Iron Recovery from Turkish and Romanian Bauxite Residues Through Magnetic Separation: Effect of Hydrothermal Processing and Separation Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Bauxite Residue Materials
2.2. Analytical Techniques
2.3. Hydrothermal Reduction
2.4. Wet Magnetic Separation
3. Results
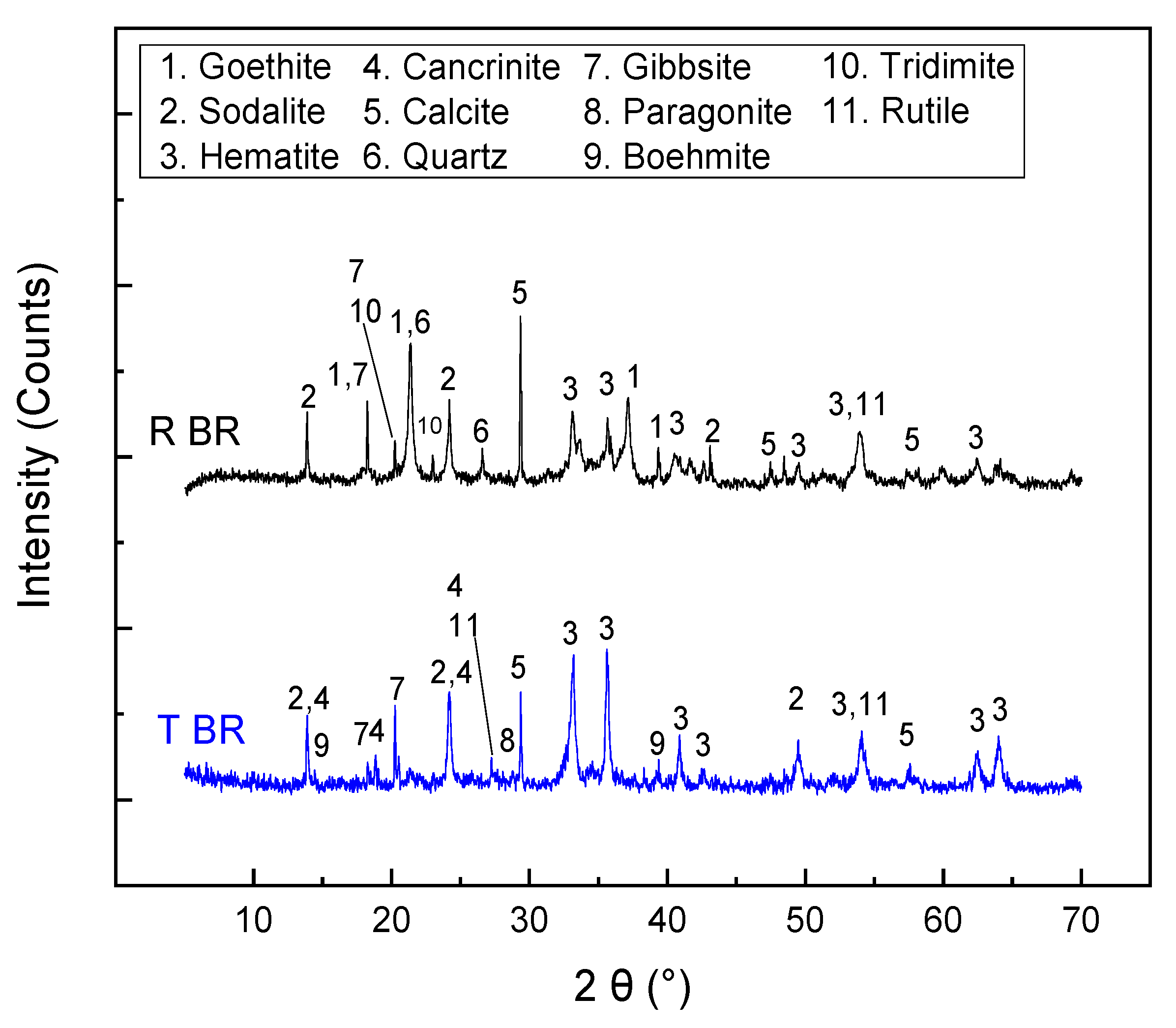
3.1. Physicochemical Characterization of the Bauxite Residue Samples
3.2. Alkaline Hydrothermal Reduction
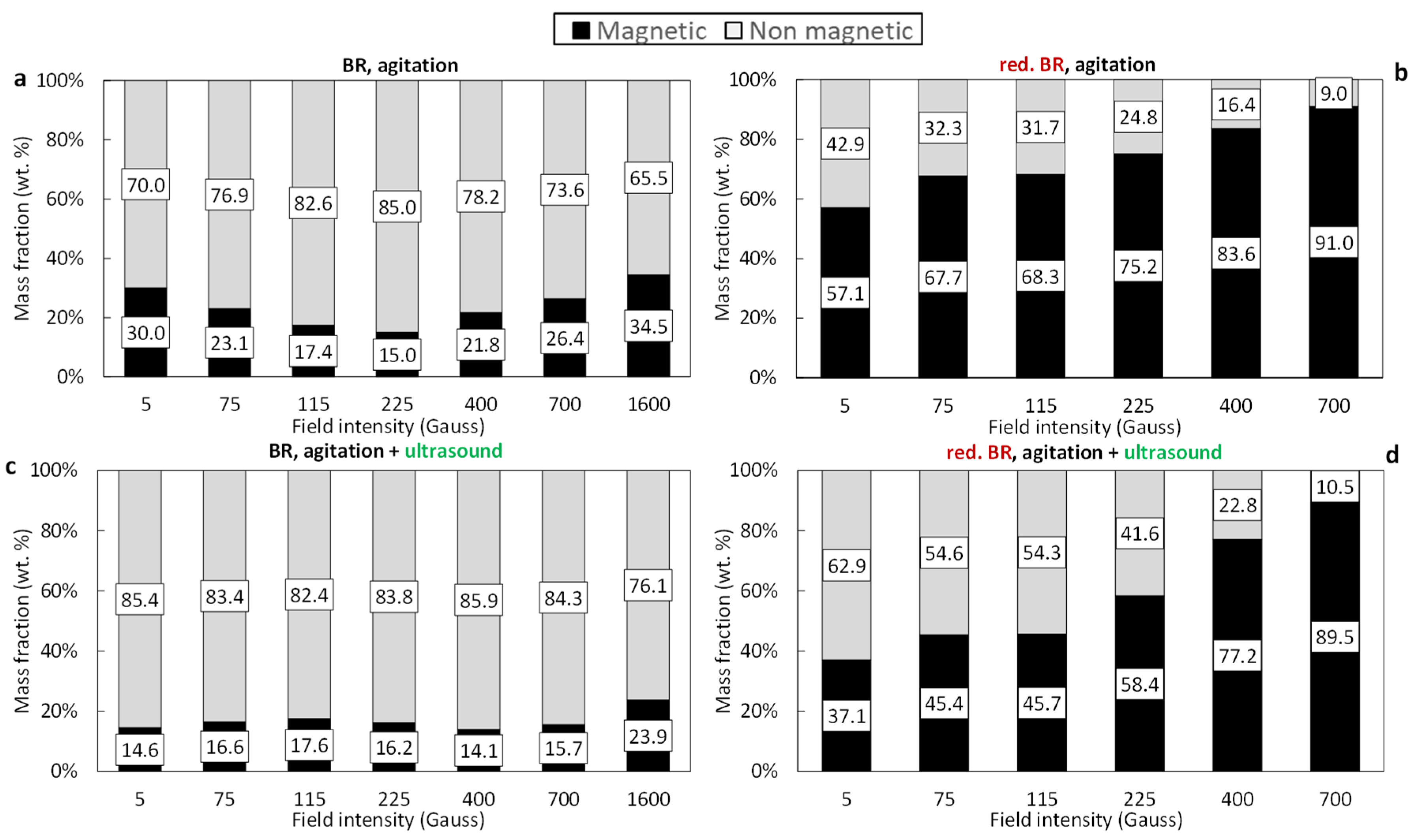
3.3. Magnetic Separation Tests
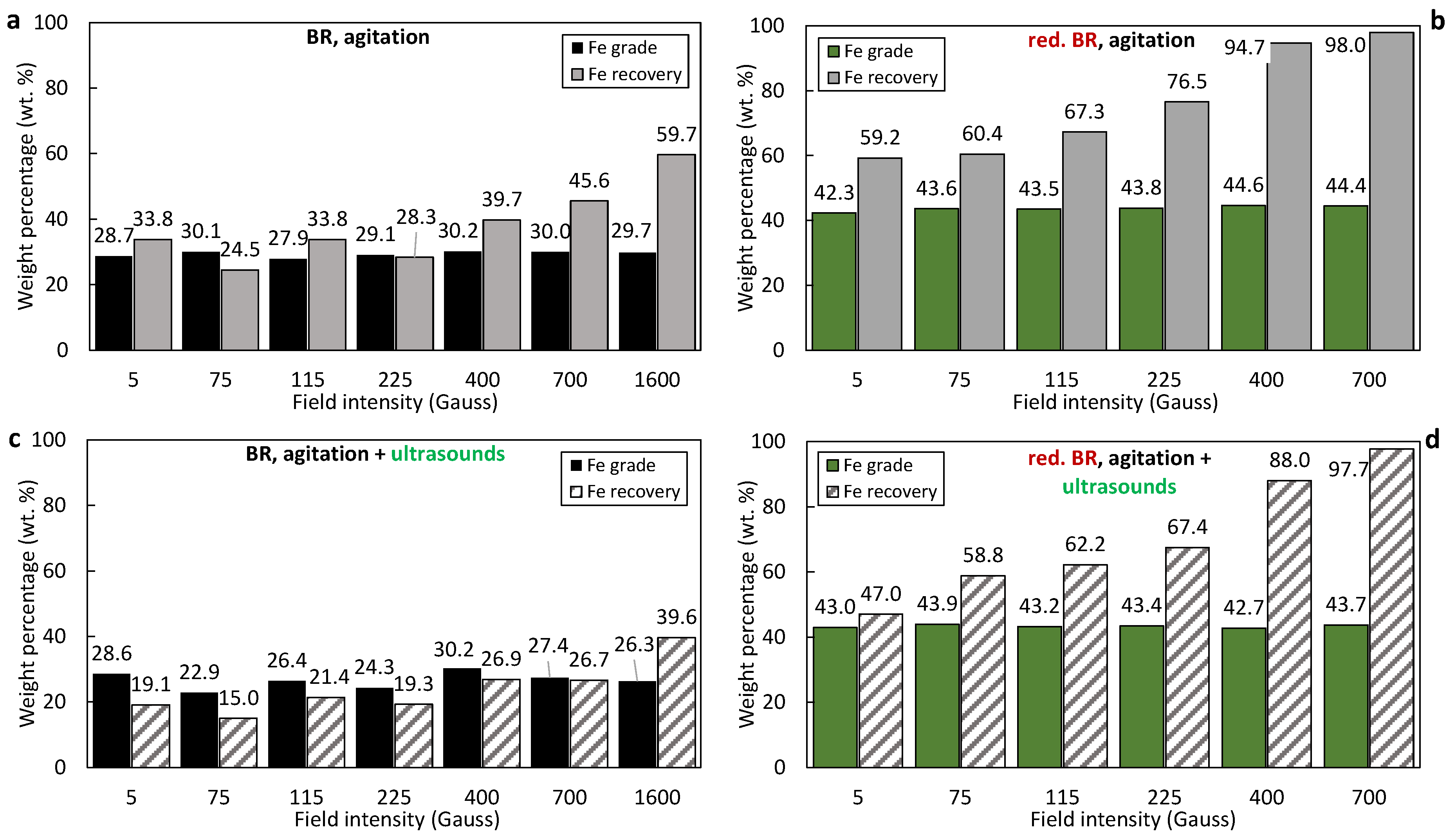
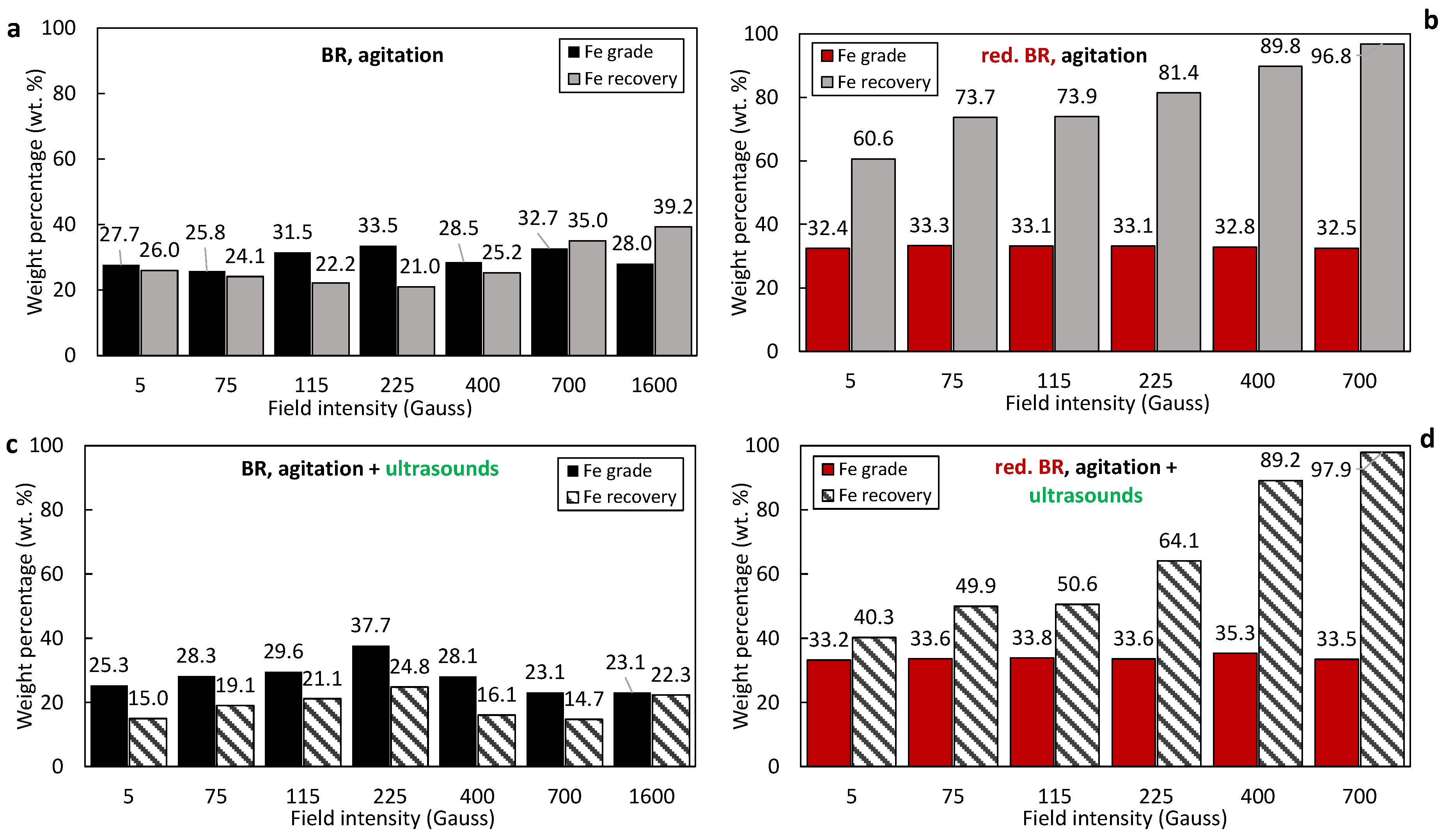
3.4. Separation Performance
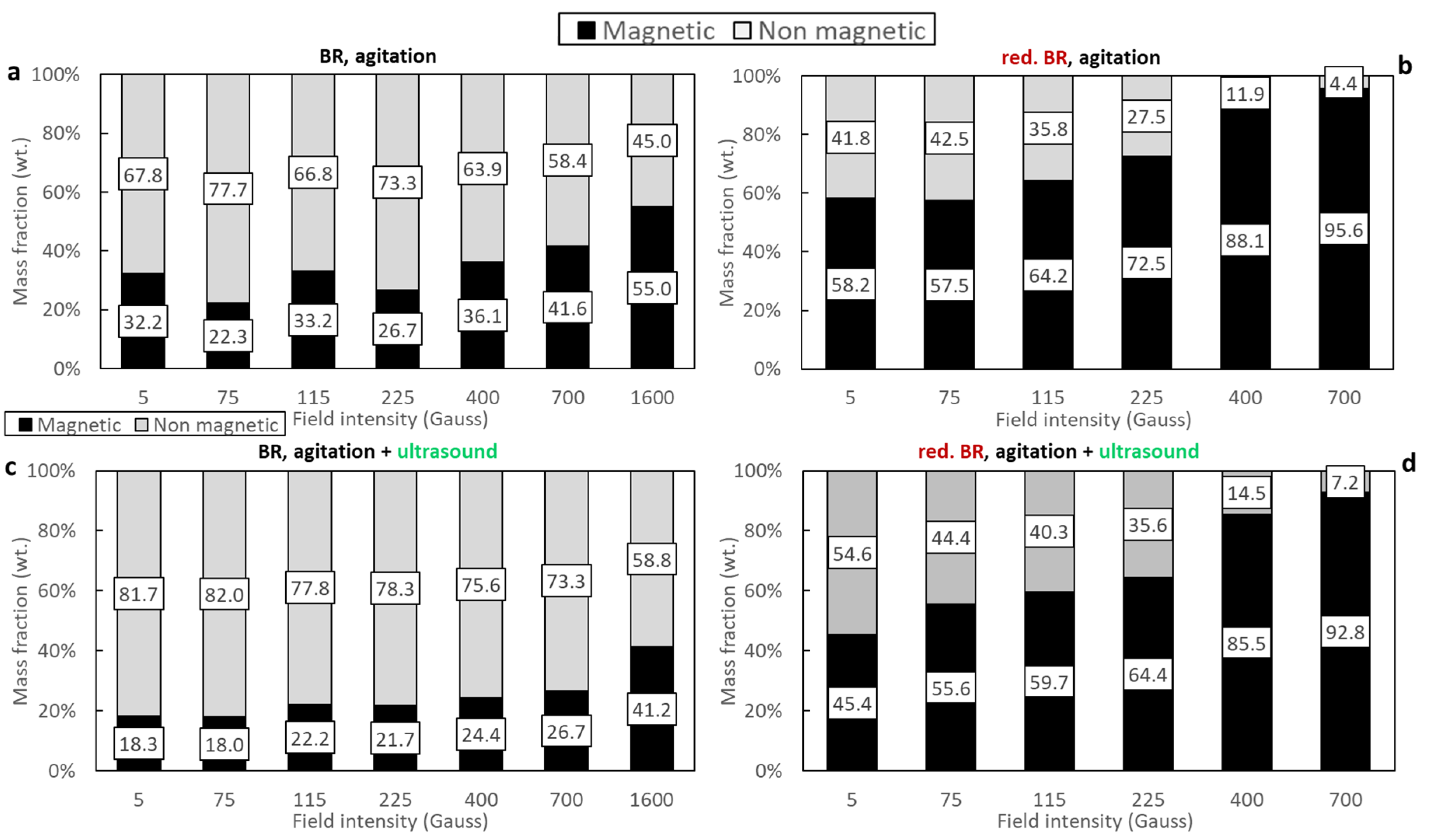
- Hydrothermal treatment: the reduction of goethite and hematite and the formation of magnetite impacts the separation performance primarily because of the higher magnetic susceptibility of the latter. In addition to this, hydrothermal treatment reduces the mass of the solids due to Al leaching, leading to enrichment of the iron-containing minerals and those minerals containing Si, Na, Ca and Ti, evidenced by comparison of the composition of raw BR samples (Table 3) and the hydrothermally treated ones (Table 5). As has been shown elsewhere, the Al-rich liquor composition for Greek BR can have more than 17g/L Al2O3 [29]. With hydrothermal treatment, under a magnetic field of 400 Gs, the Fe recovery increases from 39.7% to 94.7% for the RBR sample, and from 25.2% to 89.8% with hydrothermal treatment of the TBR sample. Fe grade increases too, but not so much; for the raw RBR, it increases from 30.2% to 44.6%, while for the TBR sample, it increases from 28.5% for raw BR to 32.8% for the concentrate obtained from the hydrothermally treated sample.
- Ultrasonication as particle dispersion method: the dispersion of the particles in the slurry that is fed into the magnetic separator is aimed at the breakage of agglomerates that would reduce the selectivity of the separation process. Indeed, as has been shown, the formation of the clusters is unavoidable due to the particle size. As seen in the SEM images (Figure 2, Figure 3 and Figure 5), a considerable portion of both the RBR and TBR samples consists of grains with sizes below 10 μm. With such a small particle size, attracting forces like surface tension, adhesion, and van der Waals forces dominate over gravity, promoting the production of clusters [29,35]. The application of ultrasounds combined with agitation for the dispersion of the particles in the slurry affected the magnetic fraction obtained; for separation under 400 Gs, the magnetic fraction is 36.1% under agitation and 21.8% when agitation and ultrasounds are combined for the raw Romanian sample; for the Turkish sample, the respective results are 24.4% and 14.1%. Ultrasound indeed broke the clusters, producing slurry with particles of lower effective diameters. This weakened the magnetic forces which are proportional to the size and allowed fluid forces (mainly drag) to prevail, enhancing the particles’ entrainment by the water stream, as has been shown elsewhere [36]. Another explanation of the reduction in the separation performance under the application of ultrasound, is that, although ultrasonication improves particle dispersion, it also causes fracturing and cracking of the iron-containing bauxite residue particles [37]. As has been demonstrated, the presence of defects (such as cracks and gaps) within the particles leads to an increase in the internal demagnetizing field, which subsequently reduces the maximum magnetic permeability and retention efficiency [38].
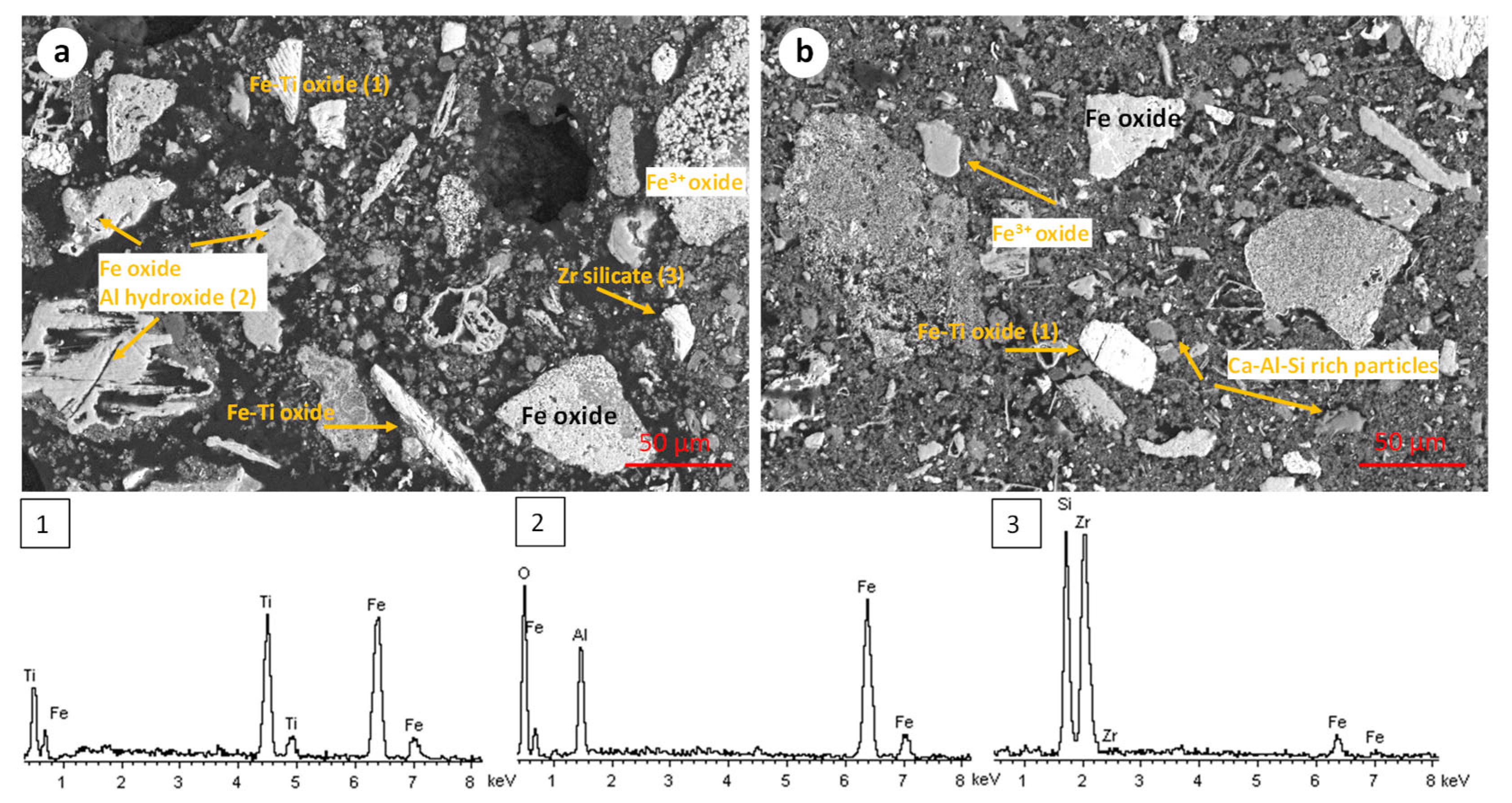
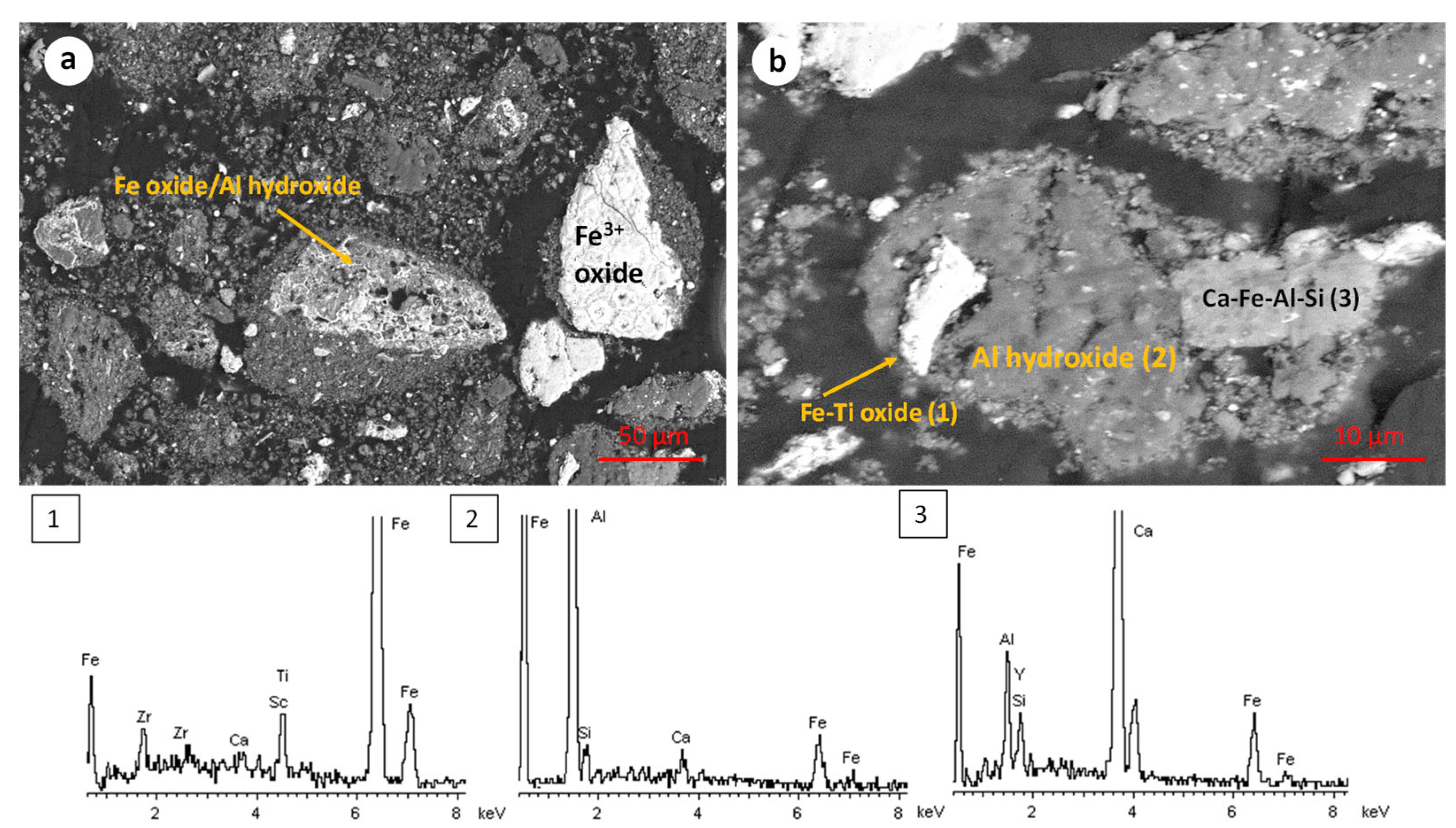
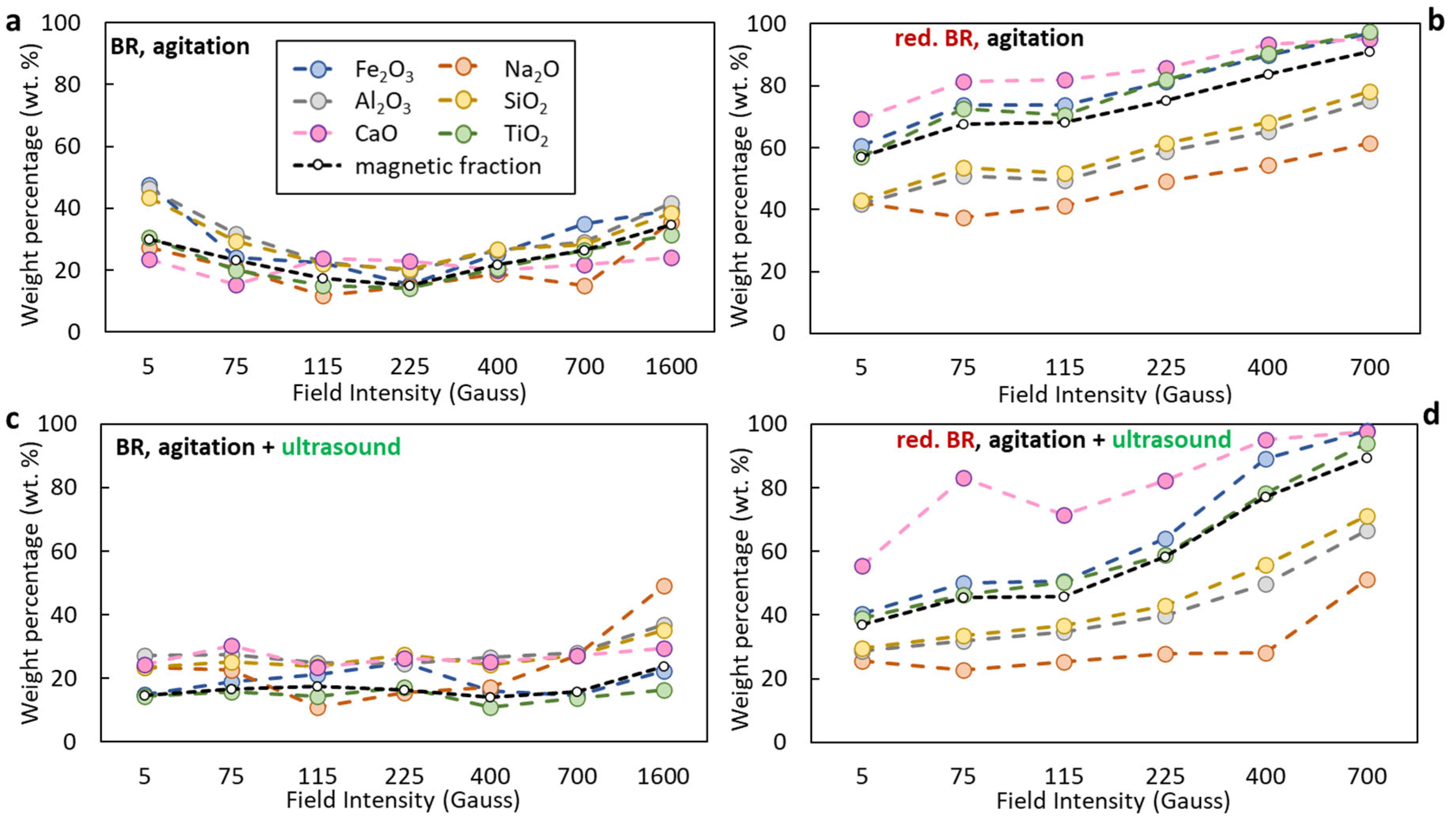
- Sample origin: bauxite ore samples of different origins present differences in their chemical and mineralogical compositions [39]. As shown in Table 3, Romanian BR has a higher Fe content, while the Turkish one has higher contents of Si and Ti. As for the mineralogical composition of the sample, RBR contains considerable amounts of Al-boehmite and hematite while the TBR one has only hematite. Also, cancrinite is identified in the Turkish sample only, while the same sample has a higher gibbsite content as well. Among these minerals, hematite is considered antiferromagnetic at ambient temperature (below its Néel temperature of around 675 °C). Goethite is weakly ferromagnetic, and can potentially show weak ferromagnetic behavior; however, its magnetic susceptibility reduces significantly with the increasing substitution of Al, rendering it weakly paramagnetic [40]. On the other hand, magnetite has high magnetic susceptibility, rendering it easy for beneficiation through magnetic separation. However, as has been shown elsewhere, the mineral magnetic susceptibilities are weakly related to the mineral recoveries either in Wet Low Intensity or in Wet High Intensity Magnetic separation, with the particle size dictating the process for fine particles [41]. It is also important to consider the composition of the grains and whether the mineral phases are associated with others and to what degree. Figure 12 presents the SEM-EDS image of the treated RBR, together with the local chemical composition at two different locations of the same particle. The particle shows a distinct magnetite region, while the surrounding part of the particle has as complex multi-element composition of calcium silicate, Ti-minerals (perovskite or rutile) and minor Fe oxide inclusions of Fe substitution. These physicochemical characteristics reflect the difficulty of efficiently and selectively separating the iron-containing particles.
- Magnetic induction and composition of magnetic fractions: the applied magnetic field in combination with the nature of the iron phases (Fe3+ oxides or magnetite) affects the magnetic fraction mass produced, either for raw and for hydrothermally treated samples. It was found that for the Romanian sample at 1600 Gs, the magnetic fraction obtained after the separation is 55% of the feed, while after hydrothermal treatment, the application of 5 Gs is enough for the magnetic fraction to be 58.2% of the feed, with similar findings for the TBR material. An increase in the magnetic fraction mass is accompanied by an enhancement of the Fe recovery, while selective distribution of certain elements either in the magnetic and non-magnetic fraction was demonstrated. More specifically, the distribution of Ti in the magnetic concentrates is very similar to, and in some cases slightly higher than, that of Fe. This can be attributed to the close association of the Ti phases with Fe oxides, as was revealed via scanning electron microscopy (Figure 2, Figure 3 and Figure 5), as well as to the fact that Ti-bearing minerals exhibit only weak paramagnetic behavior, insufficient on its own to account for their retention during magnetic separation. Furthermore, a slight enrichment of the magnetic concentrate produced from the hydrothermally treated samples occurs for calcium. For the hydrothermally treated samples, Na2O, SiO2, and Al2O3 are distributed in the non-magnetic fraction which is attributed to their association with non-magnetic minerals.
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BR | Bauxite residue |
| RBR | Romanian bauxite residue |
| TBR | Turkish bauxite residue |
References
- Khairul, M.A.; Zanganeh, J.; Moghtaderi, B. The composition, recycling and utilisation of Bayer red mud. Resour. Conserv. Recycl. 2019, 141, 483–498. [Google Scholar] [CrossRef]
- Sun, C.; Chen, J.; Tian, K.; Peng, D.; Liao, X.; Wu, X. Geochemical Characteristics and Toxic Elements in Alumina Refining Wastes and Leachates from Management Facilities. Int. J. Environ. Res. Public Health 2019, 16, 1297. [Google Scholar] [CrossRef]
- Döring, J.; Beck, T.; Beyermann, M.; Gerlier, J.; Henze, G.; Mielcarek, J.; Schkade, U.-K. Exposure and radiation protection for work areas with enhanced natural radioactivity. In Proceedings of the Naturally Occurring Radioactive Material (NORM V), Seville, Spain, 19–22 March 2007. [Google Scholar]
- Jovičević-Klug, M.; Souza Filho, I.R.; Springer, H.; Adam, C.; Raabe, D. Green steel from red mud through climate-neutral hydrogen plasma reduction. Nature 2024, 625, 703–709. [Google Scholar] [CrossRef]
- International-Aluminium.org. Available online: https://international-aluminium.org/report-reveals-global-aluminium-demand-to-reach-new-highs-after-covid/#:~:text=Report%20Reveals%20Global%20Aluminium%20Demand,Mt%20and%20Europe%204.8Mt (accessed on 10 July 2025).
- Archambo, M. New Horizons for Processing and Utilizing Red Mud. Ph.D. Thesis, School of Chemical Engineering, Michigan Technological University, Houghton, MI, USA, 2021. [Google Scholar]
- Rai, S.; Bahadure, S.; Chaddha, M.J.; Agnihotri, A. Disposal practices and utilization of red mud (bauxite residue): A review in Indian context and abroad. J. Sustain. Met. 2019, 6, 1–8. [Google Scholar] [CrossRef]
- CPCB Team. Guidelines for Handling and Management of Red Mud Generated from Alumina Plants; Central Pollution Control Board, Ministry of Environment, Forest and Climate Change: Delhi, India, 2023; p. 122. [Google Scholar]
- Ruyters, S.; Mertens, J.; Vassilieva, E.; Dehandschutter, B.; Poffijn, A.; Smolders, E. The red mud accident in Ajka (Hungary): Plant toxicity and trace metal bioavailability in red mud contaminated soil. Environ. Sci. Technol. 2011, 45, 1616–1622. [Google Scholar] [CrossRef]
- Reid, D.; Fourie, A.B. Back analyses of the August 2016 Luoyang red mud tailings failure. In Proceedings of the Tailings and Mine Waste Conference, Banff, AB, Canada, 5–8 November 2017. [Google Scholar]
- Irfan-ul-Hassan, M.; Daud, M.; Rashid, K.; Alqahtani, F.K.; Zafar, I.; Batool, U. Development and sustainability assessment of red mud-based green bricks: Techno-economic and environmental performance. J. Build. Eng. 2024, 95, 110350. [Google Scholar] [CrossRef]
- Liu, S.; Guan, X.; Zhang, S.; Dou, Z.; Feng, C.; Zhang, H.; Luo, S. Sintered bayer red mud based ceramic bricks: Microstructure evolution and alkalis immobilization mechanism. Ceram. Int. 2017, 43, 13004–13008. [Google Scholar] [CrossRef]
- Kang, S.P.; Kwon, S.J. Effects of red mud and Alkali-Activated Slag Cement on efflorescence in cement mortar. Constr. Build. Mater. 2017, 133, 459–467. [Google Scholar] [CrossRef]
- Kumar, A.; Saravanan, T.J.; Bisht, K.; Kabeer, K.I.S.A. A review on the utilization of red mud for the production of geopolymer and alkali activated concrete. Constr. Build. Mater. 2021, 302, 124170. [Google Scholar] [CrossRef]
- Mukiza, E.; Zhang, L.L.; Liu, X.; Zhang, N. Utilization of red mud in road base and subgrade materials: A review. Resour. Conserv. Recycl. 2019, 141, 187–199. [Google Scholar] [CrossRef]
- Pan, X.; Wu, H.; Lv, Z.; Yu, H.; Tu, G. Recovery of valuable metals from red mud: A comprehensive review. Sci. Total Environ. 2023, 904, 166686. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Wang, X.; Wang, B.; Luan, Z. Feasibility study of iron mineral separation from red mud by high gradient superconducting magnetic separation. Phys. C Supercond. 2011, 471, 91–96. [Google Scholar] [CrossRef]
- Yuan, S.; Wang, R.; Zhang, H.; Li, Y.; Liu, L.; Fu, Y. Investigation of mineral phase transformation technology followed by magnetic separation for recovery of iron values from red mud. Sustainability 2022, 14, 13787. [Google Scholar] [CrossRef]
- Yu, J.; Li, Y.; Lv, Y.; Han, Y.; Gao, P. Recovery of iron from high-iron red mud using suspension magnetization roasting and magnetic separation. Miner. Eng. 2022, 178, 107394. [Google Scholar] [CrossRef]
- Valeev, D.; Zinoveev, D.; Kondratiev, A.; Lubyanoi, D.; Pankratov, D. Reductive Smelting of Neutralized Red Mud for Iron Recovery and Produced Pig Iron for Heat-Resistant Castings. Metals 2019, 10, 32. [Google Scholar] [CrossRef]
- Yang, X.; Chen, X.; Zhang, T.; Ye, J.; Lv, G.; Zhang, J. Study on reductive smelting of high-iron red mud for iron recovery. Metals 2022, 12, 639. [Google Scholar] [CrossRef]
- Samouhos, M.; Taxiarchou, M.; Tsakiridis, P.E.; Potiriadis, K. Greek “red mud” residue: A study of microwave reductive roasting followed by magnetic separation for a metallic iron recovery process. J. Hazar. Mater. 2013, 254–255, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Rayapudi, V.; Dhawan, N. Microwave Reduction of Red Mud for Recovery of Iron Values. J. Sustain. Met. 2018, 4, 427–436. [Google Scholar] [CrossRef]
- Samouhos, M.; Taxiarchou, M.; Pilatos, G.; Tsakiridis, P.E.; Devlin, E.; Pissas, M. Controlled reduction of red mud by H2 followed by magnetic separation. Miner. Eng. 2017, 105, 36–43. [Google Scholar] [CrossRef]
- Sun, T.; Aslam, M.M.A.; Chen, G.; Peng, C. Low-temperature biomass pyrolytic reduction and recovery of iron oxides from red mud. Miner. Eng. 2025, 222, 109155. [Google Scholar] [CrossRef]
- Grudinsky, P.; Zinoveev, D.; Yurtaeva, A.; Kondratiev, A.; Dyubanov, V.; Petelin, A. Iron Recovery from Red Mud Using Carbothermic Roasting with Addition of Alkaline Salts. J. Sustain. Met. 2021, 7, 858–873. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Zhou, Q.; Qi, T.; Liu, G.; Peng, Z.; Wang, H. Transformation of hematite in diasporic bauxite during reductive Bayer digestion and recovery of iron. Trans. Nonferrous Met. Soc. China 2017, 27, 2715–2726. [Google Scholar] [CrossRef]
- Pasechnik, L.A.; Skachkov, V.M.; Bogdanova, E.A.; Chufarov, A.Y.; Kellerman, D.G.; Medyankina, I.S.; Yatsenko, S.P. A promising process for transformation of hematite to magnetite with simultaneous dissolution of alumina from red mud in alkaline medium. Hydrometallurgy 2020, 196, 105438. [Google Scholar] [CrossRef]
- Angelopoulos, P.M.; Oustadakis, P.; Anastassakis, G.; Pissas, M.; Taxiarchou, M. Iron recovery from bauxite residue (BR) through magnetic separation; Effect of endogenous properties and processing conditions. Miner. Eng. 2024, 217, 108954. [Google Scholar] [CrossRef]
- Doebelin, N.; Kleeberg, R. Profex: A graphical user interface for the Rietveld refinement program BGMN. J. Appl. Crystallogr. 2015, 48, 1573–1580. [Google Scholar] [CrossRef]
- Lu, J.F.; Tsai, C.J. Reduction kinetics of hematite to magnetite under hydrothermal treatments. RSC Adv. 2015, 5, 17236–17244. [Google Scholar] [CrossRef]
- Simoni, M.; Hanein, T.; Woo, C.L.; Provis, J.; Kinoshita, H. Effect of impurities on the decarbonization of calcium carbonate using aqueous sodium hydroxide. ACS Sustain. Chem. Eng. 2022, 10, 11913–11925. [Google Scholar] [CrossRef]
- Hellige, K. The Reactivity of Ferric (Hydr)Oxides Towards Dissolved Sulphide. Ph.D. Thesis, University of Bayreuth, Dortmund, Germany, 2010. [Google Scholar]
- Yan, W.; Liu, H.; Chen, R.; Xie, J.; Wei, Y. Dissolution and oriented aggregation: Transformation from lepidorocite to goethite by the catalysis of aqueous Fe(II). RSC Adv. 2015, 5, 106396. [Google Scholar] [CrossRef]
- Kendall, K. Adhesion: Molecules and Mechanics. Science 1994, 263, 1720–1725. [Google Scholar] [CrossRef] [PubMed]
- Arol, A.I.; Aydogan, A. Recovery enhancement of magnetite fines in magnetic separation. Colloids Surf. A Physicochem. Eng. Asp. 2004, 232, 151–154. [Google Scholar] [CrossRef]
- Hassanjani-Roshan, A.; Vaezi, M.R.; Koohestani, H.; Cheraghi, F. Investigating the Effect of Ultrasound Intensity on the Magnetic Properties of Magnetite Nanostructures Synthesized by Sonochemical Method. Int. J. Eng. 2023, 36, 1034–1039. [Google Scholar] [CrossRef]
- Anhalt, M. Systematic investigation of particle size dependence of magnetic properties in soft magnetic composites. J. Magn. Magn. Mater. 2008, 320, 366–369. [Google Scholar] [CrossRef]
- Angelopoulos, P.; Georgiou, M.; Oustadakis, P.; Taxiarchou, M.; Karadağ, H.; Eker, Y.; Dobra, G.; Boiangiu, A.; Demir, G.; Arslan, S.; et al. Preliminary Characterization of Three Metallurgical Bauxite Residue Samples. Mater. Proc. 2021, 5, 66. [Google Scholar]
- Ponomar, V.P. Thermomagnetic properties of the goethite transformation during high-temperature treatment. Miner. Eng. 2018, 127, 143–152. [Google Scholar] [CrossRef]
- Bazin, C.; Sista, R.; Légaré, B.; Caron, J.; Lavoie, F. Investigation of the effect of mineral composition and size on particle separation in Wet low and high intensities magnetic separators: An industrial case. Miner. Eng. 2024, 218, 109003. [Google Scholar] [CrossRef]




| Parameter | Value |
|---|---|
| Operating Temperature | 250 °C |
| Agitation Speed | 400 rpm |
| Pressure | 500 psi |
| Leaching Agent | NaOH (5 M) |
| Pulp Density | 20% |
| FeSO4⋅7H2O | Fe2+/Fetot ratio: 0.3 |
| Reaction Time | 3 h |
| Parameter | Value |
|---|---|
| Magnetic Induction | 70–1600 Gs (7 × 10−3–1.6 × 10−1 T) |
| Slurry Concentration | 2 wt.% |
| Fluid Flow Rate | 0.1 L/min |
| Ultrasound Amplitude | 75% (0.75 s ON, 0.25 s OFF) |
| Components | RBR | TBR |
|---|---|---|
| Fe2O3 | 39.2 | 35.3 |
| (Feelem) | 27.4 | 24.7 |
| Al2O3 | 17.4 | 17.9 |
| SiO2 | 11.5 | 14.7 |
| Na2O | 6.7 | 7.0 |
| CaO | 4.9 | 4.9 |
| TiO2 | 2.2 | 4.2 |
| LOI | 16.3 | 14.1 |
| Other | 1.8 | 1.9 |
| Mineral Species | Chemical Formula | Content (wt.%) | |
|---|---|---|---|
| RBR | TBR | ||
| Al-Goethite | Al0.1Fe0.9OH | 44.3 | 0.0 |
| Sodalite | Al6Na6Si6C2.4O34.32H23.04 | 17.1 | 18.2 |
| Hematite | Fe2O3 | 13.3 | 43.1 |
| Cancrinite | Al6Na7.14Si7.08O31.6H9.74 | 0.0 | 11.4 |
| Calcite | CaCO3 | 10.3 | 7.6 |
| Quartz | SiO2 | 3.2 | 0.0 |
| Gibbsite | Al(OH)3 | 3.1 | 6.7 |
| Mica-group | Al3NaSi3O12H2 | 0.0 | 3.4 |
| Boehmite | AlO(OH) | 0.0 | 1.0 |
| Ca-Na-Al oxide | Al6Ca8.25Na1.5O18 | 0.0 | 0.9 |
| Tridymite | SiO2 | 2.6 | 0.0 |
| Cristobalite-α | SiO2 | 0.0 | 0.8 |
| Rutile | TiO2 | 1.8 | 3.5 |
| Amorphous | 4.3 | 3.6 | |
| Components | Treated RBR | Treated TBR |
|---|---|---|
| Fe2O3 | 59.3 | 43.7 |
| (Feelem) | 41.5 | 30.6 |
| Al2O3 | 9.4 | 11.6 |
| SiO2 | 12.7 | 15.5 |
| Na2O | 4.9 | 8.4 |
| CaO | 5.4 | 6.8 |
| TiO2 | 3.0 | 5.0 |
| LOI | 2.7 | 6.2 |
| Other | 2.6 | 2.8 |
| Mineral Species | Chemical Formula | Content (wt.%) | |
|---|---|---|---|
| RBR | TBR | ||
| Magnetite | Fe3O4 | 47.3 | 27.9 |
| Sodalite | Al6Na6Si6C2.4O34.32H23.04 | 3.0 | 7.6 |
| Hematite | Fe2O3 | 9.8 | 19.1 |
| Cancrinite | Al6Na7.14Si7.08O31.6H9.74 | 19.4 | 23.7 |
| Calcite | CaCO3 | 2.3 | 2.0 |
| Gibbsite | Al(OH)3 | 5.3 | 5.2 |
| Mica group (potentially paragonite) | Al3NaSi3O12H2 | 2.4 | 3.5 |
| Rutile | TiO2 | 2.8 | 4.6 |
| Other | 1.3 | 1.1 | |
| Amorphous | 6.3 | 5.3 | |
| Conversion degree to magnetite | |||
| η(Fe2O3 or α–FeOOH)% | 76.7 | 49.6 | |
| Origin and Conditions | Fe2O3 | Al2O3 | SiO2 | Na2O | TiO2 | CaO | Other | LOI |
|---|---|---|---|---|---|---|---|---|
| Raw: non-reduced, Romanian Dispersion: Agitation Magnetic Field: 1600 Gs | 42.5 | 17.2 | 10.9 | 6.5 | 2.8 | 2.0 | 1.8 | 16.3 |
| Raw: reduced, Romanian Dispersion: Agitation Magnetic Field: 700 Gs | 63.5 | 7.1 | 9.8 | 2.3 | 3.1 | 6.6 | 1.7 | 5.9 |
| Raw: non-reduced, Turkish Dispersion: Agitation Magnetic Field: 1600 Gs | 40.0 | 18.2 | 12.4 | 4.0 | 4.8 | 3.5 | 1.9 | 15.2 |
| Raw: reduced, Turkish Dispersion: Agitation Magnetic Field: 700 Gs | 46.6 | 9.9 | 13.4 | 5.7 | 4.9 | 7.0 | 1.7 | 10.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angelopoulos, P.; Oustadakis, P.; Kountouris, N.; Samouhos, M.; Anastassakis, G.; Taxiarchou, M. Iron Recovery from Turkish and Romanian Bauxite Residues Through Magnetic Separation: Effect of Hydrothermal Processing and Separation Conditions. Separations 2025, 12, 252. https://doi.org/10.3390/separations12090252
Angelopoulos P, Oustadakis P, Kountouris N, Samouhos M, Anastassakis G, Taxiarchou M. Iron Recovery from Turkish and Romanian Bauxite Residues Through Magnetic Separation: Effect of Hydrothermal Processing and Separation Conditions. Separations. 2025; 12(9):252. https://doi.org/10.3390/separations12090252
Chicago/Turabian StyleAngelopoulos, Panagiotis, Paschalis Oustadakis, Nikolaos Kountouris, Michail Samouhos, Georgios Anastassakis, and Maria Taxiarchou. 2025. "Iron Recovery from Turkish and Romanian Bauxite Residues Through Magnetic Separation: Effect of Hydrothermal Processing and Separation Conditions" Separations 12, no. 9: 252. https://doi.org/10.3390/separations12090252
APA StyleAngelopoulos, P., Oustadakis, P., Kountouris, N., Samouhos, M., Anastassakis, G., & Taxiarchou, M. (2025). Iron Recovery from Turkish and Romanian Bauxite Residues Through Magnetic Separation: Effect of Hydrothermal Processing and Separation Conditions. Separations, 12(9), 252. https://doi.org/10.3390/separations12090252










