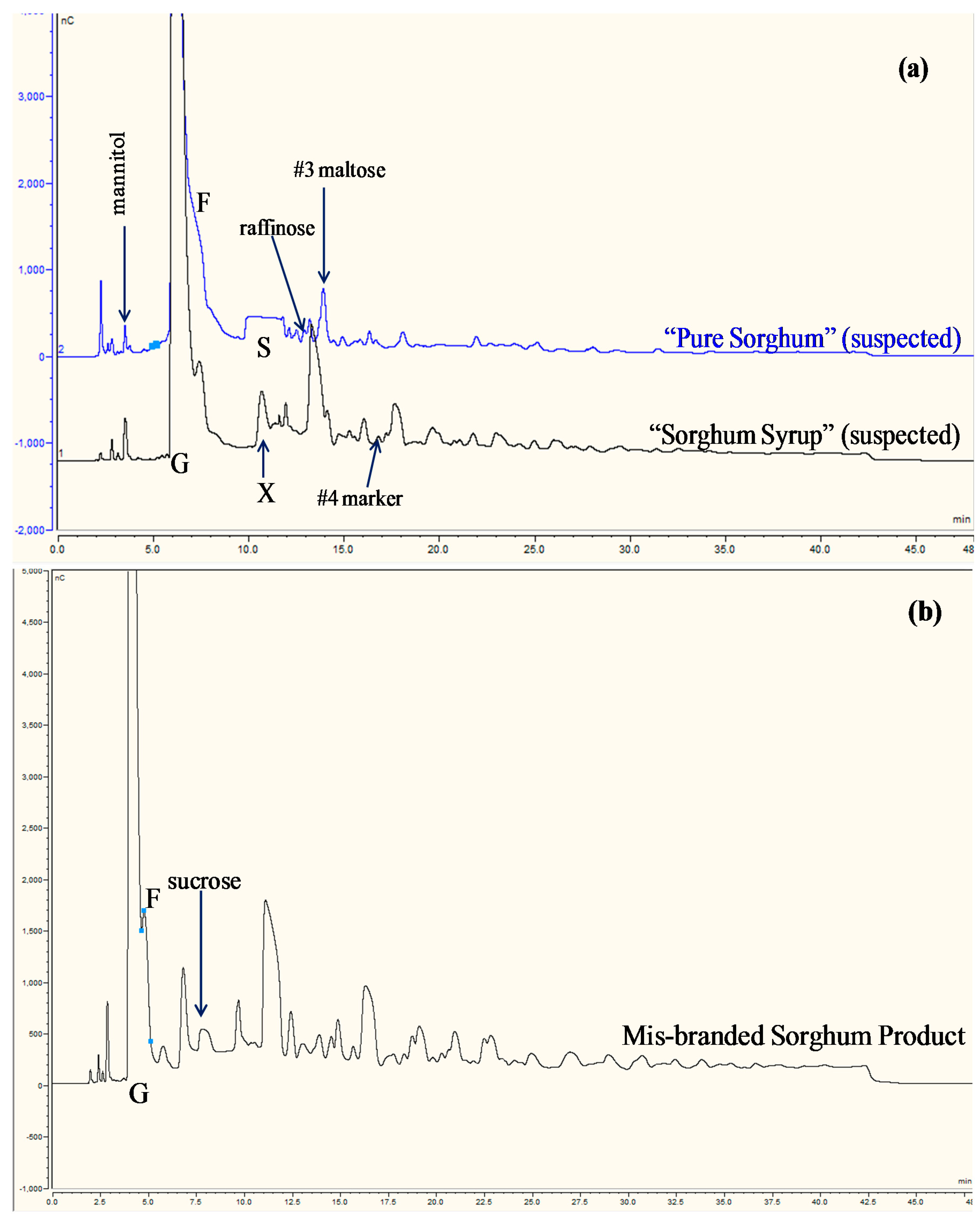
3.4. Use of Blind Samples
3.5. Practical Use of IC-IPAD Method for Authentication Purposes
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Johnson, R. Food Fraud and “Economically Motivated Adulteration” of Food and Food Ingredients; Congressional Research Service Report 7-5700; Library of Congress: Washington, DC, USA, 2014; pp. 1–40. [Google Scholar]
- Vosloo, N. Identifying Whiskey Counterfeits. Novel Application of Molecular Spectroscopic Techniques to Ensure Authenticity of Whiskies; Food Quality Safety: Hoboken, NJ, USA, 2015; pp. 40–42. [Google Scholar]
- Eggleston, G.; Cole, M.; Andrzejewski, B. New commercially viable processing technologies for the production of sugar feedstocks from sweet sorghum (Sorghum bicolor L. Moench) for biofuel and bioproducts manufacture. Sugar Tech 2013, 15, 232–249. [Google Scholar] [CrossRef]
- Andrzejewski, B.; Eggleston, G.; Powell, R. Pilot plant clarification of sweet sorghum juice and evaporation of raw and clarified juices. Ind. Crop. Prod. 2013, 49, 648–658. [Google Scholar] [CrossRef]
- Swallow, K.W.; Low, N.H. Analysis and quantitation of the carbohydrates in honey using high performance liquid chromatography. J. Agric. Food Chem. 1990, 38, 1828–1832. [Google Scholar] [CrossRef]
- Hammond, D.A. Synergy between liquid chromatography-pulsed amperometric detection and capillary-gas chromatographic methods for the detection of juice adulteration. JAOAC Int. 2001, 83, 964–975. [Google Scholar]
- Eggleston, G; Borges, E. The multiple applications of ion chromatography oligosaccharide fingerprint profiles to solve a variety of sugar and sugar-biofuel industry problems. J. Agric. Food Chem. 2015, 63, 2841–2851. [Google Scholar] [CrossRef] [PubMed]
- Eggleston, G.; Pollach, G.; Triche, R. The use of ion chromatography profiles as screening tool to differentiate cane white sugar from beet white sugar. Zuckerind 2005, 130, 611–616. [Google Scholar]
- Cordella, C.; Millit, J.; Clement, M.-C.; Drajnudel, P.; Cabrol-Bass, D. Detection and quantitation of honey adulteration via direct incorporation of sugar syrups or bee-feeding: preliminary study using high-performance anion exchange chromatography (HPAEC-PAD) and chemometrics. Anal. Chem. Acta 2005, 531, 239–248. [Google Scholar] [CrossRef]
- Stuckel, J.G.; Low, N.H. Maple syrup authenticity analysis by anion-exchange liquid chromatography with pulsed amperometric detection. J. Agric. Food Chem. 1995, 43, 3046–3051. [Google Scholar] [CrossRef]
- Lee, Y.C. Carbohydrate analyses with high-performance anion-exchange chromatography. J. Chromatogr. A 1996, 720, 137–149. [Google Scholar] [CrossRef]
- Basumallik, L.; Rohrer, J. Determination of Hydroxymethylfurfural in Honey and Biomass; Thermo Scientific Dionex Application Note Update 270; Thermo Fisher Scientific: Sunnyvale, CA, USA, 2011; pp. 1–6. [Google Scholar]
- Koizumi, K.; Kubota, Y.; Tanimoto, T.; Okada, Y. High-performance anion-exchange chromatography of homogenous d-gluco-oligosaccharides and polysaccharides polymerization degree 50 with pulsed amperometric detection. J. Chromatogr. 1989, 464, 365–373. [Google Scholar] [CrossRef]
- Hanashiro, I.; Abe, J.; Hizukuri, S. A periodic distribution of the chain length of amylopectin as revealed by high-performance anion-exchange chromatography. Carbohydr. Res. 1996, 283, 151–159. [Google Scholar] [CrossRef]
- Anon. Determination of Plant-Derived Neutral Oligo-and Polysaccharides; Thermo Scientific Dionex Application Note Update 67; Thermo Fisher Scientific: Sunnyvale, CA, USA, 1993; pp. 1–11. [Google Scholar]
- Cataldi, T.R.I.; Campa, C.; de Benedetto, G.E. Carbohydrate analysis by high-performance anion-exchange chromatography with pulsed amperometric detection: The potential is still growing. Fresenius J. Anal. Chem. 2000, 368, 739–758. [Google Scholar] [CrossRef] [PubMed]
- Eggleston, G. Determination of the Relative Activity of Commercial α-Amylase as Used in Sugar Cane Processing and Refining. ICUMSA GS7-33 Official Method; ICUMSA Methods Book; Bartens publisher: Berlin, Germany, 2013; pp. 1–4. [Google Scholar]
- Anon. Determination of plant-derived neutral oligo- and polysaccharides using the CarboPac™ PA200; Thermo Scientific Application Note Update 150; Thermo Fisher Scientific: Sunnyvale, CA, USA, 2005; pp. 1–6. [Google Scholar]
- Eggleston, G.; Clarke, M.A. Applications of HPAE-PAD in the sugar industry. Semin. Food Anal. 1997, 2, 119–127. [Google Scholar]
- Eggleston, G.; DeLucca, A.; Sklanka, S.; Dalley, C.; St. Cyr, E.; Powell, R. Investigation of the stabilization and preservation of sweet sorghum juices. Ind. Crop. Prod. 2015, 64, 258–270. [Google Scholar] [CrossRef]
- Eggleston, G.; Heckemeyer, M.; St. Cyr, E.; Wartelle, L. Case study: Commercialization of sweet sorghum juice clarification for large-scale syrup manufacture. Sugar Tech 2016, 18, 249–257. [Google Scholar] [CrossRef]
- Broadhead, D.M.; Freeman, K.C.; Zummo, N. “M81 E”-A New Variety of Sweet Sorghum; Mississippi Agricultural & Forestry Experimental Station: Meridian, MS, USA, 1981; Volume 1309, pp. 1–4. [Google Scholar]
- Low, N.H.; Wudrich, G.G. Detection of inexpensive sweetener addition to grapefruit juice by HPLC-PAD. J. Agric. Food Chem. 1993, 41, 902–909. [Google Scholar] [CrossRef]
- French, D.; Taylor, P.M.; Whelan, W.J. The mechanism of carbohydrase action. 10. Enzymic synthesis and properties of 6-α-maltosylglucose. Biochem. J. 1964, 90, 616–620. [Google Scholar] [CrossRef] [PubMed]
- Pazur, J.H.; French, D. The action of transglucosidase of Aspergillus oryzae on maltose. J. Biol. Chem. 1952, 196, 265–272. [Google Scholar] [PubMed]
- Kandler, O.; Knopf, H. The Biochemistry of Plants; Preiss, J., Ed.; Academic Press: San Diego, CA, USA, 1981; Volume 3, pp. 221–270. [Google Scholar]
- Anglani, C. Sorghum carbohydrates—A review. Plant Foods Hum. Nutr. 1998, 52, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Eggleston, G. Deterioration of cane juice—Sources and indicators. Food Chem. 2002, 78, 99–107. [Google Scholar] [CrossRef]
- Robyt, J.F.; Eklund, S.H. Stereochemistry involved in the mechanism of action of dextransucrase in the synthesis of dextran and the formation of acceptor products. Bioorg. Chem. 1982, 11, 115–132. [Google Scholar] [CrossRef]
- Richards, G.N. Initial steps in thermal degradation of sucrose. Intern. Sugar J. 1986, 88, 145–148. [Google Scholar]
- Eggleston, G.; Grisham, M. Oligosaccharides in cane and their formation on cane deterioration. In Oligosaccharides in Food and Agriculture; Eggleston, G., Côté, G.L., Eds.; ACS Symposium Series 849; Oxford University Press: Washington, DC, USA, 2003; Chapter 16; pp. 211–232. [Google Scholar]
- Kowalski, B.R.; Bender, C.F. Pattern recognition a powerful approach to interpreting chemical data. J. Am. Chem. Soc. 1972, 94, 5632–5639. [Google Scholar] [CrossRef]
- Irudayaraj, J.; Xu, F.; Tewari, J. Rapid determination of invert cane adulteration in honey using FTIR spectroscopy and multivariate analysis. J. Food Sci. 2006, 68, 2040–2045. [Google Scholar] [CrossRef]
- Salvator, R.A.; Angelino, D.; Maria, L.G.; Guiseppe, L. Classification and quantification of H1 NMR spectra of alditols binary mixtures using artificial neural networks. Anal. Chem. 1998, 70, 1249–1254. [Google Scholar]
| Syrup No. | Syrup Title on Label * | Major Ingredients on Label ‡ | Syrup Brix % | pH |
|---|---|---|---|---|
| 1 | Organic Molasses | Organic blackstrap molasses | 79.26 | 4.81 |
| 2 | 100% Pure Cane Syrup | Pure sugarcane syrup | 80.34 | 4.97 |
| 3 | Blended Syrup | Corn Syrup, pure cane syrup | 79.20 | 5.22 |
| 4 | Sorghum Syrup | Sorghum syrup | 81.06 | 4.92 |
| 5 | Sorghum † | Sorghum | 74.40 | 5.02 |
| 6 | Sorghum (Unblended) † | Sorghum | 83.22 | 4.92 |
| 7 | Pancake Syrup | Corn syrup, high fructose corn syrup | 77.62 | 4.55 |
| 8 | Dark Corn Syrup * | Corn syrup, refiners syrup | 77.06 | 4.80 |
| 9 | Light Corn Syrup * | Corn syrup | 76.54 | 4.84 |
| 10 | Lite Syrup | High fructose corn syrup | 38.97 | 4.64 |
| 11 | Honey Maple Flavored Syrup | Corn syrup, high fructose corn syrup | 67.72 | 4.65 |
| 12 | Golden Syrup | Cane sugar syrup | 80.55 | 5.24 |
| 13 | Sugar Free Low Calorie Syrup | Sorbitol | 19.97 | 5.22 |
| 14 | Maple Syrup Premium | 100% pure maple syrup | 67.74 | 6.39 |
| 15 | Blackstrap Molasses | Molasses | 79.30 | 5.12 |
| 16 | Sorghum Molasses With Cane Syrup Blend | Corn syrup, cane syrup, sorghum molasses | 82.64 | 4.81 |
| 17 | Sorghum Molasses Table Syrup † | Corn syrup, sorghum syrup | 82.72 | 4.71 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eggleston, G.; Wartelle, L.; St. Cyr, E. Detecting Adulterated Commercial Sweet Sorghum Syrups with Ion Chromatography Oligosaccharide Fingerprint Profiles. Separations 2016, 3, 20. https://doi.org/10.3390/separations3030020
Eggleston G, Wartelle L, St. Cyr E. Detecting Adulterated Commercial Sweet Sorghum Syrups with Ion Chromatography Oligosaccharide Fingerprint Profiles. Separations. 2016; 3(3):20. https://doi.org/10.3390/separations3030020
Chicago/Turabian StyleEggleston, Gillian, Lynda Wartelle, and Eldwin St. Cyr. 2016. "Detecting Adulterated Commercial Sweet Sorghum Syrups with Ion Chromatography Oligosaccharide Fingerprint Profiles" Separations 3, no. 3: 20. https://doi.org/10.3390/separations3030020
APA StyleEggleston, G., Wartelle, L., & St. Cyr, E. (2016). Detecting Adulterated Commercial Sweet Sorghum Syrups with Ion Chromatography Oligosaccharide Fingerprint Profiles. Separations, 3(3), 20. https://doi.org/10.3390/separations3030020






