Investigation on Li Recovery from Greek Coal Fly Ash Under a Circular Economy Framework: An Experimental Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Characterization
2.1.1. Mineralogical Analysis
2.1.2. Chemical Analysis and Li Content Determination
2.2. Leaching Experiments
3. Results
3.1. Sample Characterization
3.1.1. Mineralogical Analysis
3.1.2. Chemical Analysis and Li Content
3.2. Leaching Experiments
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Regulation (EU) 2024/1252 of the European Parliament and of the Council of 11 April 2024 Establishing a Framework for Ensuring a Secure and Sustainable Supply of Critical Raw Materials and Amending Regulations (EU) No 168/2013, (EU) 2018/858, (EU) 2018/1724 and (EU) 2019/1020 Text with EEA Relevance. Available online: https://eur-lex.europa.eu/eli/reg/2024/1252/oj (accessed on 2 March 2024).
- Bradley, D.C.; Stillings, L.L.; Jaskula, B.W.; Munk, L.A.; McCauley, A.D. Lithium. In Critical Mineral Resources of the United States—Economic and Environmental Geology and Prospects for Future Supply; Schulz, K.J., De Young, J.H., Jr., Seal, R.R., II, Bradley, D.C., Eds.; U.S. Geological Survey: Reston, VA, USA, 2017; pp. K1–K21. Available online: https://pubs.usgs.gov/publication/pp1802K (accessed on 2 March 2024).
- Garside, M. Average Lithium Carbonate Price from 2010 to 2021. Statista. 7 March 2022. Available online: https://www.statista.com/statistics/606350/batterygrade-lithium-carbonate-price/ (accessed on 27 November 2023).
- U.S. Geological Survey. Available online: https://www.usgs.gov/news/national-news-release/us-geological-survey-releases-2022-list-critical-minerals (accessed on 2 March 2024).
- Rudnik, E. Coal and Coal By-Products as Unconventional Lithium Sources: A Review of Occurrence Modes and Hydrometallurgical Strategies for Metal Recovery. Minerals 2024, 14, 849. [Google Scholar] [CrossRef]
- Liu, C.; Lin, J.; Cao, H.; Zhang, Y.; Sun, Z. Recycling of spent lithium-ion batteries in view of lithium recovery: A review. J. Clean. Prod. 2019, 228, 801–813. [Google Scholar] [CrossRef]
- Ratuela, R.; Yadav, B.R.; Kumar, S. A review on technologies for recovery of metals from waste lithium-ion batteries. J. Power Sourc. 2023, 580, 233248. [Google Scholar]
- Cheng, Q.; Wang, Z.; Wang, Y.; Li, J.T.; Fu, H. Recent advances in preferentially selective Li recovery from spent lithium-ion batteries: A review. J. Environ. Chem. Eng. 2024, 12, 112903. [Google Scholar] [CrossRef]
- Sun, Y.Z.; Zhao, C.L.; Li, Y.H.; Wang, J.X.; Lin, M.Y. Li distribution and mode of occurrences in Li-bearing coal seam 9 from Pingshuo Mining District, Ningwu Coalfield, northern China. Energy Educ. Sci. Technol. Part A Energy Sci. Res. 2013, 31, 27–38. [Google Scholar]
- Seredin, V.V.; Dai, S.; Sun, Y.; Chekryzhov, I.Y. Coal deposits as a promising source of rare metals for alternative power and energy-efficient technologies. Appl. Geochem. 2013, 31, 1–11. [Google Scholar] [CrossRef]
- Dai, S.; Finkelman, R.B. Coal as a promising source of critical elements: Progress and future prospects. Int. J. Coal Geol. 2018, 186, 155–164. [Google Scholar] [CrossRef]
- Talan, D.; Huang, Q.; Liang, L.; Song, X. Conceptual Process Development for Recovery of Thorium, Uranium, and Rare Earths from Coarse Coal Refuse. Miner. Process. Extr. Metall. Rev. 2022, 44, 330–345. [Google Scholar] [CrossRef]
- Bhatt, A.; Priyadarshini, S.; Mohanakrishnan, A.A.; Abri, A.; Sattler, M.; Techapaphawit, S. Physical, chemical, and geotechnical properties of coal fly ash: A global review. Case Stud. Constr. Mater. 2019, 11, e00263. [Google Scholar] [CrossRef]
- Dai, S.; Yan, X.; Ward, C.R.; Hower, J.C.; Zhao, L.; Wang, X.; Finkelman, R.B. Valuable elements in Chinese coals: A review. Int. Geol. Rev. 2018, 60, 590–620. [Google Scholar] [CrossRef]
- Finkelman, R.B.; Dai, S.F.; French, D. The importance of minerals in coal as the hosts of chemical elements: A review. Int. J. Coal Geol. 2019, 212, 103251. [Google Scholar] [CrossRef]
- Hagelüken, C.; Goldmann, D. Recycling and circular economy—Towards a closed loop for metals in emerging clean technologies. Miner. Econ. 2022, 35, 539–562. [Google Scholar] [CrossRef]
- Hu, P.; Hou, X.; Zhang, J.; Li, S.; Wu, H.; Damo, A.; Xi, X. Distribution and Occurrence of Lithium in High-Alumina-Coal Fly Ash. Int. J. Coal Geol. 2018, 189, 27–34. [Google Scholar] [CrossRef]
- Marinakis, V.; Flamos, A.; Stamtsis, G.; Georgizas, I.; Maniatis, Y.; Doukas, H. The efforts towards and challenges of Greece’s post-lignite era: The case of megalopolis. Sustainability 2020, 12, 10575. [Google Scholar] [CrossRef]
- Qin, S.; Zhao, C.; Li, Y.; Zhang, Y. Review of coal as a promising source of lithium. Int. J. Oil Gas Coal Technol. 2015, 9, 215–229. [Google Scholar] [CrossRef]
- Sahoo, P.K.; Kim, K.; Powell, M.A.; Equeenuddin, S.M. Recovery of metals and other beneficial products from coal fly ash: A sustainable approach for fly ash management. Int. J. Coal Sci. Technol. 2016, 3, 267–283. [Google Scholar] [CrossRef]
- Talan, D.; Huang, Q. A review study of rare Earth, Cobalt, Lithium, and Manganese in Coal-based sources and process development for their recovery. Miner. Eng. 2022, 189, 107897. [Google Scholar] [CrossRef]
- Zhang, W.; Honaker, R. Characterization and recovery of rare earth elements and other critical metals (Co, Cr, Li, Mn, Sr, and V) from the calcination products of a coal refuse sample. Fuel 2020, 267, 117236. [Google Scholar] [CrossRef]
- Zou, J.; Cheng, L.; Guo, Y.; Wang, Z.; Tian, H.; Li, T. Mineralogical and geochemical characteristics of lithium and rare earth elements in high-sulfur coal from the donggou mine, Chongqing, southwestern China. Minerals 2020, 10, 627. [Google Scholar] [CrossRef]
- Zhou, M.; Zhao, L.; Wang, X.; Nechaev, V.; French, D.; Spiro, B.; Dai, S. Mineralogy and Geochemistry of the Late Triassic Coal from the Caotang Mine, Northeastern’s Sichuan Basin, China, with Emphasis on the Enrichment of the Critical Element Lithium. Ore Geol. Rev. 2021, 139, 104582. [Google Scholar] [CrossRef]
- Zhao, L.; Dai, S.; Nechaev, V.; Nechaeva, E.; Graham, I.; French, D. Enrichment Origin of Critical Elements (Li and Rare Earth Elements) and A Mo-U-Se-Re Assemblage in Pennsylvanian Anthracite from the Jincheng Coalfield, Southeastern Qinshui Basin, Northern China. Ore Geol. Rev. 2019, 115, 103184. [Google Scholar] [CrossRef]
- Nsiah-gyambibi, R.; Sokama-Neuyam, Y.A.; Bokakye, P.; Ampomah, W.; Aggrey, W.N.; Wang, S. Valorization of coal fly ash (CFA): A multi-industry review. Int. J. Environ. Sci. Technol. 2023, 20, 12807–12822. [Google Scholar] [CrossRef]
- Baig, S.Y.; Yousaf, M. Coal fired power plants: Emission problems and controlling techniques. J. Earth Sci. Clim. Change 2017, 8, 104. [Google Scholar] [CrossRef]
- Pentari, D.; Vlachaki, E.; Fazaki, M.E.; Stratakis, A. Lithium in Greek coal fly ashes: Contents and characterization by sequential extraction. Sustainability 2024, 16, 1442. [Google Scholar] [CrossRef]
- Pentari, D.; Varouchakis, E.; Cheiladaki, I.; Keravnopoulou, D.; Partsalaki, E. Carboxylic acid-assisted leaching of critical elements from coal fly ash: Experimental and simulation studies. Glob. Nest J. 2024, 26, 06013. [Google Scholar] [CrossRef]
- Rezaei, H.; Shafaei, S.Z.; Abdollahi, H.; Shahidi, A.; Ghassa, S. A sustainable method for germanium, vanadium and lithium extraction from coal fly ash: Sodium salts roasting and organic acids leaching. Fuel 2022, 312, 122844. [Google Scholar] [CrossRef]
- Vilakazi, A.Q.; Ndlovu, S.; Chipise, L.; Shemi, A. The Recycling of Coal Fly Ash: A Review on Sustainable Developments and Economic Considerations. Sustainability 2022, 14, 1958. [Google Scholar] [CrossRef]
- Manurung, H.; Rosita, W.; Anggara, F.; Petrus, H.B.T.M.; Bendiyasa, I.M. Leaching of REY from non-magnetic coal fly ash with acetic acid. IOP Conf. Ser. Mater. Sci. Eng. 2020, 778, 012005. [Google Scholar] [CrossRef]
- Prihutami, P.; Prasetya, A.; Sediawan, W.B.; Petrus, H.T.; Anggara, F.Y. Study on Rare Earth Elements Leaching from Magnetic Coal Fly Ash by Citric Acid. J. Sustain. Metall. 2021, 7, 1241–1253. [Google Scholar] [CrossRef]
- Prihutami, P.; Sediawan, W.B.; Astuti, W.; Prasetya, A. Effect of temperature on rare earth elements recovery from coal fly ash using citric acid. IOP Conf. Ser. Mater. Sci. Eng. 2020, 742, 012040. [Google Scholar] [CrossRef]
- Aughenbaugh, K.L.; Stutzman, P.; Juenger, M.C.G. Identifying glass compositions in fly ash. Front. Mater. 2016, 3, 1. [Google Scholar] [CrossRef]
- Adamidou, K.; Kassoli-Fournaraki, A.; Filippidis, A.; Christanis, K.; Amanatidou, E.; Tsikritzis, L.; Patrikaki, O. Chemical investigation of lignite samples and their ashing products from Kardia lignite field of Ptolemais, Northern Greece. Int. J. Coal Geol. 2007, 86, 2502–2508. [Google Scholar] [CrossRef]
- Filippidis, A.; Georgakopoulos, A. Mineralogical and chemical investigation of fly ash from the Main and Northern lignite fields in Ptolemais. Int. J. Coal Geol. 1992, 71, 373–376. [Google Scholar] [CrossRef]
- Koukouzas, N.; Kalaitzidis, S.P.; Ward, C.R. Organic petrographical, mineralogical and geochemical features of the Achlada and Mavropigi lignite deposits. NW Macedonia, Greece. Int. J. Coal Geol. 2010, 83, 387–395. [Google Scholar] [CrossRef]
- Ward, C. Analysis, Origin and Significance of Mineral Matter in Coal: An Updated Review. Int. J. Coal Geol. 2016, 165, 1–27. [Google Scholar] [CrossRef]
- Gomes, S.; François, M. Characterization of mullite in silicoaluminous fly ash by XRD, TEM, and 29Si MAS NMR. Cem. Concr. Res. 2000, 30, 175–181. [Google Scholar] [CrossRef]
- Finkelman, R. Trace and Minor Elements in Coal. In Organic Chemistry; Engel, I.M., Macko, S., Eds.; Plenum: New York, NY, USA, 1993; pp. 693–1607. [Google Scholar] [CrossRef]
- Ketris, Y.E.; Yudovich, Y. Estimations of Clarkes for Carbonaceous biolithes: World averages for trace element contents in black shales and coals. Int. J. Coal Geol. 2009, 78, 135–148. [Google Scholar] [CrossRef]
- Xu, F.; Qin, S.; Li, S.; Wang, J.; Qi, D.; Lu, Q.; Xing, J. Distribution, occurrence mode, and extraction potential of critical elements in coal ashes of the Chongqing Power Plant. J. Clean. Prod. 2022, 342, 130910. [Google Scholar] [CrossRef]



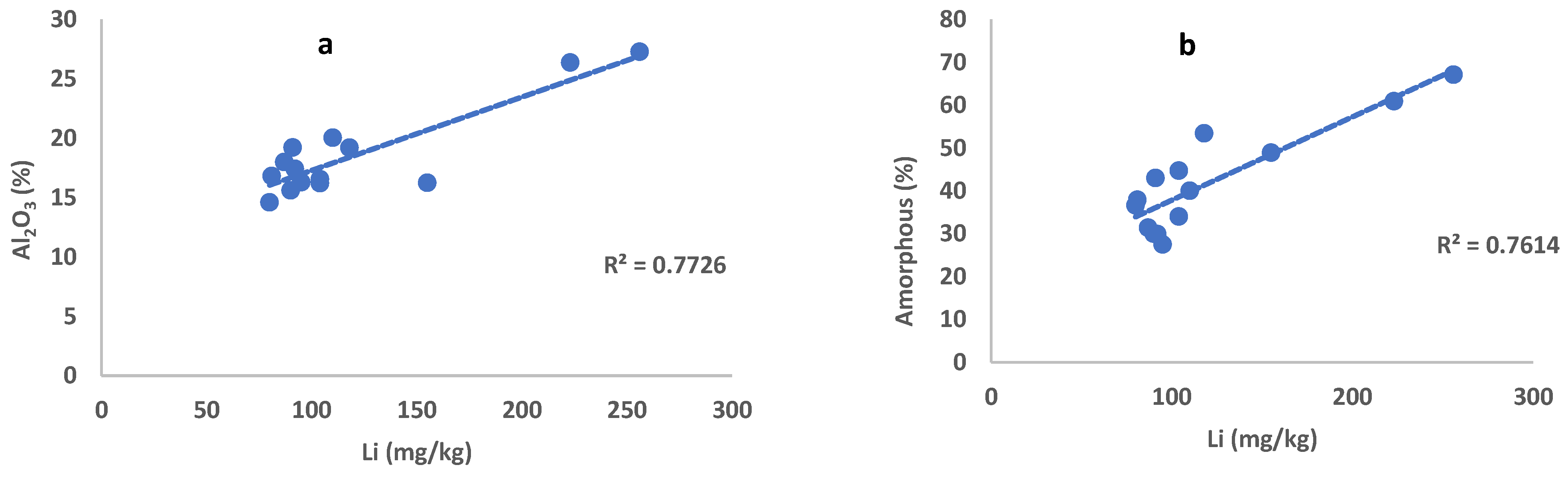
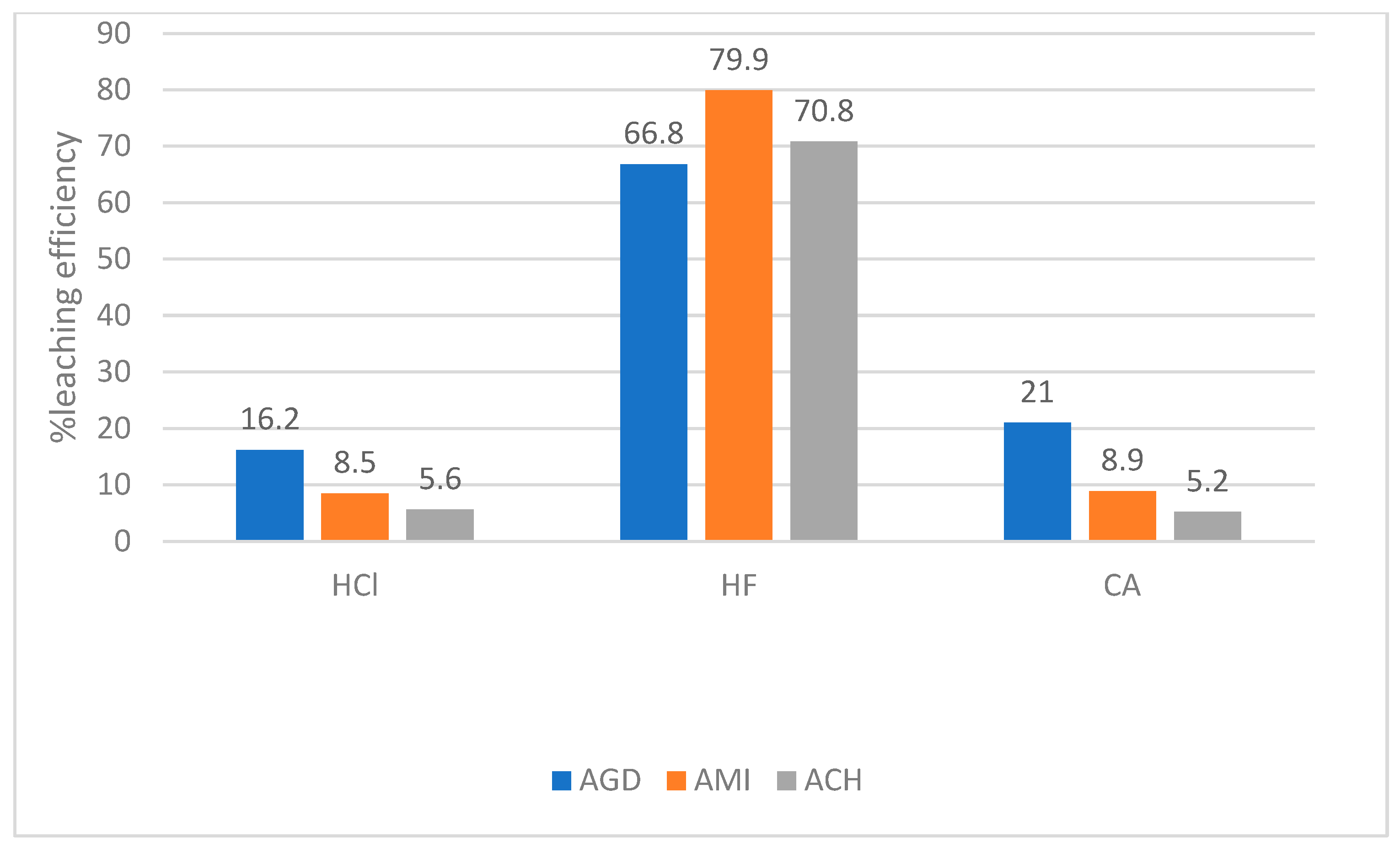

| MEG 2008 | SKP 2024 | ACH 2006 | ACH 2008 | PTL1 2024 | PTL2 2024 | PTL3 2024 | PTL4 2024 | PTL 2008 | PTL 2000 | AGD 2005 | AGD 2018 | KAR 2002 | AMI 2002 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amorphous | 53.4 | 43.0 | 60.9 | 67.1 | 29.9 | 31.4 | 27.5 | 30.0 | 36.6 | 37.9 | 40.0 | 44.7 | 34.1 | 48.9 |
| Anhydrite CaSO4 | 7.0 | 0.0 | 1.4 | 0.0 | 14.1 | 15.3 | 13.4 | 14.4 | 8.8 | 9.1 | 0.0 | 8.9 | 7.2 | 7.7 |
| Akermanite Ca2Mg(Si2O7) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 5.5 | 2.0 | 2.2 | 0.0 | 9.1 | 0.0 |
| Quartz SiO2 | 14.8 | 15.9 | 8.9 | 9.0 | 6.8 | 7.9 | 8.6 | 7.6 | 4.5 | 6.7 | 12.3 | 12.3 | 4.3 | 5.6 |
| Portlandite Ca(OH)2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 6.0 | 0.0 | 2.2 | 12.4 | 3.7 |
| Feldspars [(Ca,Na)Al1-2Si2-3O8-KAlSi3O8] | 19 | 24.9 | 10 | 9.7 | 10.6 | 13.1 | 15.9 | 13.3 | 9.4 | 9.3 | 12.6 | 4.6 | 5.6 | 16.2 |
| Calcite CaCO3 | 2.6 | 4.5 | 2.7 | 0.5 | 3.6 | 3.7 | 4.8 | 6.2 | 21.6 | 5.5 | 3.5 | 11.0 | 9.6 | 4.3 |
| Brownmillerite Ca2(Al,Fe)2O5 | 0.0 | 0.0 | 0.0 | 0.0 | 7.0 | 4.9 | 6.3 | 7.6 | 7.3 | 11.1 | 0.0 | 0.0 | 11.9 | 5.3 |
| Gehlenite Ca2Al[AlSiO7] | 0.0 | 0.0 | 0.0 | 0.0 | 14.7 | 19.6 | 13.4 | 13.9 | 6.5 | 12.4 | 5.5 | 2.6 | 0.0 | 0.0 |
| Dolomite CaMg(CO3)2 | 0.0 | 1.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Mica [KAl2(AlSi3O10)(F,OH)2] | 0.0 | 4.0 | 0.0 | 0.0 | 4.4 | 1.7 | 3.6 | 0.0 | 0.0 | 0.0 | 0.0 | 4.0 | 2.5 | 1.7 |
| Dicalcium silicate Ca2SiO4 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 5.0 |
| Calcium oxide CaO | 0.0 | 0.0 | 0.0 | 0.0 | 5.7 | 0.0 | 5.5 | 7.1 | 0.0 | 0.0 | 0.0 | 6.9 | 2.4 | 1.6 |
| Hematite Fe2O3 | 3.3 | 0.0 | 1.7 | 1.6 | 2.3 | 2.5 | 1.0 | 0.0 | 0.0 | 0.0 | 1.0 | 1.6 | 0.0 | 0.0 |
| Mullite Al6Si2O13 | 0.0 | 0.0 | 12.0 | 12.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Spinel MgAl2O4 | 0.0 | 0.0 | 2.4 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Diopside MgCaSi2O6 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 5.1 | 0.0 | 0.0 | 0.0 |
| Periclase MgO | 0.0 | 0.0 | 0.0 | 0.0 | 0.9 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1.2 | 0.8 | 0.0 | 0.0 |
| Geothite a-FeO(OH) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.3 | 0.0 | 0.0 |
| Na2O (%) | MgO (%) | K2O (%) | CaO (%) | TiO2 (%) | Al2O3 (%) | SiO2 (%) | P2O5 (%) | Cr2O3 (%) | MnO (%) | Fe2O3 (%) | ZnO (%) | SO3 (%) | BaO (%) | SrO (%) | LOI | TOTAL | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MEG 2008 | 0.7 | 3.4 | 1.6 | 14.4 | 0.5 | 19.2 | 44.5 | 0.3 | 0.0 | 0.1 | 9.5 | 0.0 | 1.8 | 0.3 | 0.1 | 3.7 | 100 |
| SKP 2024 | 0.8 | 3.2 | 1.9 | 3.4 | 0.5 | 19.2 | 49.2 | 0.0 | 0.0 | 0.1 | 6.1 | 0.0 | 0.3 | 0.3 | 0.0 | 15.1 | 100 |
| ACH 2006 | 1.0 | 4.0 | 2.6 | 4.3 | 0.6 | 26.4 | 51.1 | 0.2 | 0.0 | 0.1 | 7.9 | 0.0 | 0.3 | 0.3 | 0.0 | 2.0 | 100 |
| ACH 2008 | 0.9 | 3.9 | 2.6 | 4.3 | 0.6 | 27.3 | 51.5 | 0.1 | 0.0 | 0.1 | 7.9 | 0.0 | 0.3 | 0.4 | 0.0 | 0.6 | 100 |
| PTL1 2024 | 0.8 | 4.4 | 1.0 | 33.2 | 0.6 | 17.4 | 27.5 | 0.5 | 0.0 | 0.0 | 5.7 | 0.0 | 5.4 | 0.3 | 0.0 | 3.1 | 100 |
| PTL2 2024 | 0.7 | 4.3 | 1.0 | 30.9 | 0.6 | 18.0 | 30.3 | 0.2 | 0.0 | 0.0 | 5.5 | 0.0 | 5.5 | 0.4 | 0.0 | 2.9 | 100 |
| PTL3 2024 | 1.1 | 4.9 | 1.0 | 33.5 | 0.6 | 16.3 | 28.1 | 0.4 | 0.0 | 0.0 | 5.5 | 0.0 | 5.2 | 0.3 | 0.0 | 3.0 | 100 |
| PTL4 2024 | 1.1 | 4.4 | 1.0 | 32.1 | 0.5 | 15.6 | 30.0 | 0.4 | 0.0 | 0.0 | 5.9 | 0.0 | 5.2 | 0.1 | 0.0 | 4.1 | 100 |
| PTL 2008 | 0.8 | 4.3 | 0.8 | 31.0 | 0.6 | 14.6 | 26.3 | 0.3 | 0.0 | 0.0 | 4.9 | 0.0 | 4.1 | 0.3 | 0.0 | 11.9 | 100 |
| PTL 2000 | 0.7 | 4.3 | 1.0 | 32.6 | 0.6 | 16.8 | 25.7 | 0.4 | 0.0 | 0.0 | 5.5 | 0.0 | 5.1 | 0.3 | 0.0 | 6.9 | 100 |
| AGD 2005 | 0.9 | 3.7 | 1.6 | 15.5 | 0.4 | 20.0 | 45.0 | 0.3 | 0.0 | 0.1 | 8.9 | 0.0 | 1.5 | 0.5 | 0.1 | 1.8 | 100 |
| AGD 2018 | 1.0 | 4.4 | 0.9 | 28.7 | 0.5 | 16.5 | 32.4 | 0.4 | 0.1 | 0.0 | 4.1 | 0.0 | 3.8 | 0.3 | 0.0 | 6.7 | 100 |
| KAR 2002 | 1.1 | 4.5 | 0.8 | 38.4 | 0.4 | 16.2 | 20.2 | 0.2 | 0.0 | 0.1 | 5.3 | 0.0 | 3.8 | 0.2 | 0.0 | 9.8 | 100 |
| AMI 2002 | 0.7 | 4.1 | 1.1 | 26.5 | 0.5 | 16.2 | 36.7 | 0.4 | 0.0 | 0.1 | 5.7 | 0.0 | 3.2 | 0.4 | 0.0 | 5.0 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pentari, D.; Makri, P.; Xanthopoulos, P.; Vamvuka, D. Investigation on Li Recovery from Greek Coal Fly Ash Under a Circular Economy Framework: An Experimental Study. Separations 2025, 12, 251. https://doi.org/10.3390/separations12090251
Pentari D, Makri P, Xanthopoulos P, Vamvuka D. Investigation on Li Recovery from Greek Coal Fly Ash Under a Circular Economy Framework: An Experimental Study. Separations. 2025; 12(9):251. https://doi.org/10.3390/separations12090251
Chicago/Turabian StylePentari, Despina, Pagona Makri, Panagiotis Xanthopoulos, and Despina Vamvuka. 2025. "Investigation on Li Recovery from Greek Coal Fly Ash Under a Circular Economy Framework: An Experimental Study" Separations 12, no. 9: 251. https://doi.org/10.3390/separations12090251
APA StylePentari, D., Makri, P., Xanthopoulos, P., & Vamvuka, D. (2025). Investigation on Li Recovery from Greek Coal Fly Ash Under a Circular Economy Framework: An Experimental Study. Separations, 12(9), 251. https://doi.org/10.3390/separations12090251







