Semi-Pilot Scale Extraction of Pinocembrin and Galangin from Populus alba L. × berolinensis K. Koch via Enzymatic Pretreatment and Ultrasonication
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials and Reagents
2.2. Ultraviolet Full-Wavelength Scanning of Standard/Reference Stock Solutions
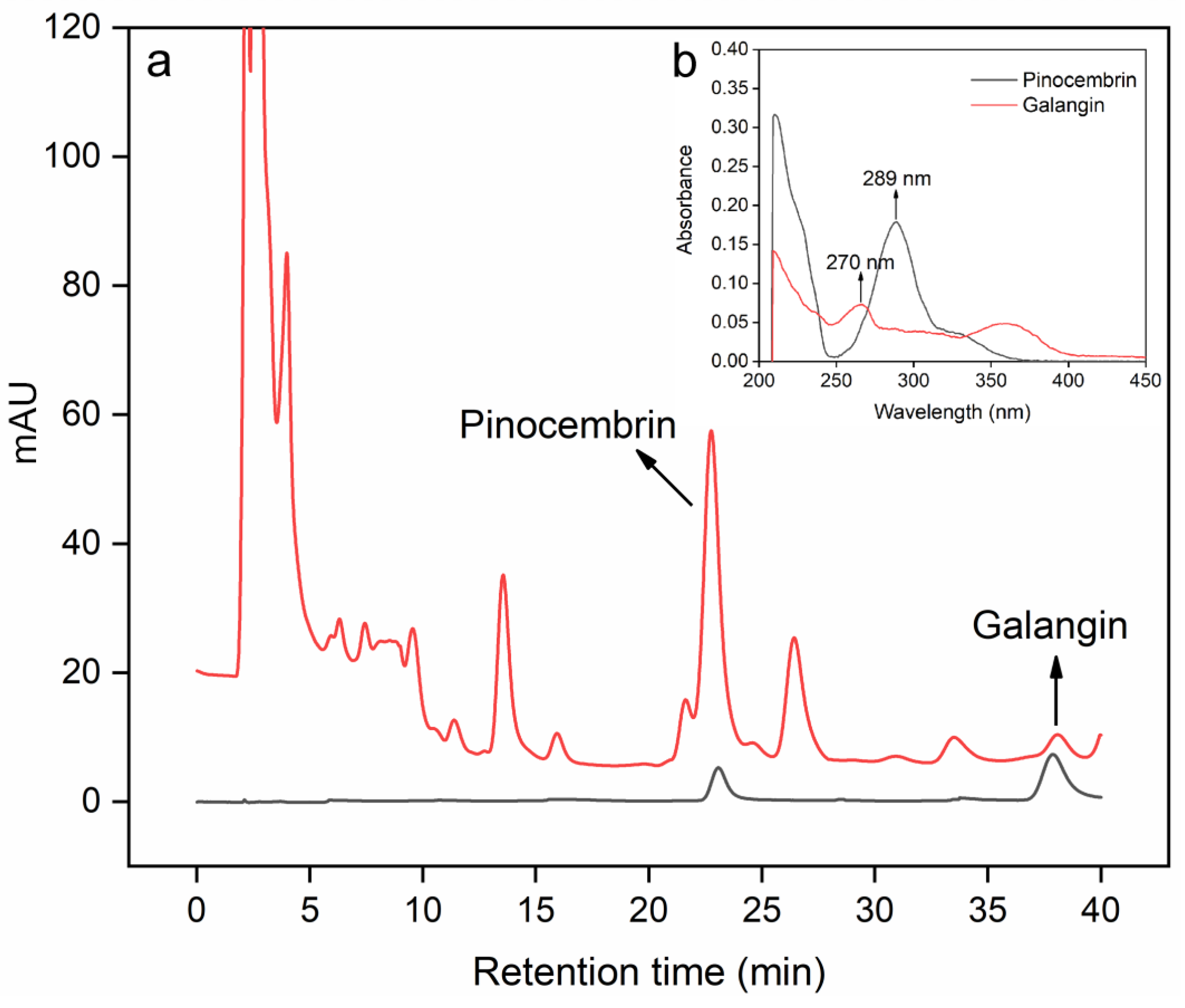
2.3. HPLC Apparatus and Quantitative Conditions
2.4. Enzymatic Pretreatment-Ultrasonic-Assisted Strategy
2.4.1. Laboratory-Scale Process
2.4.2. Semi-Pilot Scale Process
2.5. Box–Behnken Design of the RSM
2.6. Method Validation
3. Results and Discussion
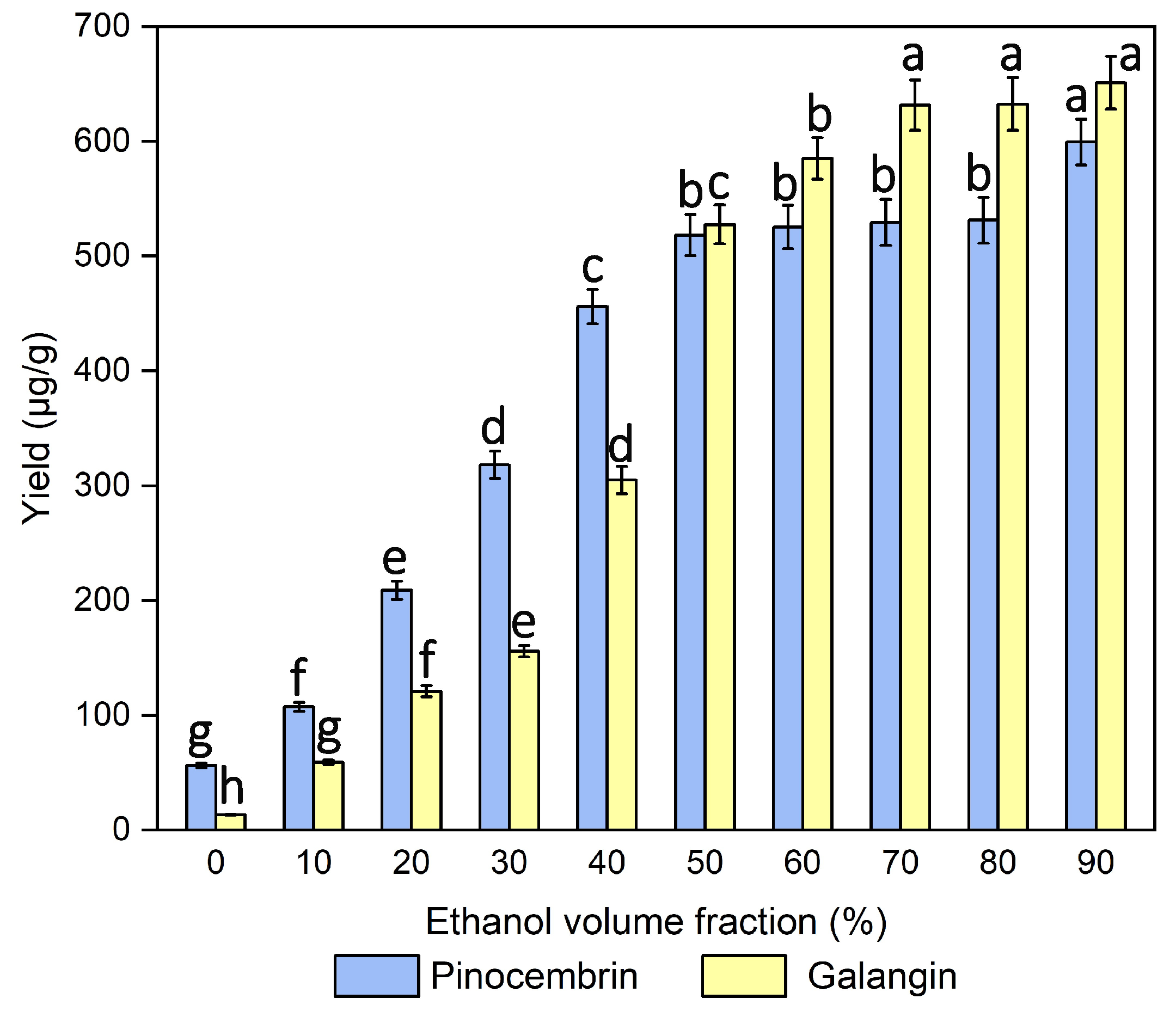
3.1. Effect of the Ethanol Volume Fraction
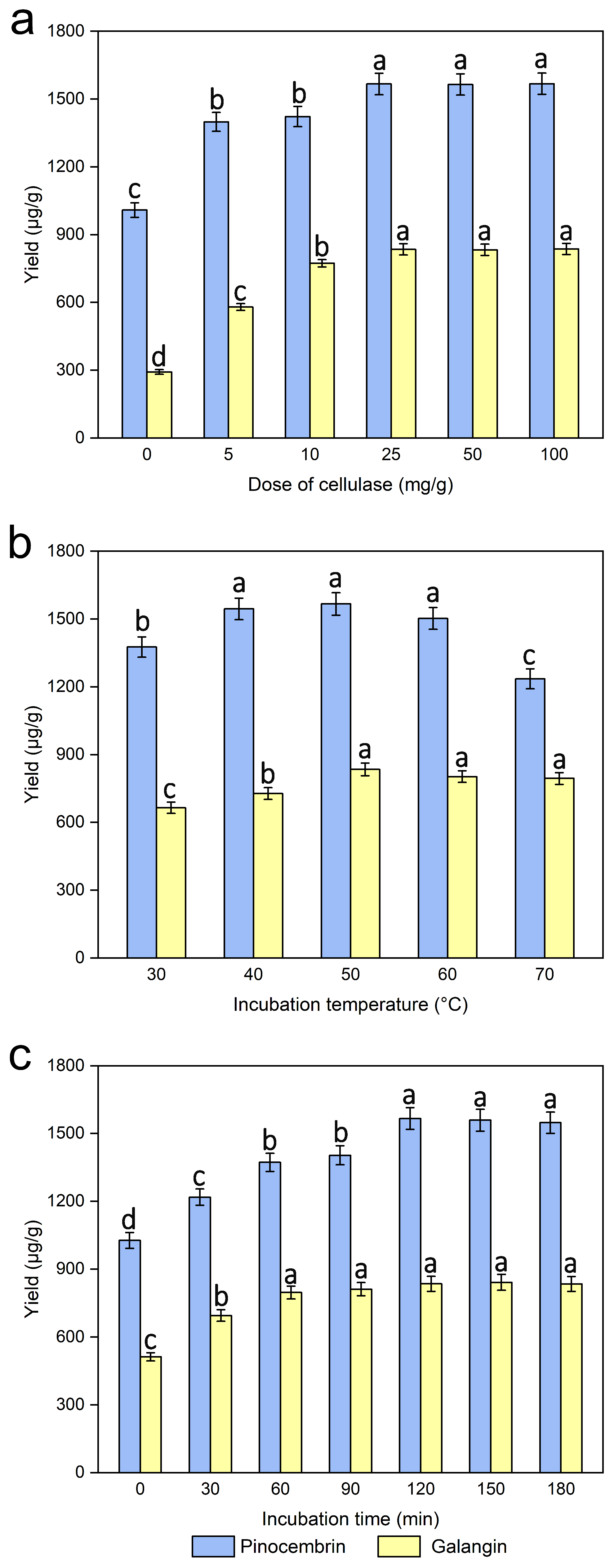
3.2. Effects of the Dose of Cellulase
3.3. Effect of the Incubation Temperature
3.4. Effect of Incubation Time
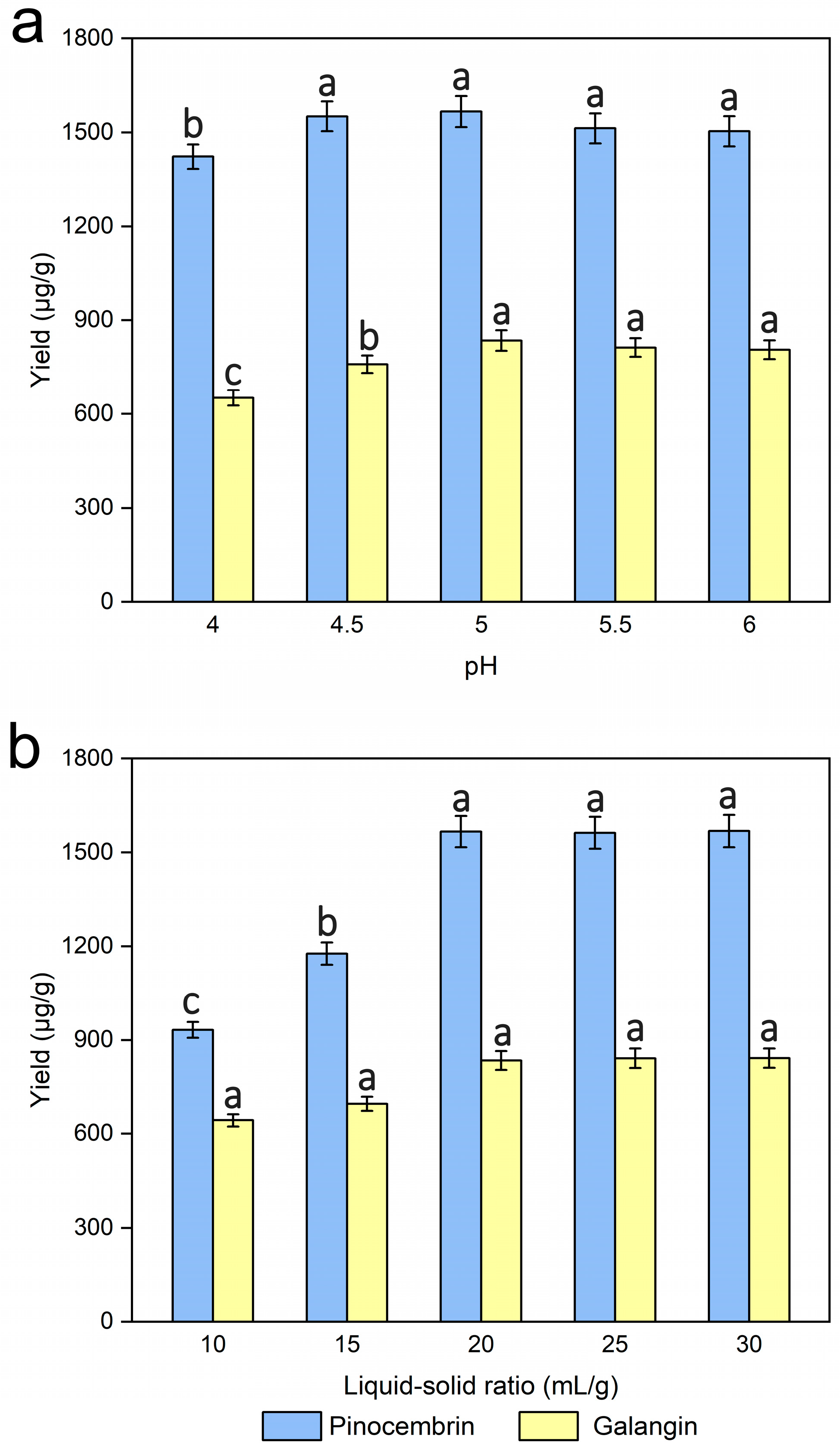
3.5. Effect of pH
3.6. Effects of the Liquid‒Solid Ratio
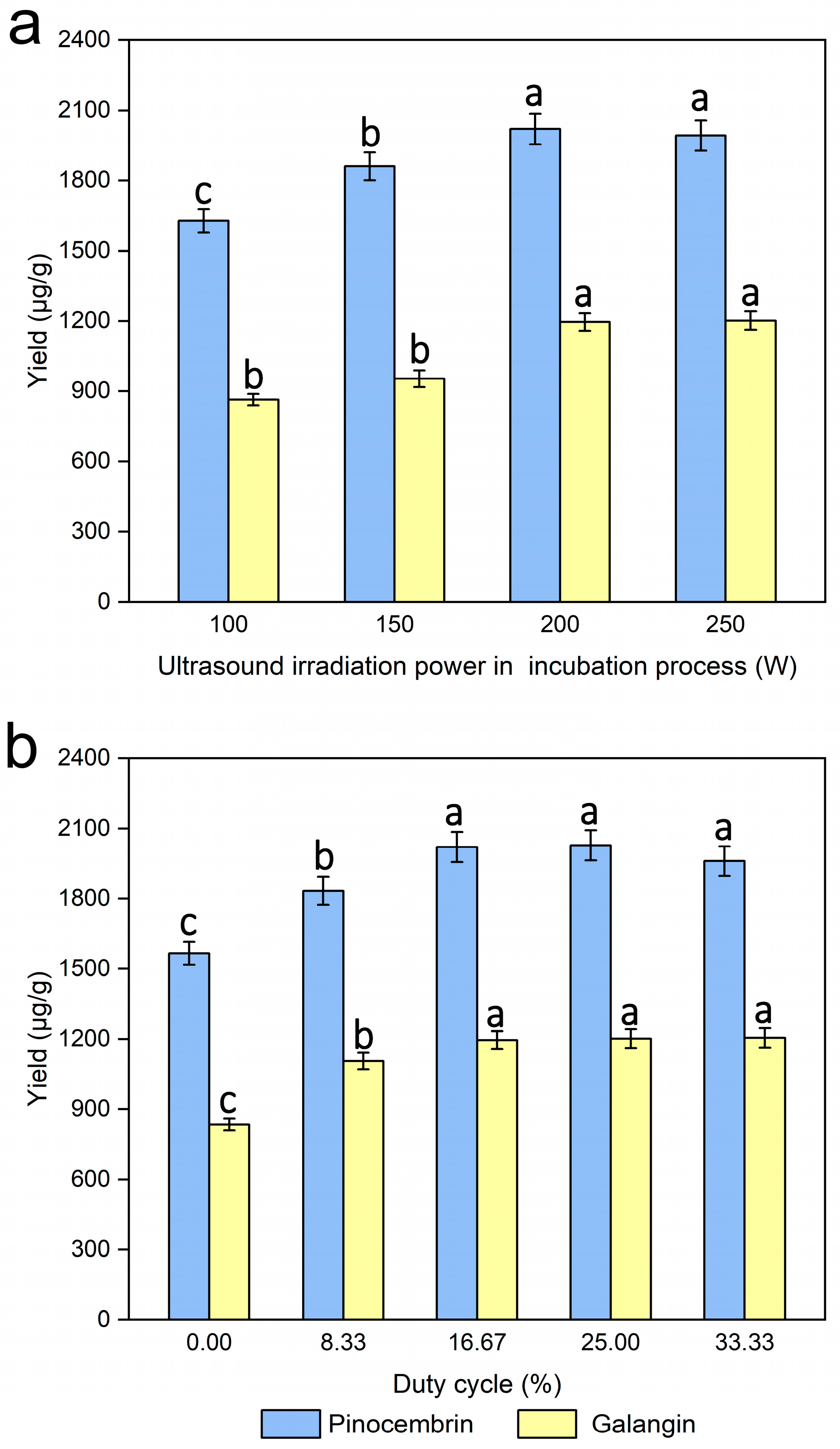
3.7. Effects of the Ultrasound Irradiation Power on the Incubation Process
3.8. Effect of the Duty Cycle
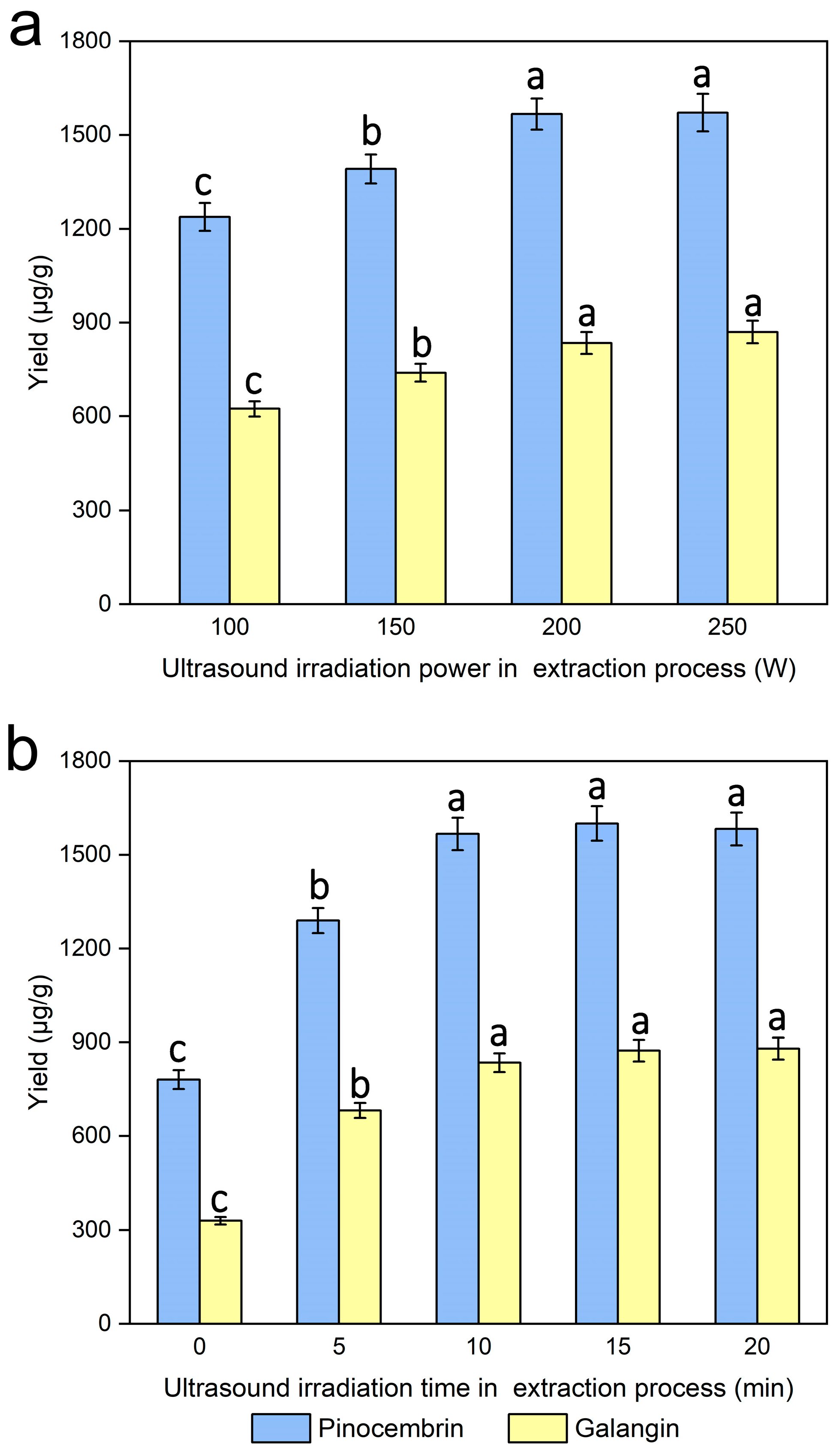
3.9. Effects of Ultrasound Irradiation Power on the Extraction Process
3.10. Effect of Ultrasound Irradiation Time on the Extraction Process
3.11. Parameter Optimization via Response Surface Methodology (RSM)
3.12. Method Validation
3.13. Semi-Pilot-Scale Verification Experiment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, H.; Liu, H.; Wang, W.; Zu, Y. Effects of Thidiazuron, basal medium and light quality on adventitious shoot regeneration from in vitro cultured stem of Populus alba × P. berolinensis. J. For. Res. 2008, 19, 257–259. [Google Scholar] [CrossRef]
- Bai, X.; Wang, W.; Ji, J.; Jiang, J.; Yu, Q.; Yang, C.; Liu, G. PabCIPK24 plays an important role in the response of hybrid poplar to salt stress. Ind. Crop. Prod. 2023, 205, 117452. [Google Scholar] [CrossRef]
- Ding, C.; Zhang, W.; Li, D.; Dong, Y.; Liu, J.; Huang, Q.; Su, X. Effect of overexpression of JERFs on intracellular K+/Na+ balance in transgenic Poplar (Populus alba × P. berolinensis) under salt stress. Front. Plant Sci. 2020, 11, 1192. [Google Scholar] [CrossRef]
- Xu, S.; He, X.; Burkey, K.; Chen, W.; Li, P.; Li, Y.; Li, B.; Wang, Y. Ethylenediurea (EDU) pretreatment alleviated the adverse effects of elevated O3 on Populus alba “Berolinensis” in an urban area. J. Environ. Sci. 2019, 84, 42–50. [Google Scholar] [CrossRef]
- Yu, Y.; He, R.; Chen, S.; Zhang, H.; Zhang, X.; Wang, X.; Liu, Z.; Li, Z.; Wang, Y.; Liu, W.; et al. The B-box transcription factor PabBBX27 in the regulation of chlorophyll biosynthesis and photosynthesis in poplar (Populus alba × P. Berolinensis). Ind. Crop. Prod. 2023, 203, 117159. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, B.; Jia, Z.; Scarlett, C.J.; Sheng, Z. Adsorption/desorption characteristics and enrichment of pinocembrin and galangin from Flos populi using macroporous resin. Rev. Bras. Farmacogn. 2019, 29, 69–76. [Google Scholar] [CrossRef]
- Wang, B.; Goldsmith, C.D.; Zhao, J.; Zhao, S.; Sheng, Z.; Yu, W. Optimization of ultrasound-assisted extraction of quercetin, luteolin, apigenin, pinocembrin and chrysin from Flos populi by Plackett-Burman design combined with Taguchi method. Chiang Mai J. Sci. 2018, 45, 427–439. [Google Scholar]
- Zhao, Y.; Tang, G.; Cai, E.; Liu, S.; Zhang, L.; Wang, S. Hypolipidemic and antioxidant properties of ethanol extract from Flos populi. Nat. Prod. Res. 2014, 28, 1467–1470. [Google Scholar] [CrossRef]
- Ni, H.; Muhammad, I.; Li, J.; Wang, B.; Sheng, Z. In vitro and in vivo antioxidant activities of the flavonoid-rich extract from Flos populus. Pak. J. Pharm. Sci. 2019, 32, 2553–2560. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Janmeda, P. Extraction, isolation and identification of flavonoid from Euphorbia neriifo leaves. Arab. J. Chem. 2017, 10, 509–514. [Google Scholar] [CrossRef]
- Darwish, S.F.; Abedel Mageed, S.S.A.; Mahmoud, A.M.A.; El-demerdash, A.A.; Doghish, A.S.; Azzam, R.K.; Mohamed, R.E.; Farouk, E.A.; Noshy, M.; Shakweer, M.M.; et al. Pinocembrin protects against cisplatin-induced liver injury via modulation of oxidative stress, TAK-1 inflammation, and apoptosis. Toxicol. Appl. Pharm. 2025, 502, 117433. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Liu, W.; Da, X.; Li, Z.; Pu, J. The natural flavonoid pinocembrin shows antithrombotic activity and suppresses septic thrombosis. Int. Immunopharmacol. 2024, 142, 113237. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Sun, Y.; Wang, J.; Wu, S.; Ren, J.; Su, D.; Tang, L.; Gong, J.; Fang, H.; Xu, S.; et al. Integrating network analysis and experimental validation to reveal the mechanism of pinocembrin in alleviating high glucose and free fatty acid-induced lipid accumulation in HepG2 cells. J. Funct. Foods. 2023, 110, 105879. [Google Scholar] [CrossRef]
- Zhang, F.; Yan, Y.; Zhang, L.; Li, D.; Li, L.; Lian, W.; Xia, C.; He, J.; Xu, J.; Zhang, W. Pharmacological activities and therapeutic potential of galangin, a promising natural flavone, in age-related diseases. Phytomedicine 2023, 120, 155061. [Google Scholar] [CrossRef]
- Wu, Y.; Luo, J.; Xu, B. Insights into the anticancer effects of galangal and galangin: A comprehensive review. Phytomedicine 2024, 135, 156085. [Google Scholar] [CrossRef]
- Jomova, K.; Cvik, M.; Orolinova, T.; Alomar, S.Y.; Alwasel, S.H.; Aldahmash, W.; Alqarzae, S.; Al-Juaimlani, A.; Nepovimova, E.; Kuca, K.; et al. Antioxidant versus prooxidant properties of the flavonoid, galangin: ROS scavenging activity, flavonoid-DNA interaction, copper-catalyzed Fenton reaction and DNA damage study. J. Agric. Food Res. 2024, 16, 101112. [Google Scholar] [CrossRef]
- Thapa, R.; Afzal, O.; Altamimi, A.S.A.; Goyal, A.; Almalki, W.H.; Alzarea, S.I.; Kazmi, I.; Jakhmola, V.; Singh, S.K.; Dua, K.; et al. Galangin as an inflammatory response modulator: An updated overview and therapeutic potential. Chem.-Biol. Interact. 2023, 378, 110482. [Google Scholar] [CrossRef]
- Rampogu, S.; Gajula, R.G.; Lee, K.W. A comprehensive review on chemotherapeutic potential of galangin. Biomed. Pharmacother. 2021, 141, 11808. [Google Scholar] [CrossRef]
- Liao, J.; Guo, Z.; Yu, G. Process intensification and kinetic studies of ultrasound-assisted extraction of flavonoids from peanut shells. Ultrason. Sonochem. 2021, 76, 105661. [Google Scholar] [CrossRef]
- Fu, Q.; Dong, W.; Ge, D.D.; Ke, Y.; Jin, Y. Supercritical fluid-based method for selective extraction and analysis of indole alkaloids from Uncaria rhynchophylla. J. Chromatogr. A 2023, 1710, 464410. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, C.; Jiang, S.; Guan, L.; Liu, X.; Wen, L. Ultrasonic-assisted extraction of a low molecular weight polysaccharide from Nostoc commune Vaucher and its structural characterization and immunomodulatory activity. Ultrason. Sonochem. 2024, 108, 106961. [Google Scholar] [CrossRef]
- Romero-Díez, R.; Matos, M.; Rodrigues, L.; Bronze, M.R.; Rodríguez-Rojo, S.; Cocero, M.J.; Matias, A.A. Microwave and ultrasound pre-treatments to enhance anthocyanins extraction from different wine lees. Food Chem. 2019, 272, 258–266. [Google Scholar] [CrossRef]
- Dessie, W.; Luo, X.; Wang, M.; Liao, Y.; Li, Z.; Khan, M.R.; Qin, Z. Enhancing the valorization efficiency of Camellia oil extraction wastes through sequential green acid pretreatment and solid-state fermentation-based enzymatic hydrolysis. Ind. Crop. Prod. 2024, 217, 118893. [Google Scholar] [CrossRef]
- Bouloumpasi, E.; Skendi, A.; Christaki, S.; Biliaderis, C.G.; Irakli, M. Optimizing conditions for the recovery of lignans from sesame cake using three green extraction methods: Microwave-, ultrasound- and accelerated-assisted solvent extraction. Ind. Crop. Prod. 2024, 207, 117770. [Google Scholar] [CrossRef]
- Hatami, T.; Johner, J.C.F.; Meireles, M.A.A. Extraction and fractionation of fennel using supercritical fluid extraction assisted by cold pressing. Ind. Crop. Prod. 2018, 123, 661–666. [Google Scholar] [CrossRef]
- Chemat, F.; Zill-e-Huma; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef]
- Zhang, Y.; Kong, X.; Wang, Z.; Sun, Y.; Zhu, S.; Li, L.; Lv, P. Optimization of enzymatic hydrolysis for effective lipid extraction from microalgae Scenedesmus sp. Renew. Energ. 2018, 125, 1049–1057. [Google Scholar] [CrossRef]
- Xu, Q.; Shen, Z.; Wang, Y.; Guo, S.; Li, F.; Wang, Y.; Zhou, C. Anti-diarrhoeal and anti-microbial activity of Flos populi (male inflorescence of Populus tomentosa Carrière) aqueous extracts. J. Ethnopharmacol. 2013, 148, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.C.; Chen, J.; Zhang, H.; Li, Z.; Zhao, L.; Qiu, H. Effective extraction of flavonoids from Lycium barbarum L. fruits by deep eutectic solvents-based ultrasound-assisted extraction. Talanta 2019, 203, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Clardy, J.; Walsh, C. Lessons from natural molecules. Nature 2004, 432, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhang, Q.; Liu, J.; Gu, H.; Yang, L. An efficient approach for the extraction of orientin and vitexin from Trollius chinensis flowers using ultrasonic circulating technique. Ultrason. Sonochem. 2017, 37, 267–278. [Google Scholar] [CrossRef]
- Yang, X.; Liu, T.; Qi, S.; Gu, H.; Li, J.; Yang, L. Tea saponin additive to extract eleutheroside B and E from Eleutherococcus senticosus by ultrasonic mediation and its application in a semi-pilot scale. Ultrason. Sonochem. 2022, 86, 106039. [Google Scholar] [CrossRef]
- Zhao, R.; Yang, X.; Zhang, A.; Zhou, T.; Zhou, Y.; Yang, L. An efficient approach for simultaneously obtaining oil and epigoitrin from Orychophragmus violaceus seeds by microwave-mediated immiscible binary solvent extraction. Food Chem. 2022, 372, 131258. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, Q.; Mo, K.; Fei, S.; Gu, H.; Yang, L. Optimization of ionic liquid-based omogenate extraction of orientin and vitexin from the flowers of Trollius chinensis and its application on a pilot scale. Sep. Purif. Technol. 2017, 175, 147–157. [Google Scholar] [CrossRef]
- Wei, M.; Yang, L. Determination of orientin in Trollius chinensis using ultrasound- assisted extraction and high-performance liquid chromatography: Several often-overlooked sample preparation parameters in an ultrasonic bath. J. Chromatogr. A. 2017, 1530, 68–79. [Google Scholar] [CrossRef]
- Hai, T.; Man, P.V.; Anh, L.T.H. Optimization of ultrasound-assisted extraction of astaxanthin from black tiger shrimp (Penaeus monodon) shells using deep eutectic solvent and ethanol as a cosolvent. LWT 2024, 212, 116965. [Google Scholar] [CrossRef]
- Machado, T.O.X.; Kodel, H.D.A.C.; dos Santos, F.A.; dos Santos Lima, M.; Costa, A.S.G.; Oliveira, M.B.P.P.; Dariva, C.; dos Santos Estevam, C.D.; Fathi, F.; Souto, E.B. Cellulase-assisted extraction followed by pressurized liquid extraction for enhanced recovery of phenolic compounds from ‘BRS Violeta’ grape pomace. Sep. Purif. Technol. 2025, 354, 129218. [Google Scholar] [CrossRef]
- Tang, Z.; Huang, G.; Huang, H. Ultrasonic/cellulase-assisted extraction of polysaccharide from Garcinia mangostana rinds and its carboxymethylated derivative. Ultrason. Sonochem. 2023, 99, 106571. [Google Scholar] [CrossRef] [PubMed]
- Muniraj, S.; Shih, H.; Chen, Y.; Hsiech, C.; Ponnusamy, V.K.; Jen, J. Novel one-step headspace dynamic in-syringe liquid phase derivatization-extraction technique for the determination of aqueous aliphatic amines by liquid chromatography with fluorescence detection. J. Chromatogr. A 2013, 1296, 104–110. [Google Scholar] [CrossRef]
- Wang, J.; Sun, B.; Liu, Y.; Zhang, H. Optimization of ultrasound-assisted enzymatic extraction of arabinoxylan from wheat bran. Food Chem. 2014, 150, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Romsaiyud, A.; Songkasiri, W.; Nopharatana, A.; Chaiprasert, P. Combination effect of pH and acetate on enzymatic cellulose hydrolysis. J. Environ. Sci. 2009, 21, 965–970. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Yan, J.; Zhu, M.; Ye, C. Ultrasound-cellulase synergy for the extraction of total flavonoids from Astragali complanati Semen and its antioxidant properties. J. Appl. Res. Med. Aroma. 2024, 43, 100597. [Google Scholar] [CrossRef]
- Fan, W.; Duan, H.; Ren, X.; Guo, X.; Zhang, Y.; Li, J.; Zhang, F.; Chen, J.; Yang, X. Optimization of ultrasound-assisted cellulase degradation method on the extraction of mulberry leaf protein and its effect on the functional characteristics. Ultrason. Sonochem. 2023, 99, 106561. [Google Scholar] [CrossRef]
- Yang, B.; Jiang, Y.; Zhao, M.; Shi, J.; Wang, L. Effects of ultrasonic extraction on the physical and chemical properties of polysaccharides from longan fruit pericarp. Polym. Degrad. Stabil. 2008, 93, 268–272. [Google Scholar] [CrossRef]
- Yuan, M.; Huan, X.; Yang, X.; Fan, M.; Yin, J.; Ma, Y.; Deng, B.; Cao, H.; Han, Y.; Xu, F. Simultaneous extraction of five heavy metal ions from root vegetables via dual-frequency ultrasound-assisted enzymatic digestion. Food Chem. 2024, 454, 139741. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, C.; Zhang, C.; Li, W. Ultrasound-assisted enzyme catalyzed hydrolysis of olive waste and recovery of antioxidant phenolic compounds. Innov. Food Sci. Emerg. 2017, 44, 224–234. [Google Scholar] [CrossRef]


| Run | Factor X1 | Factor X2 | Factor X3 | Response 1 | Response 2 | ||
|---|---|---|---|---|---|---|---|
| Dose of Cellulase (mg/g) | Incubation Temperature (°C) | Incubation Time (min) | Predicted Galangin Yield (μg/g) | Actual Galangin Yield (μg/g) | Predicted Pinocembrin Yield (μg/g) | Actual Pinocembrin Yield (μg/g) | |
| 1 | 10 | 40 | 120 | 1163.08 | 1179.63 | 1941.35 | 1933.74 |
| 2 | 40 | 40 | 120 | 1180.49 | 1182.51 | 1879.66 | 1927.96 |
| 3 | 10 | 60 | 120 | 1062.18 | 1060.15 | 1577.66 | 1731.31 |
| 4 | 40 | 60 | 120 | 1001.33 | 985.30 | 1740.96 | 1789.15 |
| 5 | 10 | 50 | 90 | 1249.38 | 1253.05 | 1971.70 | 2020.49 |
| 6 | 40 | 50 | 90 | 1221.90 | 1240.10 | 2003.47 | 2000.25 |
| 7 | 10 | 50 | 150 | 1269.80 | 1251.61 | 2094.44 | 2098.57 |
| 8 | 40 | 50 | 150 | 1253.84 | 1250.17 | 2164.29 | 2113.03 |
| 9 | 25 | 40 | 90 | 1204.17 | 1183.95 | 1921.84 | 1985.79 |
| 10 | 25 | 60 | 90 | 1016.11 | 1014.09 | 1693.22 | 1745.77 |
| 11 | 25 | 40 | 150 | 1182.31 | 1183.95 | 2086.20 | 2089.90 |
| 12 | 25 | 60 | 150 | 1090.32 | 1110.54 | 1812.42 | 1855.66 |
| 13 | 25 | 50 | 120 | 1248.16 | 1250.17 | 2095.37 | 2006.03 |
| 14 | 25 | 50 | 120 | 1248.16 | 1228.58 | 2095.37 | 2118.82 |
| 15 | 25 | 50 | 120 | 1248.16 | 1251.61 | 2095.37 | 2121.71 |
| 16 | 25 | 50 | 120 | 1248.16 | 1254.49 | 2095.37 | 2118.82 |
| 17 | 25 | 50 | 120 | 1248.16 | 1255.93 | 2095.37 | 2115.92 |
| Source | Degree of Freedom | Sum of Squares | Mean Square | F-Value | p-Value a | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Galangin | Pinocembrin | Galangin | Pinocembrin | Galangin | Pinocembrin | Galangin | Pinocembrin | Galangin | Pinocembrin | |
| Model | 9 | 9 | 124,559 | 464,642 | 13,840 | 51,627 | 37.73 | 15.43 | <0.0001 * | 0.0008 * |
| X1 | 1 | 1 | 944 | 5163 | 944 | 5163 | 2.57 | 1.54 | 0.1528 | 0.2542 |
| X2 | 1 | 1 | 39,216 | 126,200 | 39,216 | 126,200 | 106.91 | 37.71 | <0.0001 * | 0.0005 * |
| X3 | 1 | 1 | 1370 | 40,202 | 1370 | 40,202 | 3.74 | 12.01 | 0.0945 | 0.0105 * |
| X1X2 | 1 | 1 | 1531 | 12,655 | 1531 | 12,655 | 4.17 | 3.78 | 0.0804 | 0.0929 |
| X1X3 | 1 | 1 | 33 | 362 | 33 | 362 | 0.09 | 0.11 | 0.7724 | 0.7517 |
| X2X3 | 1 | 1 | 2307 | 510 | 2307 | 510 | 6.29 | 0.15 | 0.0405 * | 0.7079 |
| X12 | 1 | 1 | 459 | 17,902 | 459 | 17,902 | 1.25 | 5.35 | 0.3002 | 0.0540 |
| X22 | 1 | 1 | 77,816 | 253,269 | 77,816 | 253,269 | 212.14 | 75.67 | <0.0001 * | <0.0001 * |
| X32 | 1 | 1 | 511 | 3374 | 511 | 3374 | 1.39 | 1.01 | 0.2764 | 0.3488 |
| Residual | 7 | 7 | 2568 | 23,428 | 367 | 3347 | ||||
| Lack of fit | 3 | 3 | 2068 | 13,317 | 689 | 4439 | 5.52 | 1.76 | 0.0663 | 0.2940 |
| Pure error | 4 | 4 | 500 | 10,112 | 125 | 2528 | ||||
| Cor total | 16 | 16 | 127,127 | 488,071 | ||||||
| Credibility analysis of the regression equations | Index mark | Standard deviation | Mean | CV% | Press | R2 | Adjust R2 | Predicted R2 | Adequacy precision | |
| Y1 | 19.15 | 1184.45 | 1.62 | 33,866.76 | 0.9798 | 0.9538 | 0.7336 | 18.28 | ||
| Y2 | 57.85 | 1962.59 | 2.95 | 228,868.46 | 0.9520 | 0.8903 | 0.5311 | 13.22 | ||
| Stability studies of pinocembrin and galangin standards under the following EP-UAS a conditions: ethanol volume fraction of 70%, cellulase dosage of 40 mg/mL, incubation temperature of 45 °C, incubation time of 150 min, pH of 5, liquid‒solid ratio of 20 mL/g, ultrasonic irradiation power during incubation of 200 W, duty cycle of 16.67%, ultrasonic irradiation power during extraction of 200 W and ultrasonic irradiation time during extraction of 10 min. | |||||||||
| Compounds | Initial concentration (mg/mL) | Recovered concentration after EP-UAS (mg/mL) | RSD% (n = 3) | Average recovery (%) | Recovered concentration after 7 days (mg/mL) | RSD% (n = 3) | Average recovery (%) | ||
| Pinocembrin | 0.1090 | 0.1080 | 0.98 | 99.25 | 0.1065 | 0.99 | 97.67 | ||
| Galangin | 0.1091 | 0.1082 | 0.97 | 98.64 | 0.1060 | 0.96 | 96.93 | ||
| Recovery of pinocembrin and galangin from the male inflorescences of Populus alba × berolinensis | |||||||||
| Sample | Content of the sample (mg) | Mass of added standard (mg) | Mass of the sample analyzed with added standard (mg) | Recovery (%) | |||||
| Pinocembrin | Galangin | Pinocembrin | Galangin | Pinocembrin | Galangin | Pinocembrin | Galangin | ||
| 1 | 1.25 | 2.31 | 0.50 | 2.00 | 1.64 | 4.82 | 99.74 | 99.86 | |
| 2 | 1.25 | 2.31 | 1.00 | 3.00 | 2.13 | 5.78 | 99.26 | 98.72 | |
| 3 | 1.25 | 2.31 | 1.50 | 4.00 | 2.61 | 6.75 | 98.45 | 98.23 | |
| Average | 99.15 | 98.93 | |||||||
| Precision results as peak area of different determinations on 3 different days (pinocembrin 0.1090 mg/mL, galangin 0.1091 mg/mL (n = 3), acceptance limit RSD% < 2) | |||||||||
| Sample | Day 1 | Day 2 | Day 3 | ||||||
| Pinocembrin | Galangin | Pinocembrin | Galangin | Pinocembrin | Galangin | ||||
| 1 | 3688 | 4042 | 3636 | 4021 | 3617 | 4015 | |||
| 2 | 3680 | 4016 | 3621 | 4016 | 3602 | 3956 | |||
| 3 | 3566 | 4010 | 3510 | 3927 | 3524 | 3892 | |||
| Mean | 3645 | 4004 | 3589 | 3988 | 3581 | 3954 | |||
| Standard deviation | 68.24 | 45.21 | 68.83 | 52.89 | 49.93 | 61.52 | |||
| RSD% | 1.87 | 1.13 | 1.92 | 1.33 | 1.39 | 1.56 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, R.; Li, X.; Bao, Y.; Xu, W.; Xu, C.; Wang, R.; Xia, T.; Liu, T.; Ben, A. Semi-Pilot Scale Extraction of Pinocembrin and Galangin from Populus alba L. × berolinensis K. Koch via Enzymatic Pretreatment and Ultrasonication. Separations 2025, 12, 249. https://doi.org/10.3390/separations12090249
Zhao R, Li X, Bao Y, Xu W, Xu C, Wang R, Xia T, Liu T, Ben A. Semi-Pilot Scale Extraction of Pinocembrin and Galangin from Populus alba L. × berolinensis K. Koch via Enzymatic Pretreatment and Ultrasonication. Separations. 2025; 12(9):249. https://doi.org/10.3390/separations12090249
Chicago/Turabian StyleZhao, Ru, Xiaoli Li, Yazhou Bao, Wenjun Xu, Chen Xu, Rongrong Wang, Tianlan Xia, Tingli Liu, and Ailing Ben. 2025. "Semi-Pilot Scale Extraction of Pinocembrin and Galangin from Populus alba L. × berolinensis K. Koch via Enzymatic Pretreatment and Ultrasonication" Separations 12, no. 9: 249. https://doi.org/10.3390/separations12090249
APA StyleZhao, R., Li, X., Bao, Y., Xu, W., Xu, C., Wang, R., Xia, T., Liu, T., & Ben, A. (2025). Semi-Pilot Scale Extraction of Pinocembrin and Galangin from Populus alba L. × berolinensis K. Koch via Enzymatic Pretreatment and Ultrasonication. Separations, 12(9), 249. https://doi.org/10.3390/separations12090249







