Carp Scales Modified with Cerium Oxide Nanoparticles as a New Bio-Adsorbent for Arsenic and Chromium Separation from Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Optimization of Adsorbent Synthesis
- The carp fish scales, washed and mechanically milled (PFS—pure fish scales);
- The carp fish scales modified with cerium dioxide nanoparticles (FS-CeO2);
- The porous carbonized carp fish scales modified with cerium dioxide nanoparticles (CFS-CeO2). Detailed data on the optimization procedure of both adsorbent syntheses are given in the Supplementary Materials (Section S2.2).
2.3. The Optimization of Experimental Conditions of Adsorption and Desorption
2.4. Material Characterization Methods
2.5. Batch Adsorption Experiments
2.6. Bed Column Experiments
2.7. Desorption Study
3. Results and Discussion
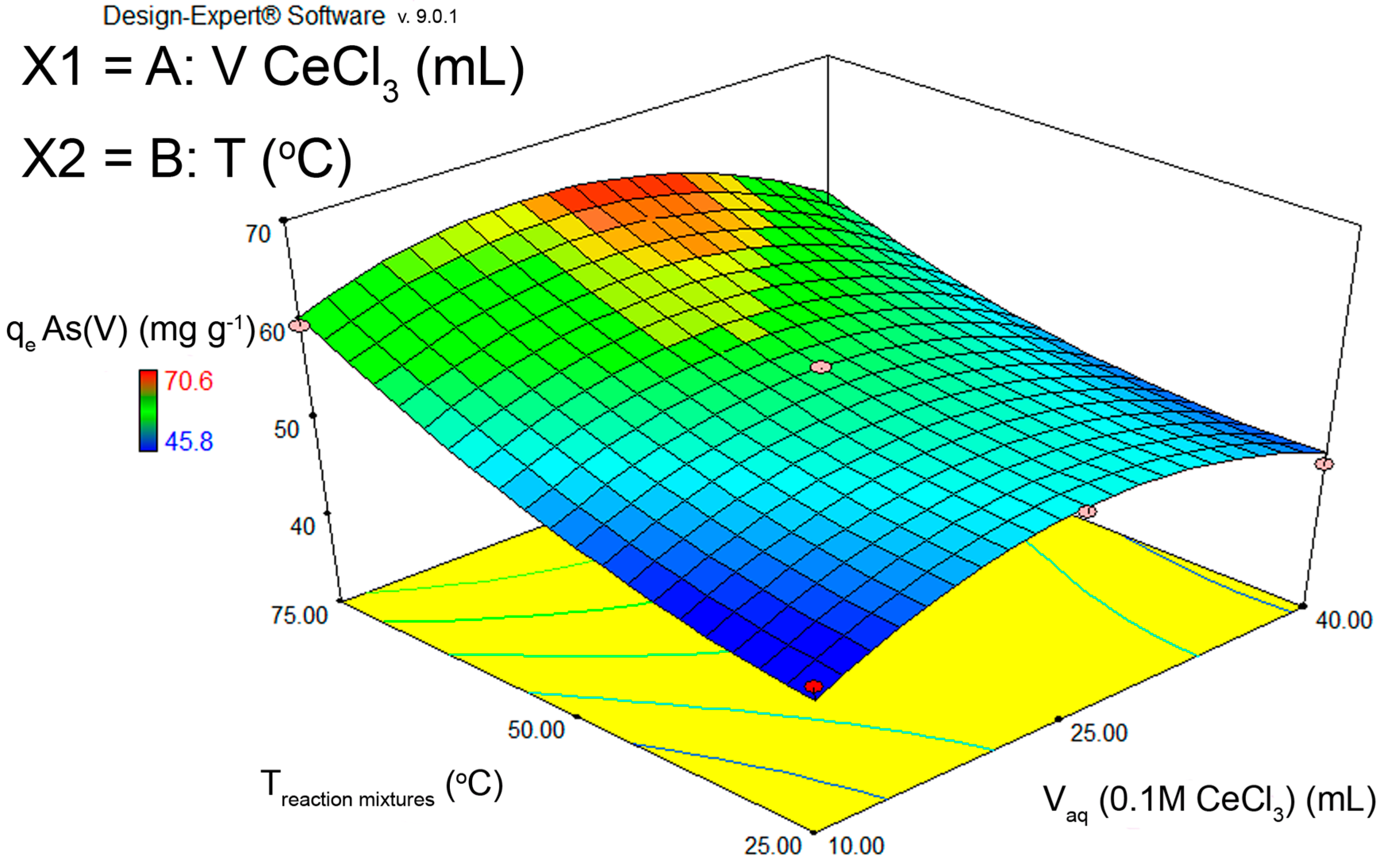
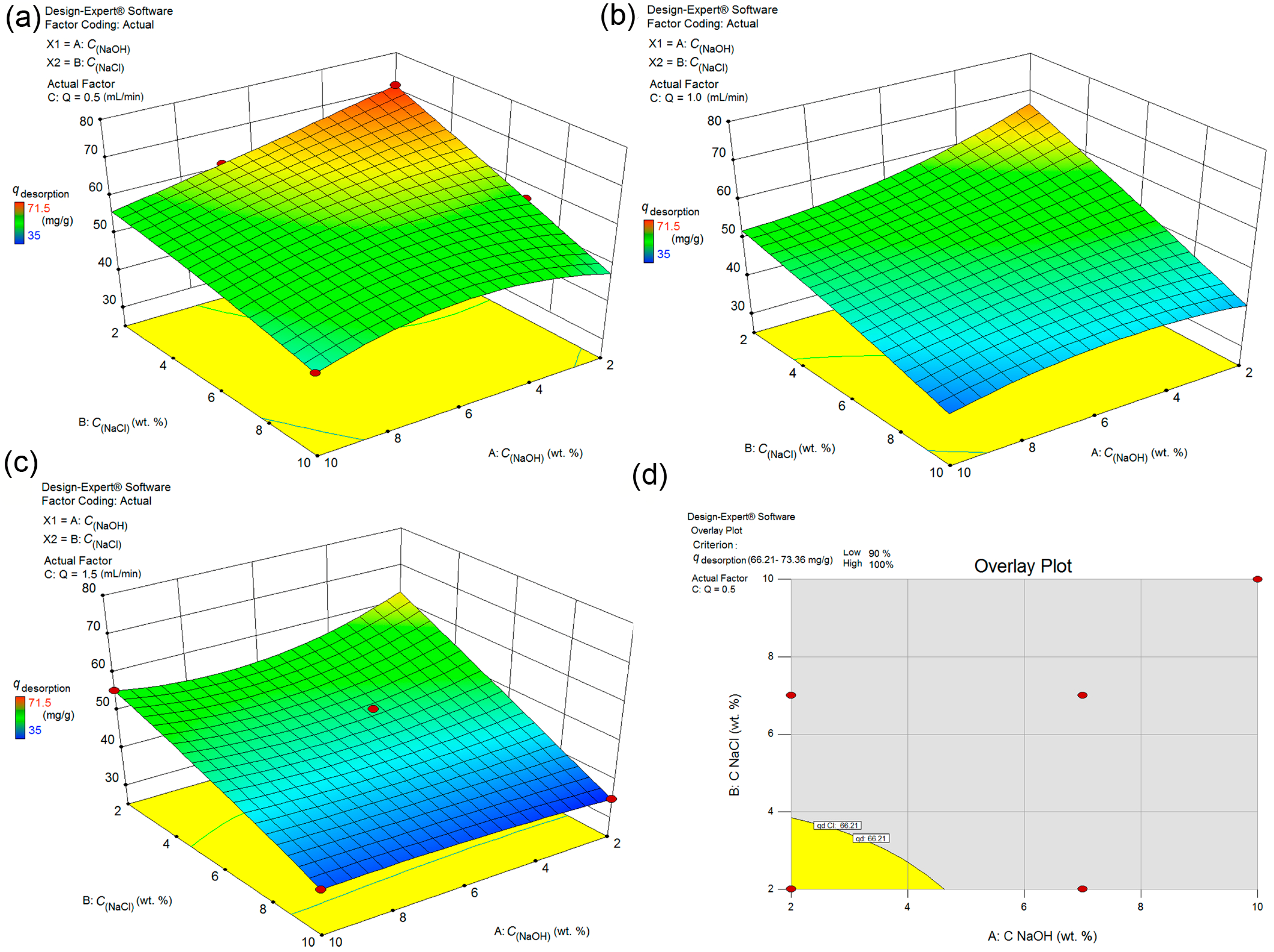
3.1. Optimization of FS-CeO2 Synthesis
3.2. Physico-Chemical Characterization
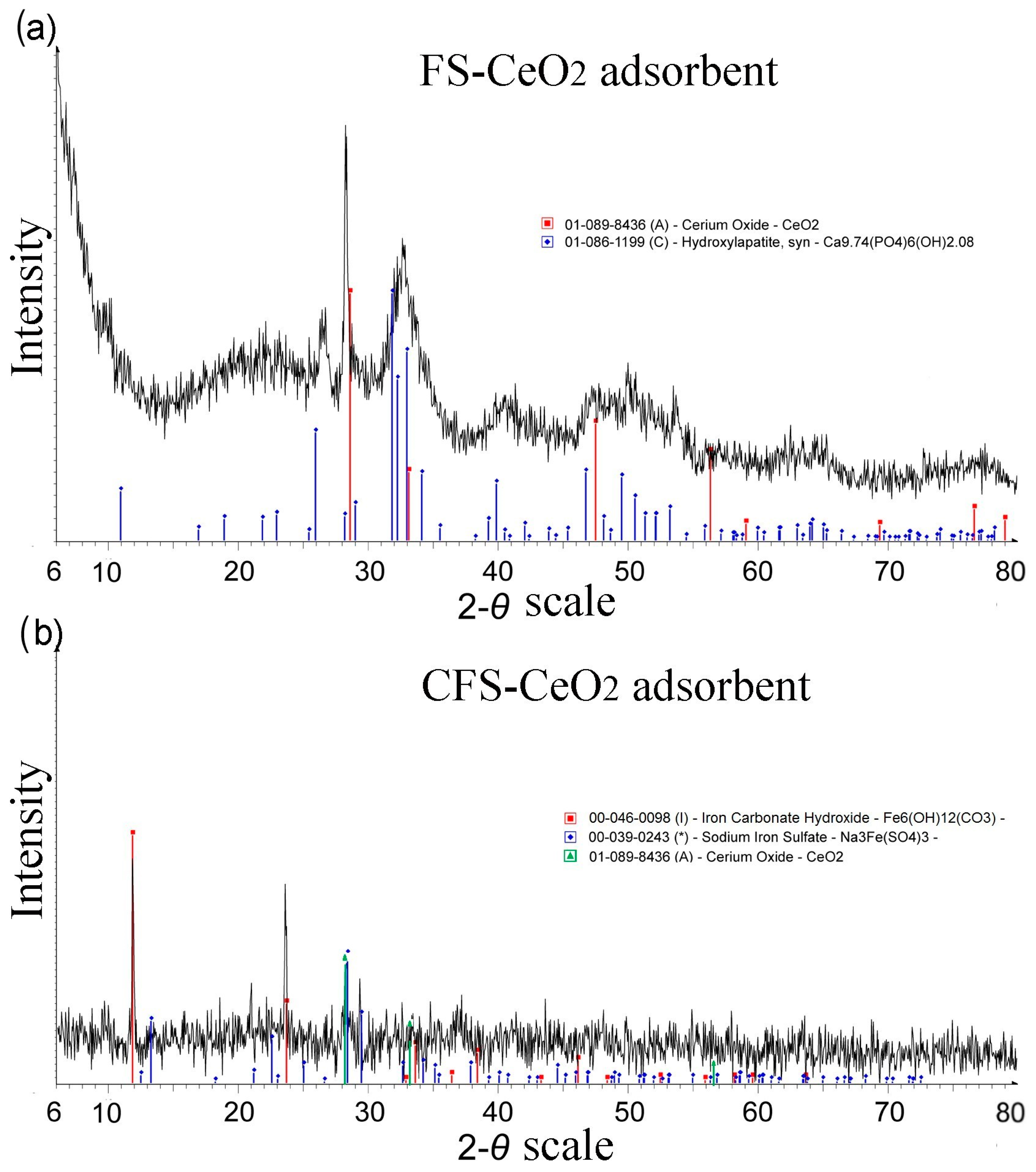
3.3. X-Ray Diffraction (XRD)
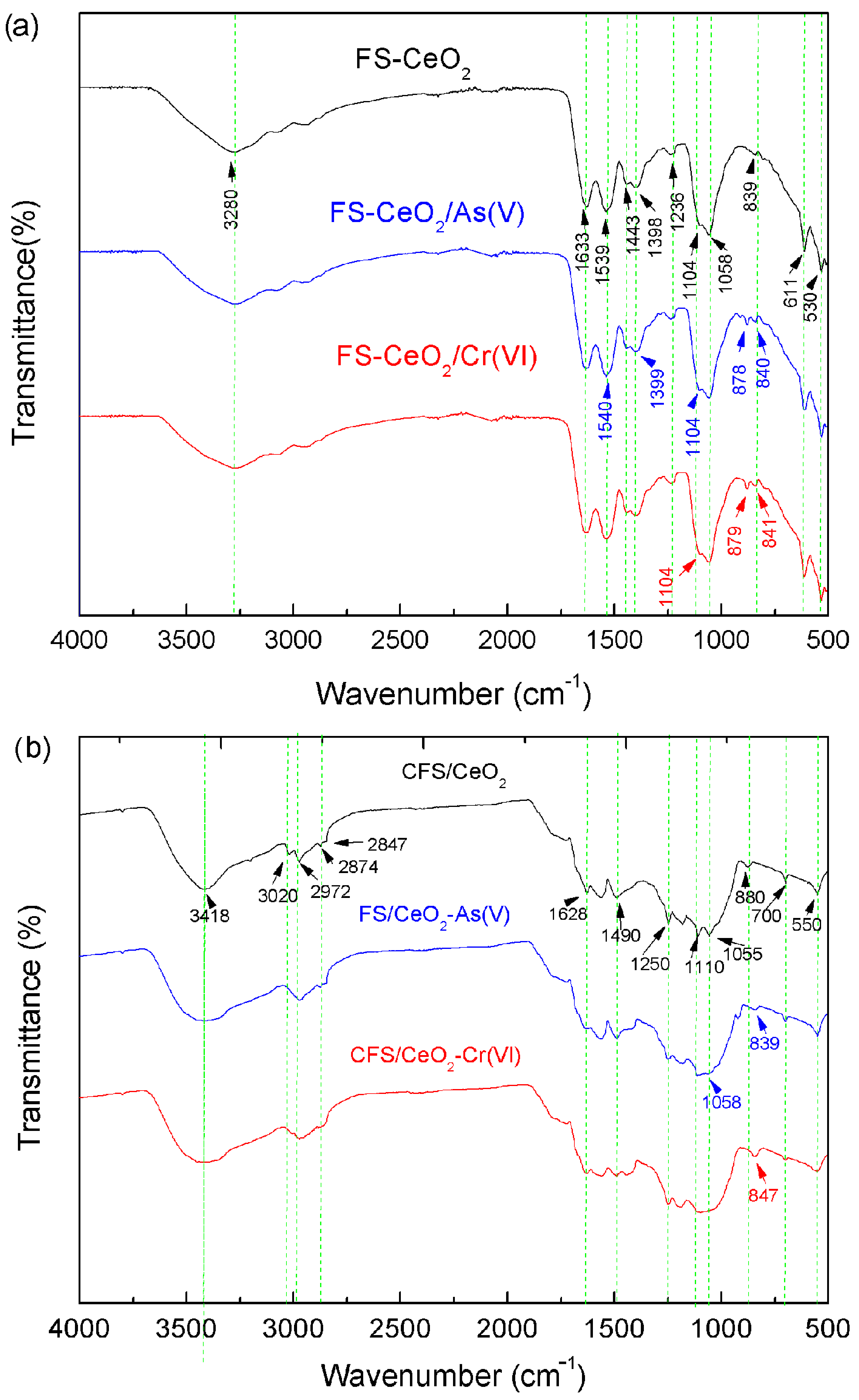
3.4. Fourier Transform Infrared Spectroscopy
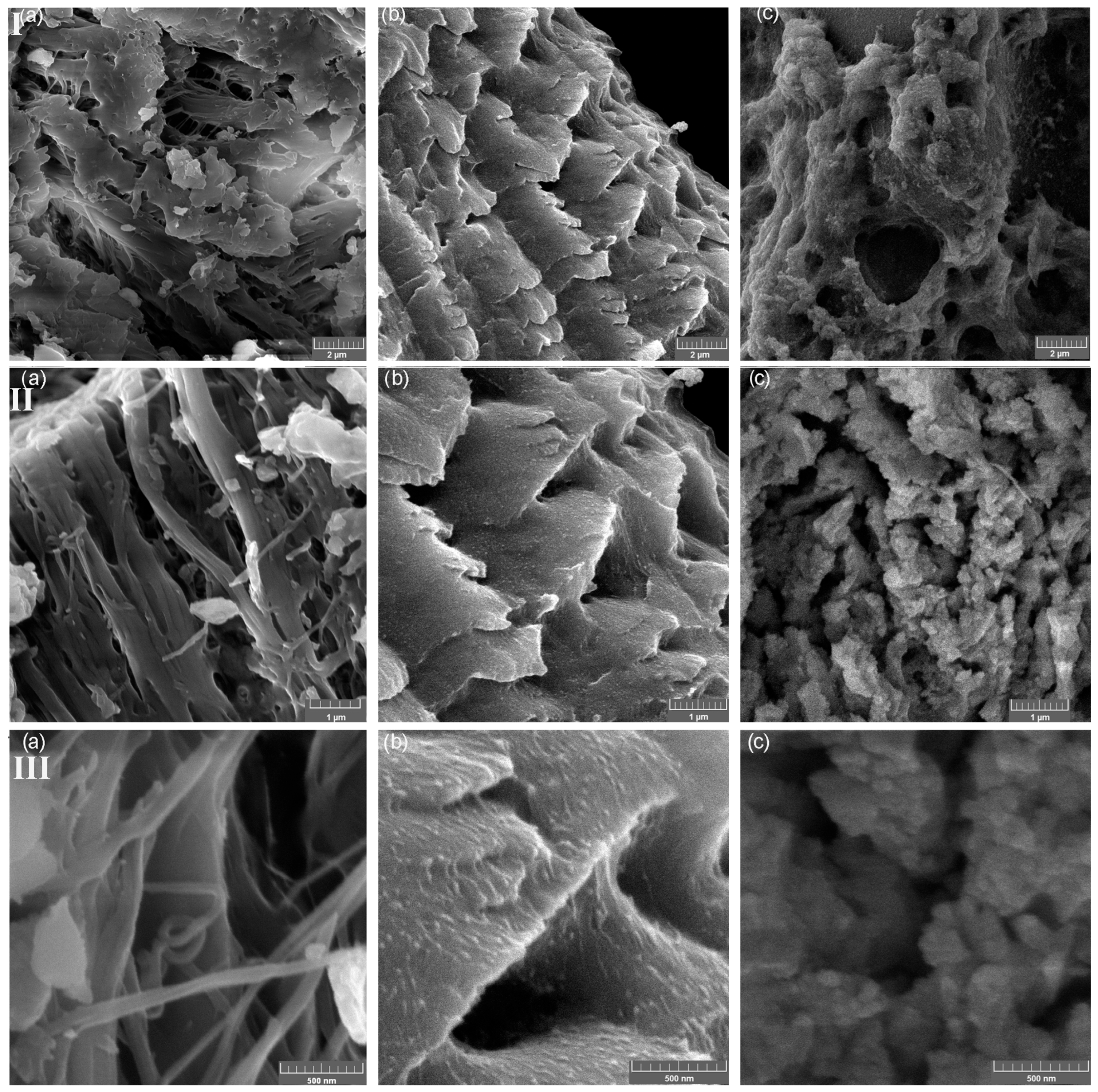
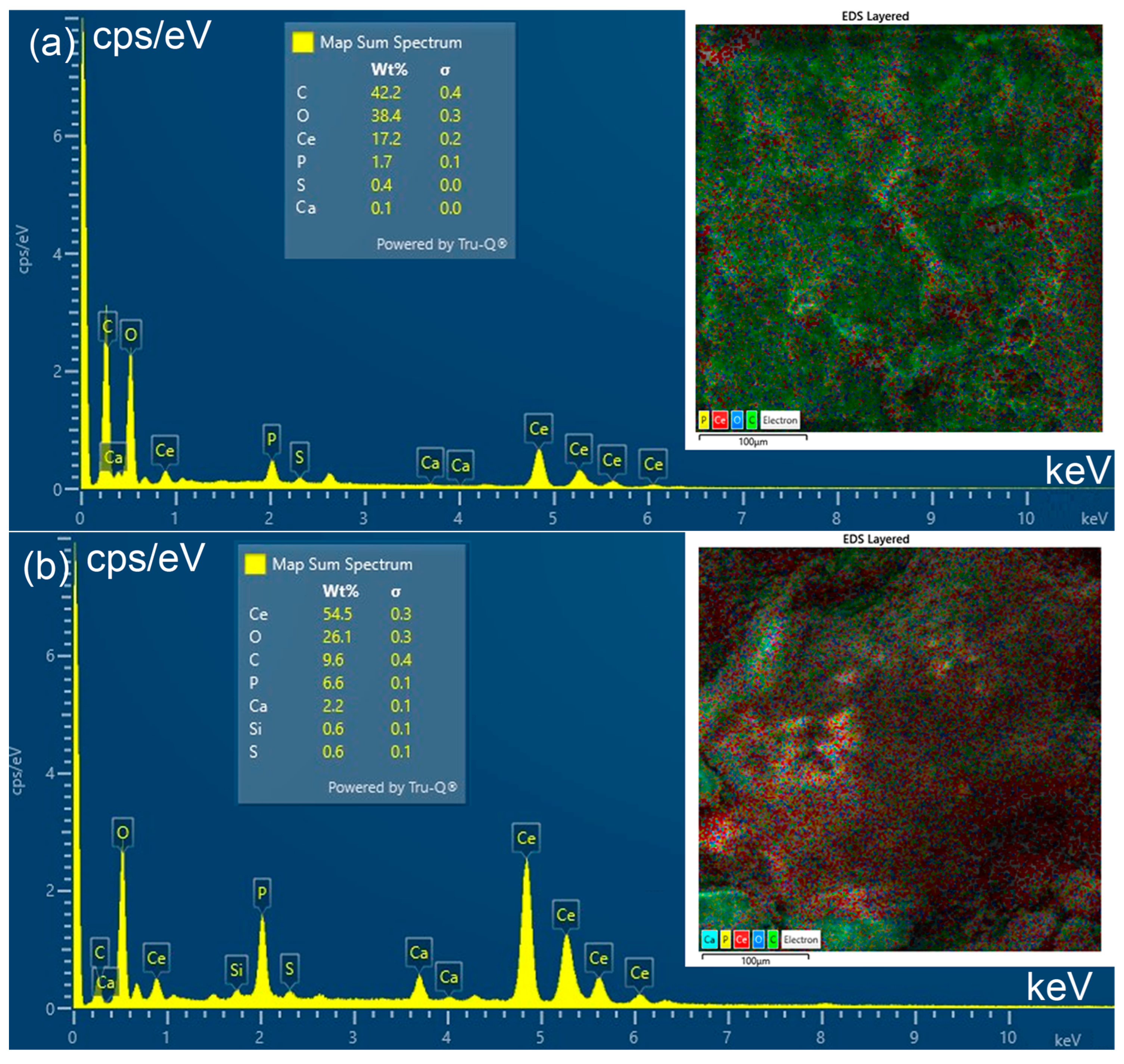
3.5. Scanning Electron Microscopy (SEM) and EDS Analysis
3.6. Adsorption Study
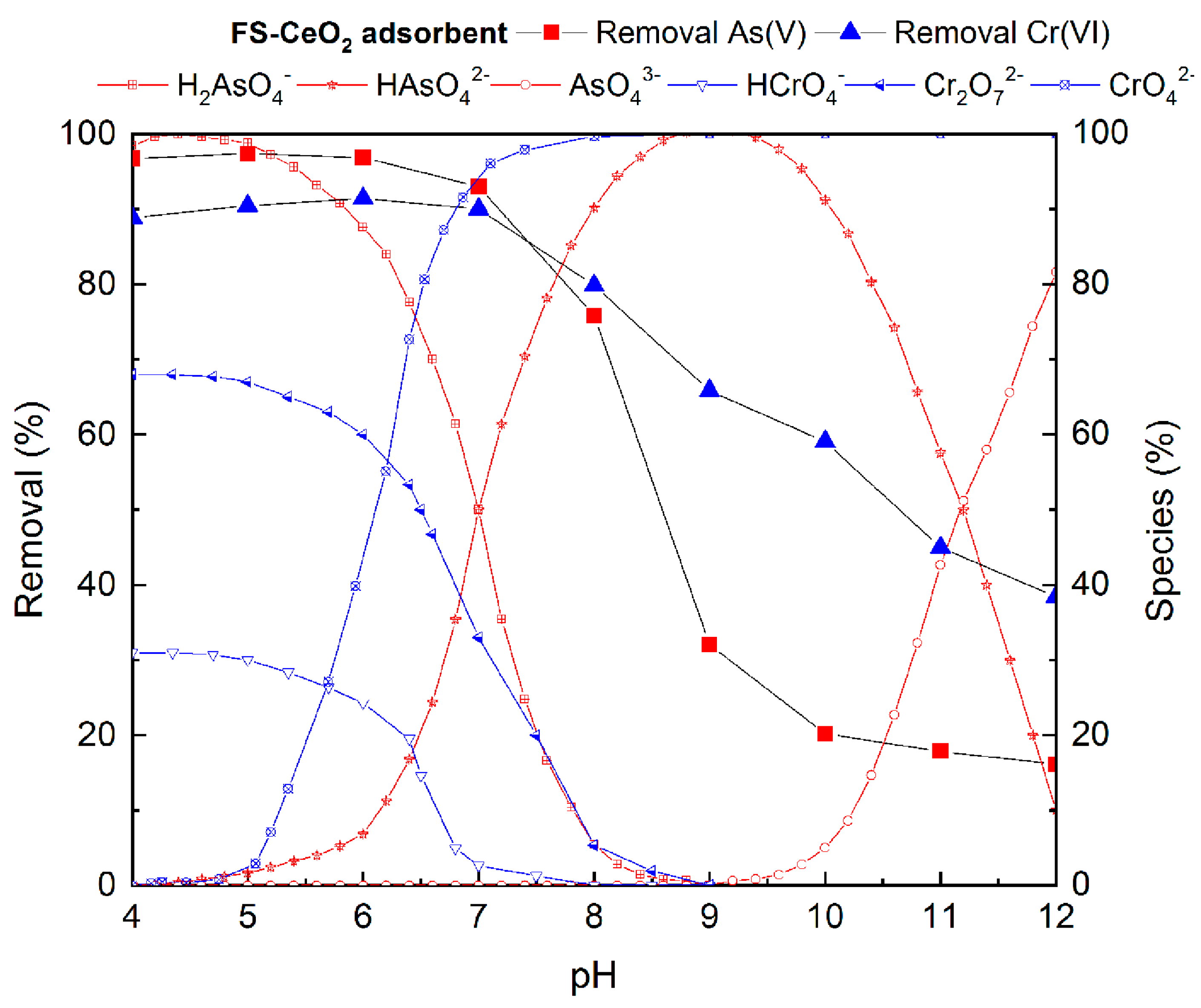
3.6.1. The Influence of pH
3.6.2. Optimization of Adsorption Using FS-CeO2 and CFS-CeO2 Adsorbents
3.6.3. Adsorption Equilibrium Study
3.6.4. Adsorption Thermodynamics
3.6.5. Adsorption Kinetics
3.6.6. The Adsorption Activation Energy
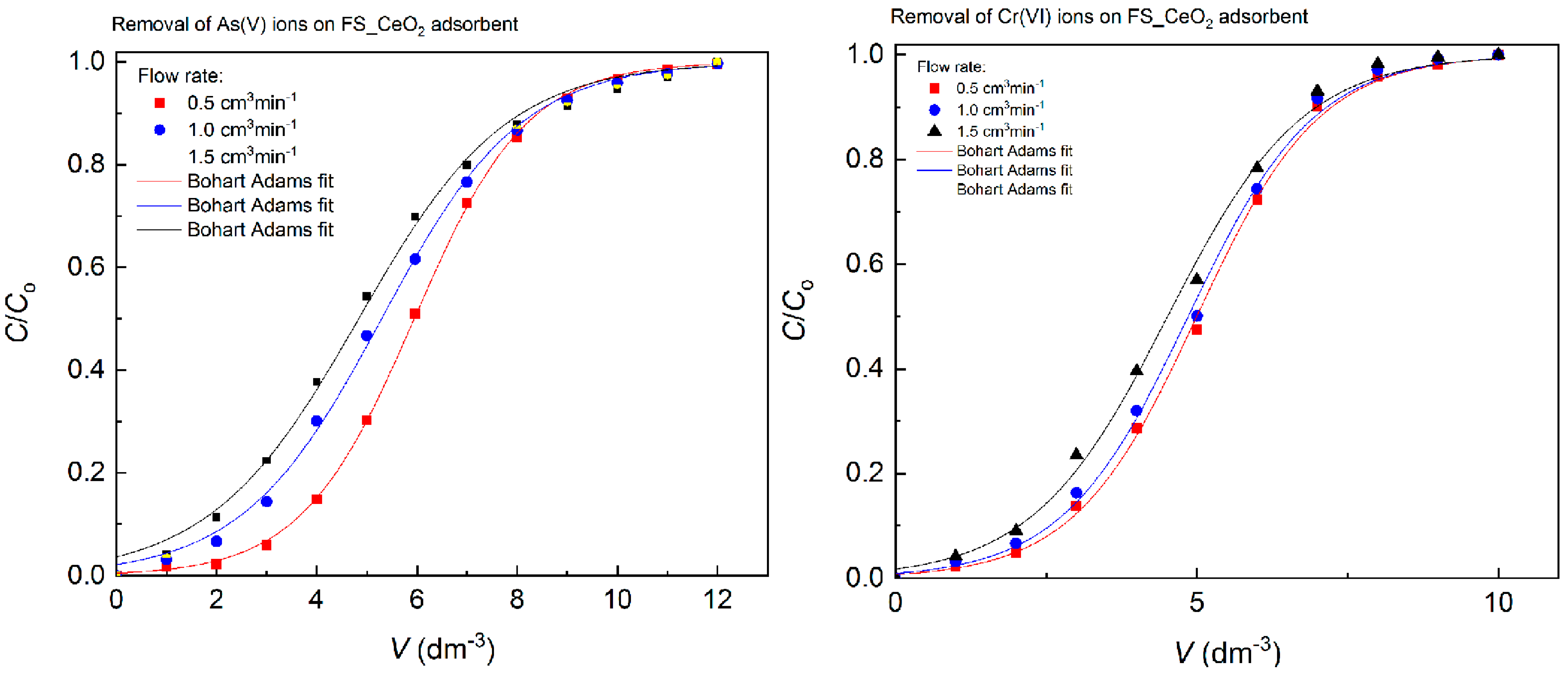
3.6.7. Bed Column Study
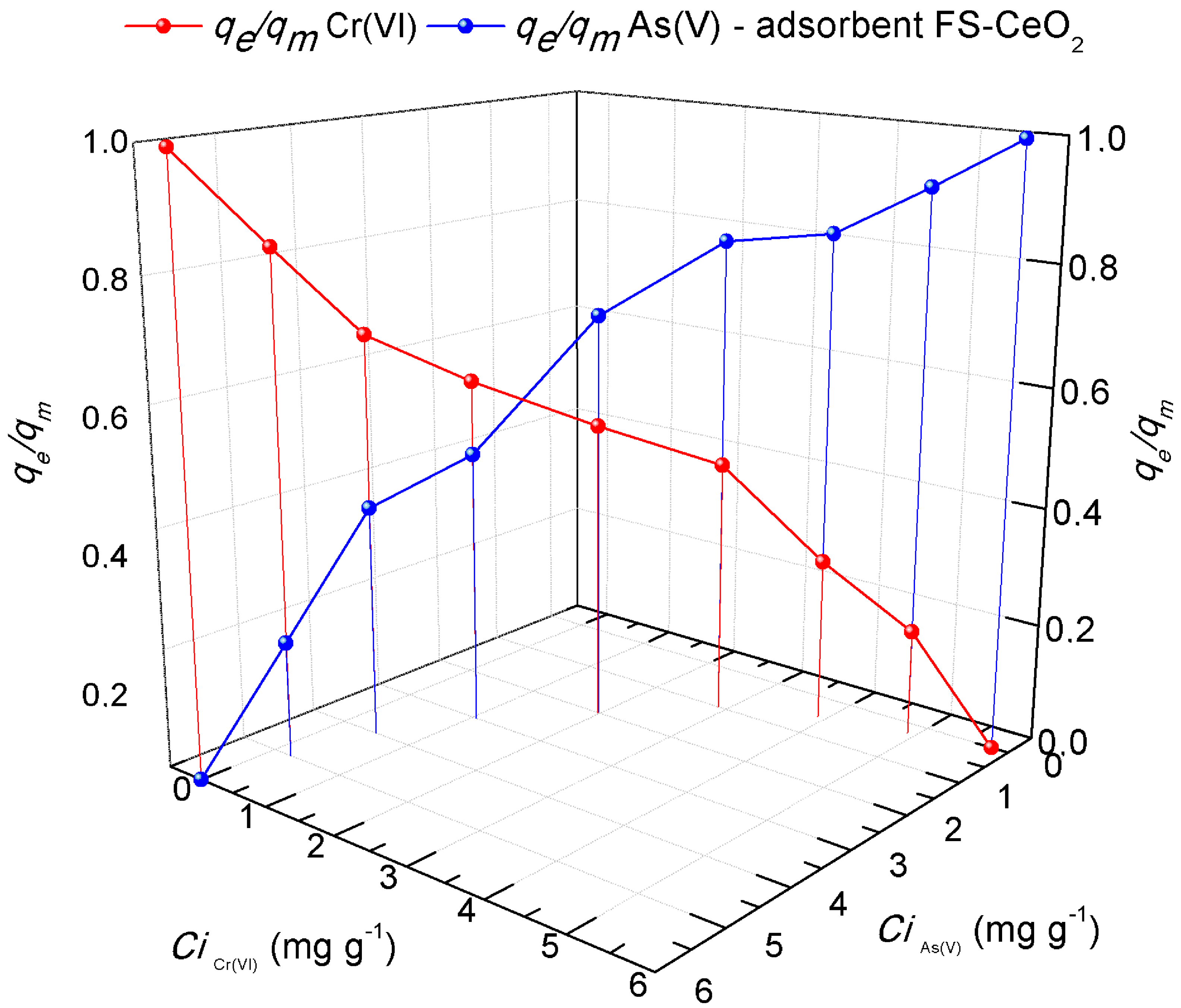
3.6.8. Competitive Arsenate and Chromate Ion Adsorption
3.6.9. Desorption Study
3.7. Comparative Review of Adsorbents Performances
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Muntean, S.G.; Halip, L.; Nistor, M.A.; Păcurariu, C. Removal of Metal Ions via Adsorption Using Carbon Magnetic Nanocomposites: Optimization through Response Surface Methodology, Kinetic and Thermodynamic Studies. Magnetochemistry 2023, 9, 163. [Google Scholar] [CrossRef]
- Fertu, D.I.; Bulgariu, L.; Gavrilescu, M. Modeling and Optimization of Heavy Metals Biosorption by Low-Cost Sorbents Using Response Surface Methodology. Processes 2022, 10, 523. [Google Scholar] [CrossRef]
- Alguacil, F.J.; Alcaraz, L.; García-Díaz, I.; López, F.A. Removal of Pb2+ in Wastewater via Adsorption onto an Activated Carbon Produced from Winemaking Waste. Metals 2018, 8, 697. [Google Scholar] [CrossRef]
- Perendija, J.; Velickovic, Z.S.; Cvijetic, I.; Rusmirovic, J.D.; Ugrinovic, V.; Marinkovic, A.D.; Onjia, A. Batch and column adsorption of cations, oxyanions and dyes on a magnetite modified cellulose-based membrane. Cellulose 2020, 27, 8215–8235. [Google Scholar] [CrossRef]
- Popovic, A.L.; Rusmirovic, J.D.; Velickovic, Z.; Radovanovic, Z.; Ristic, M.; Pavlovic, V.P.; Marinkovic, A.D. Novel amino-functionalized lignin microspheres: High performance biosorbent with enhanced capacity for heavy metal ion removal. Int. J. Biol. Macromol. 2020, 156, 1160–1173. [Google Scholar] [CrossRef]
- Georgaki, J.M.-N.; Charalambous, M.; Kazakis, N.; Talias, M.A.; Georgakis, C.; Papamitsou, T.; Mytiglaki, C. Chromium in Water and Carcinogenic Human Health Risk. Environments 2023, 10, 33. [Google Scholar] [CrossRef]
- Shaji, E.; Santosh, M.; Sarath, K.; Prakash, P.; Deepchand, V.; Divya, B. Arsenic contamination of groundwater: A global synopsis with focus on the Indian Peninsula. Geosci. Front. 2021, 12, 101079. [Google Scholar] [CrossRef]
- Bajić, Z.J.; Pamučar, D.S.; Bogdanov, J.Đ.; Bučko, M.M.; Veličković, Z.S. Optimization of arsenite adsorption on hydroxy apatite based adsorbent using the adaptive neuro-fuzzy inference system. Mil. Tech. Cour. 2019, 67, 735–752. [Google Scholar]
- Sharma, S.K.; Petrusevski, B.; Amy, G. Chromium removal from water: A review. J. Water Supply Res. Technol. AQUA 2008, 57, 541–553. [Google Scholar] [CrossRef]
- Sarkar, A.; Paul, B. The global menace of arsenic and its conventional remediation—A critical review. Chemosphere 2016, 158, 37–49. [Google Scholar] [CrossRef]
- Bajić, Z.J.; Veličković, Z.S.; Djokić, V.R.; Perić-Grujić, A.A.; Ersen, O.; Uskoković, P.S.; Marinković, A.D. Adsorption Study of Arsenic Removal by Novel Hybrid Copper Impregnated Tufa Adsorbents in a Batch System. Clean Soil. Air Water 2016, 44, 1477–1488. [Google Scholar] [CrossRef]
- Pantić, K.; Bajić, Z.J.; Veličković, Z.S.; Tomić, N.Z.; Marinković, A.D. Arsenic removal by copper-impregnated natural mineral tufa part II: A kinetics and column adsorption study. Environ. Sci. Pollut. Res. 2019, 26, 24143–24161. [Google Scholar] [CrossRef]
- Karanac, M.; Đolić, M.; Veljović, Đ.; Rajaković-Ognjanović, V.; Veličković, Z.; Pavićević, V.; Marinković, A. The removal of Zn2+, Pb2+, and As(V) ions by lime activated fly ash and valorization of the exhausted adsorbent. Waste Manag. 2018, 78, 366–378. [Google Scholar] [CrossRef]
- Veličković, Z.S.; Karkalić, R.; Bajić, Z.J.; Marinković, A.; Nikolić, A.; Otrisal, P.; Florus, S. Cerium supported on high porous carbon from fish scales carp, as a novel low cost adsorbent to remove As (V) ions from water. Mater. Methods Technol. 2018, 12, 110–122. [Google Scholar]
- Bajić, Z.J.; Djokić, V.R.; Veličković, Z.S.; Vuruna, M.M.; Ristić, M.Đ.; Ben Issa, N.; Marinković, A.D. Equilibrium, kinetic and thermodynamic studies on removal of Cd(II), Pb(II) and As(V) from wastewater using carp (Cyprinus Carpio) scales. Dig. J. Nanomater. Biostruct. 2013, 8, 1581–1590. [Google Scholar]
- Teshale, F.; Karthikeyan, R.; Sahu, O. Synthesized bioadsorbent from fish scale for chromium (III) removal. Micron 2020, 130, 102817. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Eletta, O.A.A. Recent advances in the biosorption of pollutants by fish scales: A mini-Review. Chem. Eng. Commun. 2021, 208, 1301–1312. [Google Scholar] [CrossRef]
- Damian, G.; Varvara, S. Assessment of Cyprinus carpio Scales as a Low-Cost and Effective Biosorbent for the Removal of Heavy Metals from the Acidic Mine Drainage Generated at Rosia Montana Gold Mine (Romania). Water 2022, 14, 3734. [Google Scholar] [CrossRef]
- Nagai, T.; Izumi, M.; Ishii, M. Fish scale collagen. Preparation and partial characterization. Int. J. Food Sci. Technol. 2004, 39, 239–244. [Google Scholar] [CrossRef]
- Li, R.; Li, Q.; Gao, S.; Shang, J.K. Exceptional arsenic adsorption performance of hydrous cerium oxide nanoparticles: Part A. Adsorption capacity and mechanism. Chem. Eng. J. 2012, 185, 127–135. [Google Scholar] [CrossRef]
- Gran, S.; Aziz, R.; Rafiq, M.T.; Abbasi, M.; Qayyum, A.; Elnaggar, A.Y.; Elganzory, H.H.; El-Bahy, Z.M.; Hussein, E.E. Development of cerium oxide/corncob nanocomposite: A cost-effective and eco-friendly adsorbent for the removal of heavy metals. Polymers 2021, 13, 4464. [Google Scholar] [CrossRef]
- Yi, L.; Mehrab, M. Photocatalytic Treatment of An Actual Confectionery Wastewater Using Ag/TiO2/Fe2O3: Optimization of Photocatalytic Reactions Using Surface Response Methodology. Catalysts 2018, 8, 409. [Google Scholar] [CrossRef]
- Pantić, K.; Bajić, Z.J.; Veličković, Z.S.; Djokića, V.; Rusmirović, J.; Marinković, A.; Perić-Grujić, A. Adsorption performances of branched aminated waste polyacrylonitrile fibers: Experimental versus modelling study. Desalination Water Treat. 2019, 171, 223–249. [Google Scholar] [CrossRef]
- Sawana, R.; Somasundar, Y.; Iyer, V.S.; Baruwati, B. Ceria modified activated carbon: An efficient arsenic removal adsorbent for drinking water purification. Appl. Water Sci. 2017, 7, 1223–1230. [Google Scholar] [CrossRef]
- Knežević, N.; Milanović, J.; Veličković, Z.; Milošević, M.; Vuksanović, M.M.; Onjia, A.; Marinković, A. A closed cycle of sustainable development: Effective removal and desorption of lead and dyes using an oxidized cellulose membrane. J. Ind. Eng. Chem. 2023, 126, 520–536. [Google Scholar] [CrossRef]
- Aichour, A.; Zaghouane-Boudiaf, H. Single and competitive adsorption studies of two cationic dyes from aqueous mediums onto cellulose-based modified citrus peels/calcium alginate composite. Int. J. Biol. Macromol. 2020, 154, 1227–1236. [Google Scholar] [CrossRef]
- Magsi, S.K.; Kandhar, I.A.; Brohi, R.-O.-Z.; Channa, A. Removal of metals from water using fish scales as a bio adsorbent. AIP Conf. Proc. 2019, 2119, 20023. [Google Scholar] [CrossRef]
- Parlayici, Ş.; Pehlivan, E. Comparative study of Cr(VI) removal by bio-waste adsorbents: Equilibrium, kinetics, and thermodynamic. J. Anal. Sci. Technol. 2019, 10, 15. [Google Scholar] [CrossRef]
- Zhang, X.; Ren, B.; Wu, X.; Yan, X.; Sun, Y.; Gao, H.; Qu, F. Efficient Removal of Chromium(VI) Using a Novel Waste Biomass Chestnut Shell-Based Carbon Electrode by Electrosorption. ACS Omega 2021, 6, 25389–25396. [Google Scholar] [CrossRef]
- Oke, I.A.; Olarinoye, N.O.; Adewusi, S.R.A. Adsorption Kinetics for Arsenic Removal from Aqueous Solutions by Untreated Powdered Eggshell. Adsorption 2008, 14, 73–83. [Google Scholar] [CrossRef]
- Ouédraogo, I.W.K.; Pehlivan, E.; Tran, H.T.; Bonzi-Coulibaly, Y.L.; Zachmann, D.; Bahadir, M. Synthesis of iron oxyhydroxide-coated rice straw (IOC-RS) and its application in arsenic(V) removal from water. J. Water Health 2015, 13, 726–736. [Google Scholar] [CrossRef]
- Guo, X.; Chen, F. Removal of Arsenic by Bead Cellulose Loaded with Iron Oxyhydroxide from Groundwater. Environ. Sci. Technol. 2005, 39, 6808–6818. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, X.; Xionghui, J.; Ma, L. Efficient arsenate removal by magnetite-modified water hyacinth biochar. Environ. Pollut. 2016, 216, 575–583. [Google Scholar] [CrossRef]
- Kongsri, S.; Janpradit, K.; Buapa, K.; Techawongstien, S.; Chanthai, S. Nanocrystalline hydroxyapatite from fish scale waste: Preparation, characterization and application for selenium adsorption in aqueous solution. Chem. Eng. J. 2013, 215–216, 522–532. [Google Scholar] [CrossRef]
- Shourije, S.M.J.S.; Dehghan, P.; Bahrololoom, M.E.; Cobley, A.J.; Vitry, V.; Azar, G.T.P.; Kamyab, H.; Mesbah, M. Using fish scales as a new biosorbent for adsorption of nickel and copper ions from wastewater and investigating the effects of electric and magnetic fields on the adsorption process. Chemosphere 2023, 317, 137829. [Google Scholar] [CrossRef]
- Selimin, M.A.; Latif, A.F.A.; Lee, C.W.; Muhamad, M.S.; Basri, H.; Lee, T.C. Adsorption efficiency of hydroxyapatite synthesised from black tilapia fish scales for chromium (VI) removal. Mater. Today Proc. 2022, 57, 1142–1146. [Google Scholar] [CrossRef]
- Kashyap, K.; Moharana, M.; Pattanayak, S.K.; Khan, F. Effective Removal of Pb(II), Cr(VI), and Cd(II) Ions from Water Using Environmentally Friendly Cerium Oxide Nanoparticles Synthesized from Pods of Pisum sativum. Water Air Soil Pollut. 2024, 235, 276. [Google Scholar] [CrossRef]
- Chen, B.; Zhu, Z.; Hong, J.; Wen, Z.; Ma, J.; Qiu, Y.; Chen, J. Nanocasted synthesis of ordered mesoporous cerium iron mixed oxide and its excellent performances for As(V) and Cr(VI) removal from aqueous solutions. Dalton Trans. 2014, 43, 10767–10777. [Google Scholar] [CrossRef]
- Hoang, V.A.; Yoshizuka, K.; Nishihama, S. Oxidative adsorption of arsenic from water environment by activated carbon modified with cerium oxide/hydroxide. Chem. Eng. Res. Des. 2022, 186, 161–173. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, C.; Yang, L.; Paul, C.J. Cerium oxide modified activated carbon as an efficient and effective adsorbent for the rapid uptake of arsenate and arsenite: Material development and study of performance and mechanisms. Chem. Eng. J. 2017, 315, 630–638. [Google Scholar] [CrossRef]
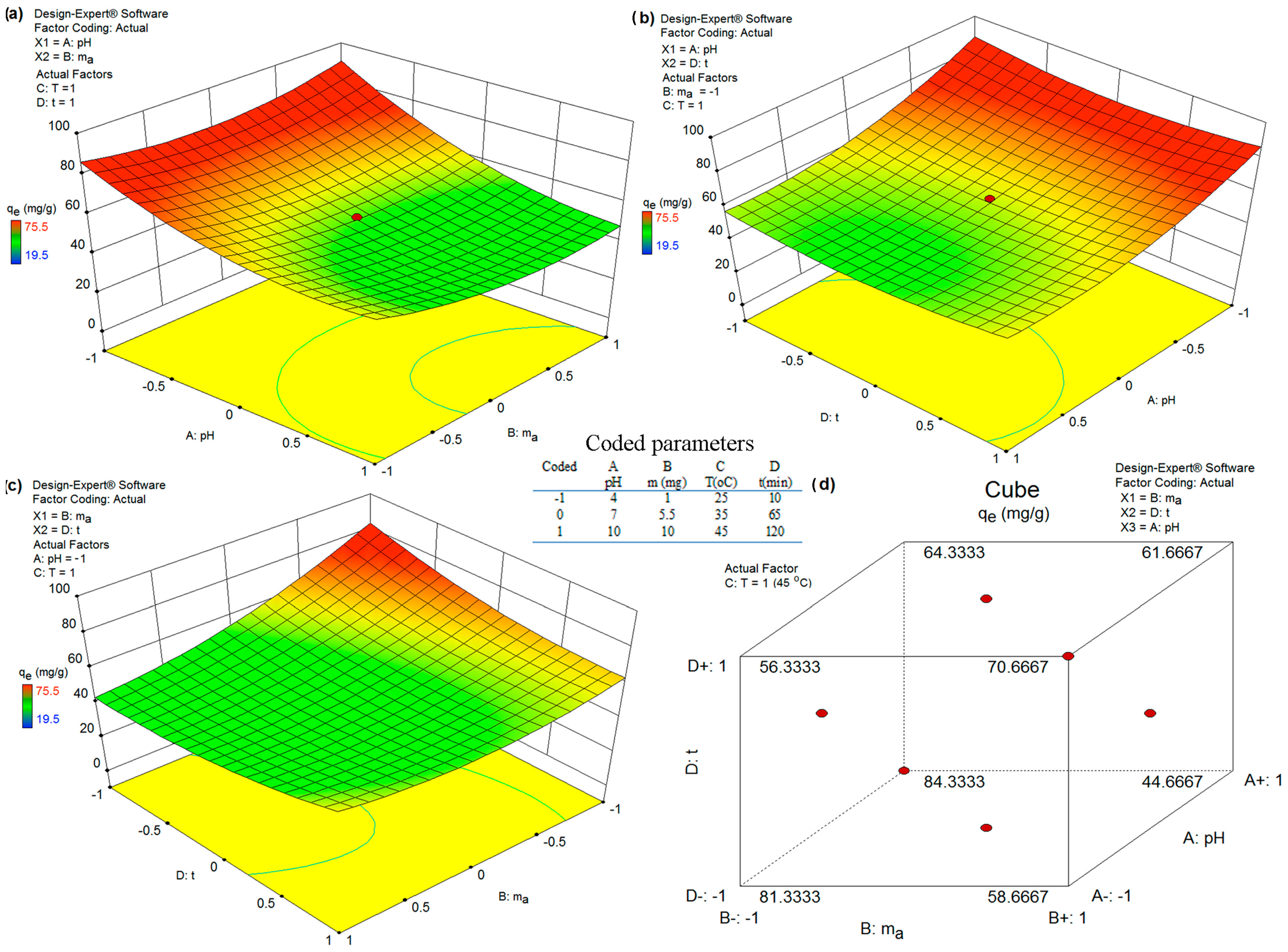
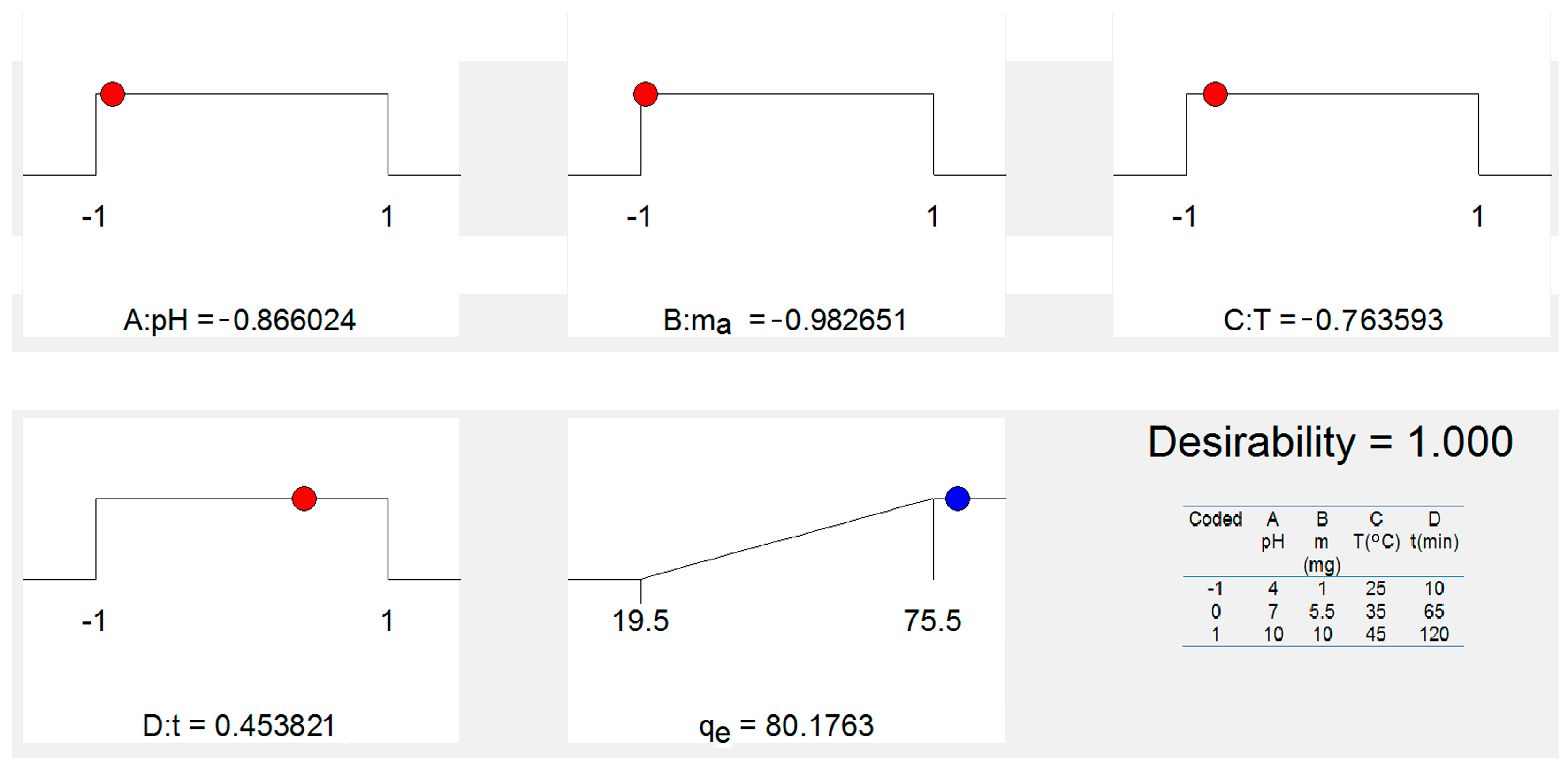
| Coded | A pH | B m (mg) | C T (°C) | D t (min) |
|---|---|---|---|---|
| −1 | 4 | 1 | 25 | 10 |
| 0 | 7 | 5.5 | 35 | 65 |
| 1 | 10 | 10 | 45 | 120 |
| Run No. | A pH | B m (mg) | C T (°C) | D t (min) | qe for As(V) (mg g−1) | qe for Cr(VI) (mg g−1) |
|---|---|---|---|---|---|---|
| 1 | 0 | 0 | 1 | −1 | 40.545 | 33.655 |
| 2 | −1 | 0 | 0 | 1 | 45.550 | 37.810 |
| 3 | 1 | 1 | 0 | 0 | 35.539 | 29.502 |
| 4 | 1 | 0 | −1 | 0 | 45.550 | 37.810 |
| 5 | −1 | 1 | 0 | 0 | 35.539 | 29.502 |
| 6 | 1 | −1 | 0 | 0 | 37.541 | 31.162 |
| 7 | −1 | 0 | −1 | 0 | 52.557 | 43.627 |
| 8 | −1 | −1 | 0 | 0 | 48.553 | 40.303 |
| 9 | 0 | 0 | 0 | 0 | 36.540 | 30.331 |
| 10 | 0 | 1 | −1 | 0 | 54.559 | 45.289 |
| 11 | 0 | −1 | 0 | 1 | 57.563 | 47.782 |
| 12 | 0 | 0 | 1 | 1 | 57.563 | 47.782 |
| 13 | 0 | 0 | −1 | −1 | 63.569 | 52.768 |
| 14 | 0 | 0 | 0 | 0 | 50.555 | 41.965 |
| 15 | 0 | 1 | 0 | −1 | 35.539 | 29.502 |
| 16 | 1 | 0 | 0 | 1 | 35.539 | 29.502 |
| 17 | 0 | −1 | −1 | 0 | 61.567 | 51.106 |
| 18 | 0 | 0 | −1 | 1 | 61.567 | 51.106 |
| 19 | −1 | 0 | 1 | 0 | 45.479 | 27.753 |
| 20 | 1 | 0 | 1 | 0 | 36.540 | 30.331 |
| 21 | 0 | 0 | 0 | 0 | 33.536 | 27.838 |
| 22 | 0 | 0 | 0 | 0 | 67.574 | 56.092 |
| 23 | 0 | 0 | 0 | 0 | 56.562 | 46.951 |
| 24 | 0 | −1 | 0 | −1 | 75.583 | 62.740 |
| 25 | 0 | −1 | 1 | 0 | 52.557 | 43.627 |
| 26 | −1 | 0 | 0 | −1 | 32.535 | 27.007 |
| 27 | 0 | 1 | 1 | 0 | 48.553 | 40.303 |
| 28 | 0 | 1 | 0 | 1 | 57.563 | 42.782 |
| 29 | 1 | 0 | 0 | −1 | 35.539 | 29.502 |
| Element | C | N | Ca | P | Na | Mg | Mineral Content |
|---|---|---|---|---|---|---|---|
| Content (%) | 26.5 | 11.1 | 14.5 | 6.7 | 0.4 | 0.3 | 46.26 |
| Adsorbent | Specific Surface (m2 g−1) | Pore Volumes (cm3 g−1) | Average Pore Diameter (nm) | pHPZC | Zeta Potential (mV) |
|---|---|---|---|---|---|
| FS * | 18.5 | 0.356 | 26.7 | 7.21 | / |
| FS-CeO2 | 36.9 | 0.488 | 6.8 | 7.05 | −17.92 (pH 5.14) |
| CFS-CeO2 | 106.7 | 0.638 | 2.5 | 6.84 | −17.40 (pH 5.02) |
| Source Model | Sum of Squares | df | Mean Square | F Value | p-Value |
|---|---|---|---|---|---|
| Prob > F | |||||
| Model | 4013.89 | 14 | 286.71 | 6.36 | 0.0007 |
| A-pH | 1140.75 | 1 | 1140.75 | 25.29 | 0.0002 |
| B-ma | 936.33 | 1 | 936.33 | 20.76 | 0.0004 |
| C-T | 21.33 | 1 | 21.33 | 0.47 | 0.5028 |
| D-t | 168.75 | 1 | 168.75 | 3.74 | 0.0735 |
| AB | 72.25 | 1 | 72.25 | 1.60 | 0.2263 |
| AC | 272.25 | 1 | 272.25 | 6.04 | 0.0277 |
| AD | 6.25 | 1 | 6.25 | 0.14 | 0.7153 |
| BC | 25.00 | 1 | 25.00 | 0.55 | 0.4689 |
| BD | 342.25 | 1 | 342.25 | 7.59 | 0.0155 |
| CD | 132.25 | 1 | 132.25 | 2.93 | 0.1089 |
| A2 | 545.05 | 1 | 545.05 | 12.08 | 0.0037 |
| B2 | 368.93 | 1 | 368.93 | 8.18 | 0.0126 |
| C2 | 236.77 | 1 | 236.77 | 5.25 | 0.0380 |
| D2 | 208.29 | 1 | 208.29 | 4.62 | 0.0496 |
| Residual | 631.42 | 14 | 45.10 | ||
| Lack of Fit | 631.42 | 10 | 63.14 | ||
| Pure Error | 0.000 | 4 | 0.000 | ||
| Cor Total | 4645.31 | 28 | |||
| Std. Dev. | 6.72 | R-Squared | 0.9689 | ||
| Mean | 47.26 | Adj R2 | 0.9387 | ||
| C.V.% | 14.21 | Pred R2 | 0.8771 | ||
| PRESS | 3636.96 | Adeq Precision | 18.385 |
| Adsorbent | Isotherm Models and Parameters | Temperature | |||||
|---|---|---|---|---|---|---|---|
| 25 °C | 35 °C | 45 °C | |||||
| As(V) | Cr(VI) | As(V) | Cr(VI) | As(V) | Cr(VI) | ||
| Langmuir isotherm | |||||||
| FS-CeO2 | qm (mg g−1) | 92.61 | 65.30 | 94.72 | 70.70 | 96.58 | 71.82 |
| KL (dm3 mg−1) | 2.45 | 2.51 | 2.69 | 2.80 | 3.00 | 3.21 | |
| R2 | 0.949 | 0.961 | 0.957 | 0.956 | 0.964 | 0.958 | |
| CFS-CeO2 | qm (mg g−1) | 89.67 | 57.30 | 92.07 | 58.05 | 94.44 | 58.73 |
| KL (dm3 mg−1) | 1.39 | 1.74 | 1.44 | 1.83 | 1.49 | 1.93 | |
| R2 | 0.984 | 0.941 | 0.983 | 0.946 | 0.980 | 0.938 | |
| Freundlich isotherm | |||||||
| FS-CeO2 | KF (dm3 mg−1)1/n | 65.84 | 45.04 | 70.36 | 47.92 | 75.48 | 51.06 |
| n | 1.94 | 1.95 | 1.94 | 1.94 | 1.95 | 1.93 | |
| R2 | 0.998 | 0.998 | 0.999 | 0.998 | 0.999 | 0.998 | |
| CFS-CeO2 | KF (dm3 mg−1)1/n | 48.80 | 32.47 | 51.17 | 33.51 | 53.75 | 34.60 |
| n | 1.80 | 2.33 | 1.79 | 2.35 | 1.78 | 2.37 | |
| R2 | 0.998 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 | |
| Adsorbent/ion | ΔGΘ (kJ mol−1) | ΔHΘ (kJ mol−1) | ΔSΘ (J mol−1 K−1) | R2 | ||
|---|---|---|---|---|---|---|
| 25 °C | 35 °C | 45 °C | ||||
| FS-CeO2/As(V) | −39.10 | −40.64 | −42.26 | 7.97 | 157.82 | 0.994 |
| FS-CeO2/Cr(V) | −39.16 | −40.75 | −42.43 | 9.67 | 163.73 | 0.993 |
| CFS-CeO2/As(V) | −37.70 | −39.04 | −40.40 | 2.59 | 135.13 | 0.996 |
| CFS-CeO2/Cr(V) | −38.25 | −39.66 | −41.08 | 3.93 | 141.47 | 0.998 |
| Adsorbent | Parameters of Model | Pseudo-First Order | Pseudo-Second Order | Second Order | |||
|---|---|---|---|---|---|---|---|
| As(V) | Cr(VI) | As(V) | Cr(VI) | As(V) | Cr(VI) | ||
| FS-CeO2 | qe | 6.01 | 9.19 | 9.22 | 9.98 | 9.22 | 9.98 |
| k (k1, k2) | 0.076 | 0.086 | 0.016 | 0.008 | 0.088 | 0.049 | |
| R2 | 0.933 | 0.931 | 0.998 | 0.994 | 0.869 | 0.834 | |
| CFS-CeO2 | qe | 5.31 | 8.15 | 8.67 | 9.61 | 8.67 | 9.61 |
| k (k1, k2) | 0.070 | 0.074 | 0.019 | 0.0099 | 0.027 | 0.029 | |
| R2 | 0.969 | 0.968 | 0.998 | 0.994 | 0.882 | 0.883 | |
| Model and Parameters | Ions | Q (cm3 min−1) | |||
|---|---|---|---|---|---|
| 0.5 | 1.0 | 1.5 | |||
| Bohart–Adams | KBA (dm3 mg−1 min−1) | As(V) | 0.153 | 0.248 | 0.349 |
| qo (mg g−1) | 73.36 | 65.33 | 56.68 | ||
| R2 | 0.999 | 0.998 | 0.997 | ||
| KBA (dm3 mg−1 min−1) | Cr(VI) | 0.164 | 0.320 | 0.446 | |
| qo (mg g−1) | 63.80 | 61.98 | 57.62 | ||
| R2 | 0.998 | 0.997 | 0.997 | ||
| Yoon–Nelson | kYN (min−1) | As(V) | 0.888 | 0.719 | 0.675 |
| θ (min) | 5.94 | 5.29 | 4.84 | ||
| R2 | 0.999 | 0.997 | 0.998 | ||
| kYN (min−1) | Cr(VI) | 0.983 | 0.957 | 0.981 | |
| θ (min) | 4.99 | 4.88 | 4.51 | ||
| R2 | 0.999 | 0.998 | 0.997 | ||
| Cycle | Adsorption (mg g−1) * | Flow Rate (mL min−1) | Desorption (mg g−1) * | Desorption Efficiency (%) | C (ppm) ** | Δq (mg g−1) *** |
|---|---|---|---|---|---|---|
| I | 73.36 | 0.50 | 71.23 | 97.1 | 334.8 | 2.1 |
| 1.00 | 65.36 | 89.1 | 307.2 | 8.0 | ||
| 1.50 | 58.61 | 79.9 | 275.5 | 14.7 | ||
| III | 69.45 | 0.50 | 66.05 | 95.1 | 310.4 | 3.4 |
| 1.00 | 60.28 | 86.8 | 283.3 | 9.2 | ||
| 1.50 | 54.73 | 78.8 | 257.2 | 14.7 | ||
| V | 65.75 | 0.50 | 60.95 | 92.7 | 286.5 | 4.8 |
| 1.00 | 55.29 | 84.1 | 259.9 | 10.5 | ||
| 1.50 | 49.84 | 75.8 | 234.3 | 15.9 |
| Adsorbent | Ci (mg dm−3) | Oxyanions/qmax (mg g−1) | Isotherm or Kinetic Model | Ref. |
|---|---|---|---|---|
| fish scales | 0.25 mg dm−3 | As(V)/64% (dose 200 mg dm−3) | exp. Batch Adsorption Studies | [27] |
| 10.00 mg dm−3 | Cr(VI)/first litre of 96.25% removed (dose 20 g dm−3) | exp. Column Study | ||
| cranberry kernel shell (CKS) | 1000 mg dm−3 | Cr(VI)/10.42 | Langmuir | [28] |
| rosehip seed shell (RSS) | 1000 mg dm−3 | Cr(VI)/15.17 | Langmuir | |
| banana peel (BP) | 1000 mg dm−3 | Cr(VI)/6.81 | Langmuir | |
| carbonized waste biomass chestnut shells (CPC)—electrosorption | 30 mg dm−3 | Cr(VI)/43.18- 89.71 | exp. | [29] |
| Untreated powdered eggshell | 0.5 mg dm−3 | As(V)/43.18 | Langmuir | [30] |
| Rice straw—treated Fe(NO3)3 | 50.0 mg dm−3 | As(V)/21.74 | Langmuir | [31] |
| Bead Cellulose (Cotton)—treated FeCl3·6H2O | / | As(III)/99.6, As(V)/33.2 | Langmuir | [32] |
| Magnetite-modified water hyacinth Biochar (MW2501) | 5 mg dm−3 | As(V)/8.19 | Langmuir | [33] |
| Nanocrystalline hydroxyapatite prepared from fish scale waste (FHAp) | 0.01 mg dm−3 | Se(IV)/1.94 | Langmuir | [34] |
| fish scale waste (FS) | 0.01 mg dm−3 | Se(IV)/1.02 | Langmuir | |
| fish scale waste | 150 mg dm−3 | Cr(III)/99.75% (dose 800 mg dm−3) | Pseudo 2nd order, qe = 18.83 mg g−1 | [35] |
| FS-CeO2 | 5.78 mg dm−3 | As(V)/92.61 | Langmuir | this study |
| FS-CeO2 | 6.00 mg dm−3 | Cr(IV)/65.30 | Langmuir | |
| CFS-CeO2 | 5.78 mg dm−3 | As(V)/89.67 | Langmuir | |
| CFS-CeO2 | 6.00 mg dm−3 | Cr(IV)/57.30 | Langmuir |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bajić, Z.; Veličković, U.Z.; Djokić, V.; Bučko, M.; Bogdanov, J.; Pantić, K.; Marinković, A.D. Carp Scales Modified with Cerium Oxide Nanoparticles as a New Bio-Adsorbent for Arsenic and Chromium Separation from Water. Separations 2025, 12, 253. https://doi.org/10.3390/separations12090253
Bajić Z, Veličković UZ, Djokić V, Bučko M, Bogdanov J, Pantić K, Marinković AD. Carp Scales Modified with Cerium Oxide Nanoparticles as a New Bio-Adsorbent for Arsenic and Chromium Separation from Water. Separations. 2025; 12(9):253. https://doi.org/10.3390/separations12090253
Chicago/Turabian StyleBajić, Zoran, Uroš Z. Veličković, Veljko Djokić, Mihael Bučko, Jovica Bogdanov, Krstimir Pantić, and Aleksandar D. Marinković. 2025. "Carp Scales Modified with Cerium Oxide Nanoparticles as a New Bio-Adsorbent for Arsenic and Chromium Separation from Water" Separations 12, no. 9: 253. https://doi.org/10.3390/separations12090253
APA StyleBajić, Z., Veličković, U. Z., Djokić, V., Bučko, M., Bogdanov, J., Pantić, K., & Marinković, A. D. (2025). Carp Scales Modified with Cerium Oxide Nanoparticles as a New Bio-Adsorbent for Arsenic and Chromium Separation from Water. Separations, 12(9), 253. https://doi.org/10.3390/separations12090253







