Synthesis and CO2 Capture Properties of Co- and Nd-Modified ZIF-8 Materials Loaded onto Electrospun Polyacrylonitrile Fibers
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Co- and Nd-Doped ZIF-8 Powders
2.2. Polyacrylonitrile Preparation
2.3. Synthesis of ZIF-8/PAN Microfibers
2.4. In Situ Secondary Growth of ZIF-8 on ZIF-8/PAN Microfibers
2.5. Materials Characterization
2.6. Gas Adsorption Measurements
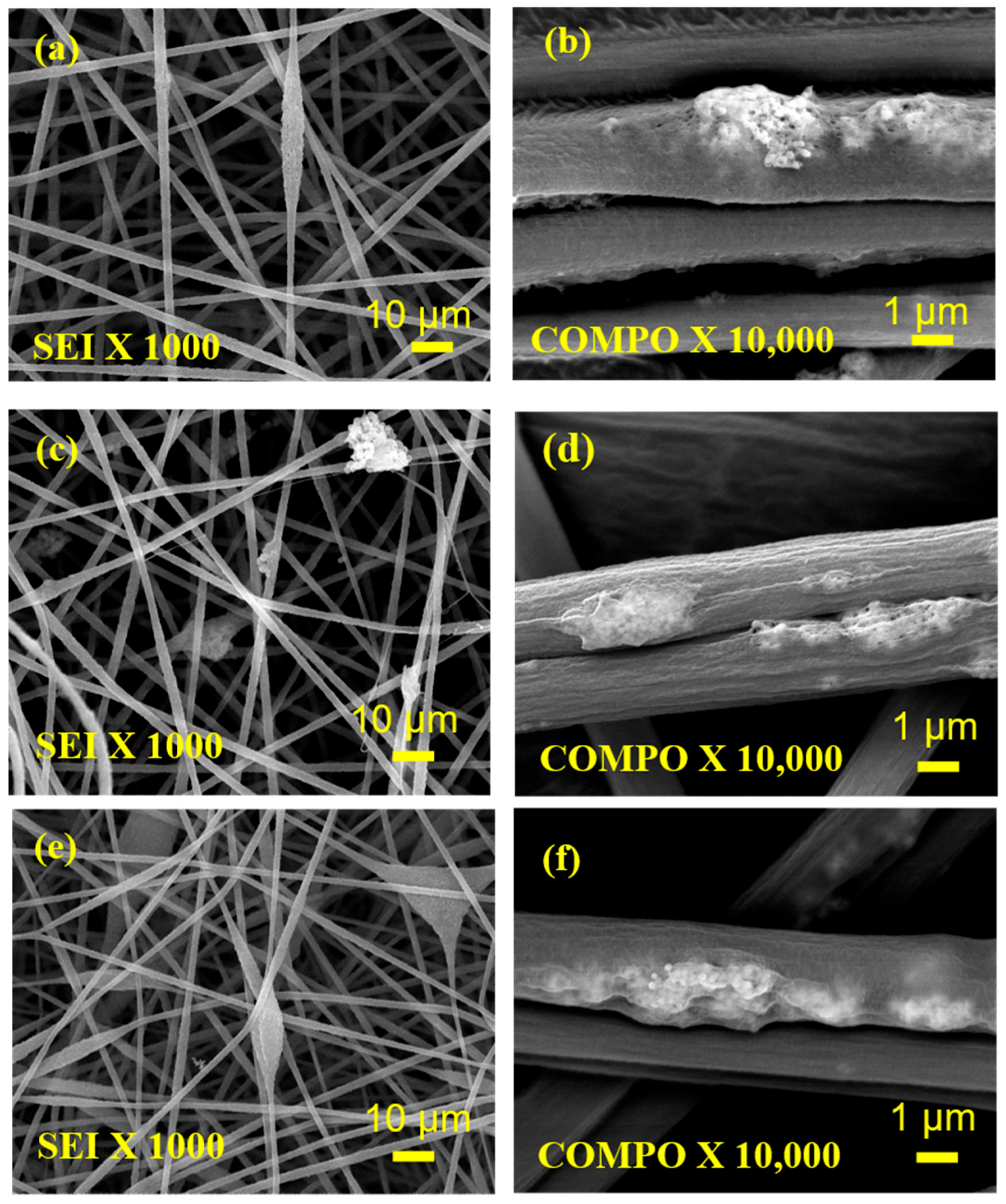
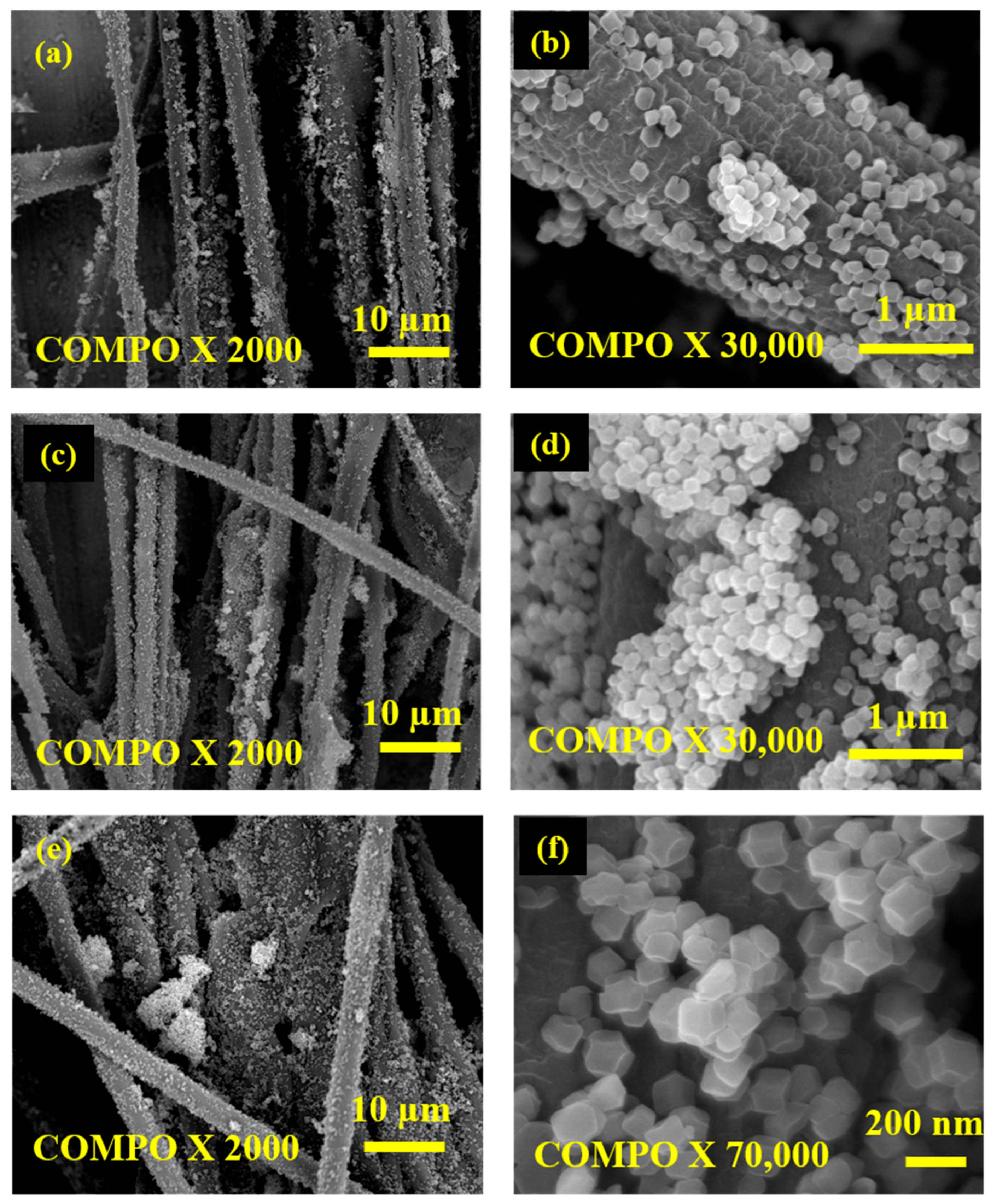
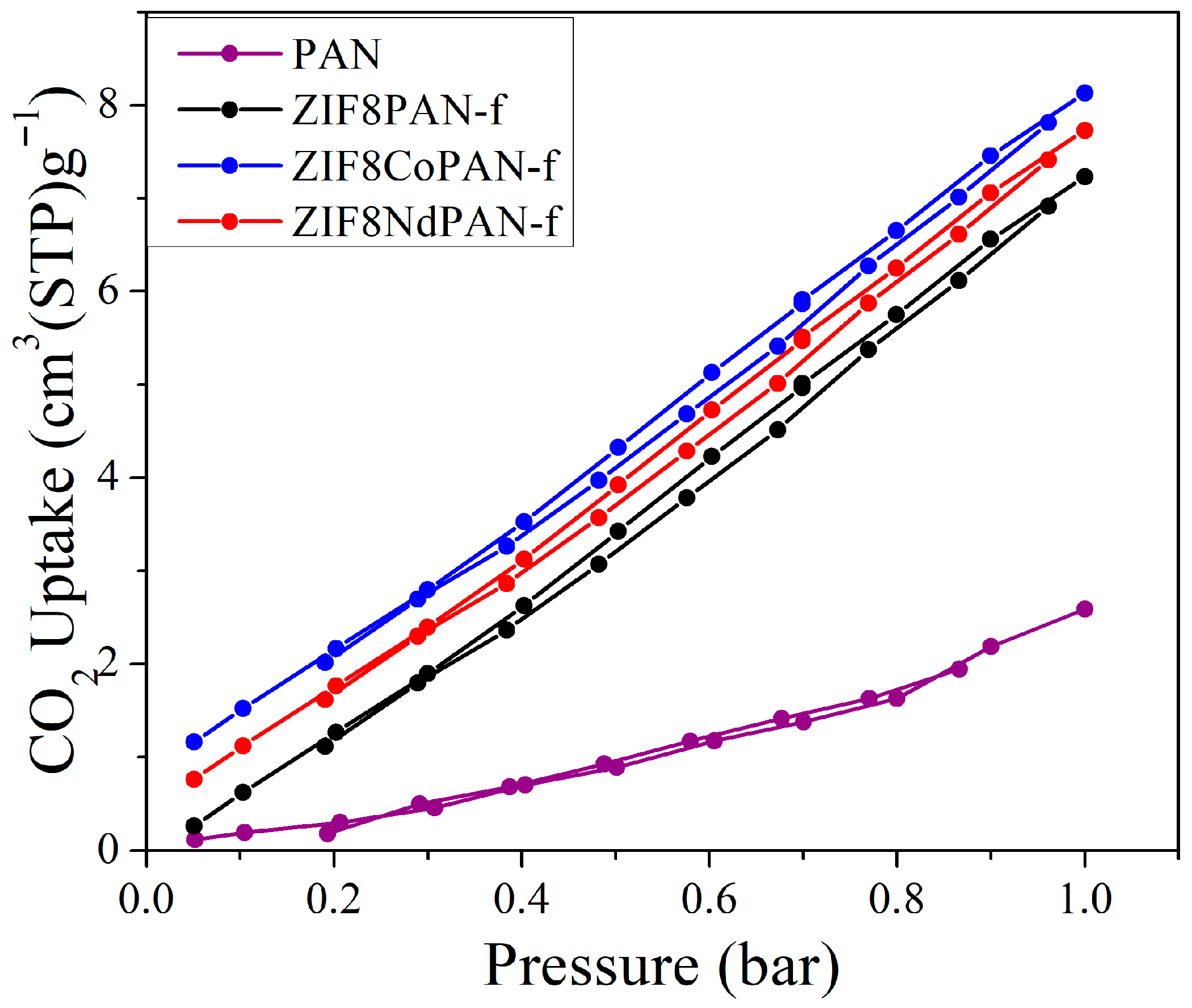
3. Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, H.; Lu, H.; Hu, H. CO2 capture and mineral storage: State of the art and future challenges. Renew. Sustain. Energy Rev. 2024, 189, 113908. [Google Scholar] [CrossRef]
- Aminu, M.D.; Nabavi, S.A.; Rochelle, C.A.; Manovic, V. A review of developments in carbon dioxide storage. Appl. Energy 2017, 208, 1389–1419. [Google Scholar] [CrossRef]
- NASA, National Aeronautics and Space Administration. Carbon Dioxide. Available online: https://climate.nasa.gov/vital-signs/carbon-dioxide/?intent=121 (accessed on 20 February 2024).
- Obi, D.; Onyekuru, S.; Orga, A. Recent Material Advances in Carbon Dioxide (CO2) Capture from Power Plant Flue Gases: Toward Achieving Net Zero Emissions. Energy Sci. Eng. 2025, 13, 980–994. [Google Scholar] [CrossRef]
- Rana, A.; Andino, J.M. A Review of Materials for Carbon Dioxide Capture. Catalysts 2025, 15, 273–298. [Google Scholar] [CrossRef]
- Lee, G.; Ahmed, I.; Hwa Jhung, S. CO2 adsorption using functionalized metal-organic frameworks under low pressure: Contribution of functional groups, excluding amines, to adsorption. Chem. Eng. J. 2023, 453, 148440. [Google Scholar] [CrossRef]
- Francis, J.C.; Nighojkar, A.; Kandasubramanian, B. Relevance of wood biochar on CO2 adsorption: A review. Hybrid Adv. 2023, 3, 100056. [Google Scholar] [CrossRef]
- Hwang, J.; Azzan, H.; Pini, R.; Petit, C. H2, N2, CO2, and CH4 Unary Adsorption Isotherm Measurements at Low and High Pressures on Zeolitic Imidazolate Framework ZIF-8. J. Chem. Eng. Data 2022, 67, 1674–1686. [Google Scholar] [CrossRef]
- Kalauni, K.; Vedrtnam, A.; Wdowin, M.; Chaturvedi, S. ZIF for CO2 capture: Structure, mechanism, optimization, and modeling. Processes 2022, 10, 2689. [Google Scholar] [CrossRef]
- Khan, I.U.; Othman, M.H.D.; Jilani, A.; Ismail, A.F.; Hashim, H.; Jaafar, J.; Rahman, M.A.; Rehman, G.U. Economical, environmental friendly synthesis, characterization for the production of zeolitic imidazolate framework-8 (ZIF-8) nanoparticles with enhanced CO2 adsorption. Arab. J. Chem. 2018, 11, 1072–1083. [Google Scholar] [CrossRef]
- Yu, J.; Qi, K.; Li, X.; Gao, L.; Wang, J.; Zeng, J.; Du, S. Insights into zeolite imidazole frameworks (ZIFs) for CO2 capture and separation: A short review. J. Environ. Sci. 2025, 157, 690–709. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Vedrtnam, A.; Kalauni, K.; Singh, A.; Wdowin, M. Synthesis routes of zeolitic imidazolate framework-8 for CO2 capture: A review. AIMS Mater. Sci. 2025, 12, 118–164. [Google Scholar] [CrossRef]
- Payra, S.; Challagulla, S.; Indukuru, R.R.; Chakraborty, C.; Tarafder, K.; Ghosh, B.; Roy, S. The structural and surface modification of zeolitic imidazolate frameworks towards reduction of encapsulated CO2. New J. Chem. 2018, 42, 19205–19213. [Google Scholar] [CrossRef]
- Neubertová, V.; Švorčík, V.; Kolská, Z. Amino-modified ZIF-8 for enhanced CO2 capture: Synthesis, characterization and performance evaluation. Microporous Mesoporous Mater. 2024, 366, 112956. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, P.; Zhao, T.; Xia, Y. Enhanced CO2 Adsorption and Selectivity of CO2/N2 on Amine@ZIF-8 Materials. Adsorpt. Sci. Technol. 2022, 2022, 3207986. [Google Scholar] [CrossRef]
- Abraha, Y.W.; Tsai, C.W.; Niemantsverdriet, J.H.; Langner, E.H. Optimized CO2 Capture of the Zeolitic Imidazolate Framework ZIF-8 Modified by Solvent-Assisted Ligand Exchange. ACS Omega 2021, 6, 21850–21860. [Google Scholar] [CrossRef]
- Song, F.; Cao, Y.; Zhao, Y.; Jiang, R.; Xu, Q.; Yan, J.; Zhong, Q. Ion-Exchanged ZIF-67 Synthesized by One-Step Method for Enhancement of CO2 Adsorption. J. Nanomater. 2020, 2020, 1508574. [Google Scholar] [CrossRef]
- Awadallah-F, A.; Hillman, F.; Al-Muhtaseb, S.A.; Jeong, H.K. Influence of doped metal center on morphology and pore structure of ZIF-8. MRS Commun. 2019, 9, 288–291. [Google Scholar] [CrossRef]
- Qi, B.; Wang, X.; Cheng, J.; Shang, Y. Synthesis and H2S-Sensing Properties of MOF-Derived Cu-Doped ZnO Nanocages. Nanomaterials 2022, 12, 2579. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Yan, Z.; Mo, X.; Peng, X.; Chen, L. Hierarchical Co/ZIF-8 as an efficient catalyst for cycloaddition of CO2 and epoxide. Microporous Mesoporous Mater. 2020, 294, 109917. [Google Scholar] [CrossRef]
- Ramadurgam, A.; Vasa, M.; Inkollu, S.; Chetan, M.P. Bimetallic ZIFs based on Ce/Zn and Ce/Co combinations for stable and enhanced carbon capture. J. Clean. Prod. 2022, 350, 131478. [Google Scholar] [CrossRef]
- Da Rocha, T.R.; Veríssimo dos Santos, T.; Da Silva Viana, R.; Plentz-Meneghetti, S.M.; Do Espírito Santo-Barbosa, C.A. Study of the morphological, structural and photophysical properties of dual emission europium-doped ZIF-8 particles. Opt. Mater. 2021, 111, 110581. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, C.; Pan, Y.; Xiang, L.; Liu, Y. Preparation of Y3+- and La3+-doped ZIF-8 Crystals and the Fluorescence Sensing of Amines. Chem. Lett. 2015, 44, 887–889. [Google Scholar] [CrossRef]
- Pullumbi, P.; Brandani, F.; Brandani, S. Gas separation by adsorption: Technological drivers and opportunities for improvement. Curr. Opin. Chem. Eng. 2019, 24, 131–142. [Google Scholar] [CrossRef]
- Rezaei, F.; Webley, P. Optimum structured adsorbents for gas separation processes. Chem. Eng. Sci. 2009, 64, 5182–5191. [Google Scholar] [CrossRef]
- Choi, C.; Kadam, R.L.; Gaikwad, S.; Hwang, K.S.; Han, S. Metal organic frameworks immobilized polyacrylonitrile fiber mats with polyethyleneimine impregnation for CO2 capture. Microporous Mesoporous Mater. 2020, 296, 110006. [Google Scholar] [CrossRef]
- Su, Q.; Guo, Q.; Wang, H.; Liu, M.; Zuo, C. Research progress of MOF-based materials in the photocatalytic CO2 reduction. Carbon Resour. Convers. 2024, 7, 100211. [Google Scholar] [CrossRef]
- Nedoma, M.; Azzan, H.; Yio, M.; Danaci, D.; Itskou, I.; Kia, A.; Pini, R.; Petit, C. The effect of adsorbent shaping on the equilibrium and kinetic CO2 adsorption properties of ZIF-8. Microporous Mesoporous Mater. 2024, 380, 113303. [Google Scholar] [CrossRef]
- Sutrisna, P.D.; Prasetya, N.; Himma, N.F.; Wenten, I.G. A mini-review and recent outlooks on the synthesis and applications of zeolite imidazolate framework-8 (ZIF-8) membranes on polymeric substrate. J. Chem. Technol. Biotechnol. 2020, 95, 2767–2774. [Google Scholar] [CrossRef]
- Niu, B.; Zhai, Z.; Wang, J.; Li, C. Preparation of ZIF-8/PAN composite nanofiber membrane and its application in acetone gas monitoring. Nanotechnology 2023, 34, 245710. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, Y.A.; Abdel-Mohsen, A.M.; Zhang, Q.J.; Younas, M.; Zhong, L.B.; Yang, J.C.E.; Zheng, Y.M. Facile synthesis of ZIF-8 incorporated electrospun PAN/PEI nanofibrous composite membrane for efficient Cr(VI) adsorption from water. Chem. Eng. J. 2023, 461, 141972. [Google Scholar] [CrossRef]
- Vedrtnam, A.; Singh, A.; Singh, A.; Kalauni, K.; Wdowin, M. High-Performance Carbon Dioxide Adsorption with Zeolitic Imidazolate Framework-8-Based Cellulose Air Filters. Appl. Sci. 2024, 14, 11019. [Google Scholar] [CrossRef]
- Wu, Y.; Li, F.; Liu, H.; Zhu, W.; Teng, M.; Jiang, Y.; Li, W.; Xu, D.; He, D.; Hannam, P.; et al. Electrospun Fibrous Mats as Skeletons to Produce Free-Standing MOF Membranes. J. Mater. Chem. 2012, 22, 16971–16978. [Google Scholar] [CrossRef]
- Efome, J.E.; Rana, D.; Matsuura, T.; Lan, C.Q. Insight Studies on Metal–Organic Framework Nanofibrous Membrane Adsorption and Activation for Heavy Metal Ions Removal from Aqueous Solution. ACS Appl. Mater. Interfaces 2018, 10, 18619–18629. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A Fascinating Fiber Fabrication Technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef]
- Li, Z.; Cao, Z.; Grande, C.; Zhang, W.; Dou, Y.; Li, X.; Kaiser, A. A Phase Conversion Method to Anchor ZIF-8 onto a PAN Nanofiber Surface for CO2 Capture. RSC Adv. 2022, 12, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef]
- Zheng, W.; Li, Z.; Sun, T.; Ruan, X.; Dai, Y.; Li, X.; He, G. PAN Electrospun Nanofiber Skeleton Induced MOFs Continuous Distribution in MMMs to Boost CO2 Capture. J. Membr. Sci. 2022, 650, 120330. [Google Scholar] [CrossRef]
- Bergaoui, M.; Khalfaoui, M.; Awadallah-F, A.; Al-Muhtaseb, S. A Review of the Features and Applications of ZIF-8 and Its Derivatives for Separating CO2 and Isomers of C3- and C4-Hydrocarbons. J. Nat. Gas Sci. Eng. 2021, 96, 104289. [Google Scholar] [CrossRef]
- Caro-Briones, R.; García-Pérez, B.E.; Báez-Medina, H.; San Martín-Martínez, E.; Martínez-Mejía, G.; Jiménez-Juárez, R.; Corea, M. Influence of Monomeric Concentration on Mechanical and Electrical Properties of Poly(Styrene-Co-Acrylonitrile) and Poly(Styrene-Co-Acrylonitrile/Acrylic Acid) Yarns Electrospun. J. Appl. Polym. Sci. 2020, 137, 49166. [Google Scholar] [CrossRef]
- Kida, K.; Okita, M.; Fujita, K.; Tanaka, S.; Miyake, Y. Formation of Highly Crystalline ZIF-8 in an Aqueous Solution. CrystEngComm 2013, 15, 1794–1801. [Google Scholar] [CrossRef]
- Cao, J.; Sun, S.; Li, X.; Yang, Z.; Xiong, W.; Wu, Y.; Jia, M.; Zhou, Y.; Zhou, C.; Zhang, Y. Efficient Charge Transfer in Aluminum-Cobalt Layered Double Hydroxide Derived from Co-ZIF for the Enhanced Catalytic Degradation of Tetracycline through Peroxymonosulfate Activation. Chem. Eng. J. 2020, 382, 122802. [Google Scholar] [CrossRef]
- Zhang, M.; Shi, X.; Dai, X.; Huo, C.; Xie, J.; Li, X.; Wang, X. Improving the crystallization and fire resistance of poly(lactic acid) with nano-ZIF-8@GO. J. Mater. Sci. 2018, 53, 7083–7093. [Google Scholar] [CrossRef]
- Shekhah, O.; Swaidan, R.; Belmabkhout, Y.; Du Plessis, M.; Jacobs, T.; Barbour, L.J.; Pinnau, I.; Eddaoudi, M. The liquid phase epitaxy approach for the successful construction of ultra-thin and defect-free ZIF-8 membranes: Pure and mixed gas transport study. Chem.Commun. 2014, 50, 2089. [Google Scholar] [CrossRef]
- Sardar, P.; Bhattacharya, G.; Manna, R.; Raj, S.; Rahut, S.; Nath Samanta, A. Excellent CO2 Adsorption Performance of Amine-Impregnated Highly Porous ZIF-8 Adsorbent: Experimental and Isotherm Modeling Studies. Adv. Powder Technol. 2024, 35, 104344. [Google Scholar] [CrossRef]
- Butova, V.V.; Budnyk, A.P.; Bulanova, E.A.; Lamberti, C.; Soldatov, A.V. Hydrothermal Synthesis of High Surface Area ZIF-8 with Minimal Use of TEA. Solid State Sci. 2017, 69, 13–21. [Google Scholar] [CrossRef]
- Alamdari, S.; Sasani Ghamsari, M.; Lee, C.; Han, W.; Park, H.H.; Tafreshi, M.J.; Ara, M.H.M. Preparation and Characterization of Zinc Oxide Nanoparticles Using Leaf Extract of Sambucus ebulus. Appl. Sci. 2020, 10, 3620. [Google Scholar] [CrossRef]
- Bashir, S.; Awan, M.S.; Farrukh, M.A.; Naidu, R.; Khan, S.A.; Rafique, N.; Ali, S.; Hayat, I.; Hussain, I.; Khan, M.Z. In-Vivo (Albino Mice) and In-Vitro Assimilation and Toxicity of Zinc Oxide Nanoparticles in Food Materials. Int. J. Nanomed. 2022, 17, 4073–4085. [Google Scholar] [CrossRef]
- Jayarambabu, N.; Kumari, B.S.; Rao, K.V.; Prabhu, Y.T. Germination and Growth Characteristics of Mungbean Seeds (Vigna radiata L.) Affected by Synthesized Zinc Oxide Nanoparticles. Int. J. Curr. Eng. Technol. 2014, 4, 3411–3416. [Google Scholar]
- Phan, A.; Doonan, C.J.; Uribe-Romo, F.J.; Knobler, C.B.; O’Keeffe, M.; Yaghi, O.M. Synthesis, Structure, and Carbon Dioxide Capture Properties of Zeolitic Imidazolate Frameworks. Acc. Chem. Res. 2010, 43, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xian, S.; Xi, H.; Wang, H.; Li, Z. Improvement of CO2 Adsorption on ZIF-8 Crystals Modified by Enhancing Basicity of Surface. Chem. Eng. Sci. 2011, 66, 4073–4080. [Google Scholar] [CrossRef]
- Ye, Y.; Ma, Z.; Lin, R.; Krishna, R.; Zhou, W.; Lin, Q.; Zhang, Z.; Xiang, S.; Chen, B. Pore space partition within a metal-organic framework for highly efficient C2H2/CO2 separation. J. Am. Chem. Soc. 2019, 141, 4130–4136. [Google Scholar] [CrossRef]
- Chen, C.; Feng, N.; Guo, Q.; Li, Z.; Li, X.; Ding, J.; Wang, L.; Wan, H.; Guan, G. Template-directed fabrication of MIL-101(Cr)/mesoporous silica composite: Layer-packed structure and enhanced performance for CO2 capture. J. Colloid Interf. Sci. 2018, 513, 891–902. [Google Scholar] [CrossRef]
- Fan, W.; Wang, X.; Liu, X.; Xu, B.; Zhang, X.; Wang, W.; Wang, X.; Wang, Y.; Dai, F.; Yuan, D.; et al. Regulating C2H2 and CO2 storage and separation through pore environment modification in a microporous Ni-MOF. ACS Sustain. Chem. Eng. 2018, 7, 2134–2140. [Google Scholar] [CrossRef]
- Lv, D.; Chen, J.; Yang, K.; Wu, H.; Chen, Y.; Duan, C.; Wu, Y.; Xiao, J.; Xi, H.; Li, Z.; et al. Ultrahigh CO2/CH4 and CO2/N2 adsorption selectivities on a cost-effectively L-aspartic acid-based metal-organic framework. Chem. Eng. J. 2019, 375, 122074. [Google Scholar] [CrossRef]
- Qian, X.; Ren, Q.; Wu, X.; Sun, J.; Wu, H.; Lei, J. Enhanced water stability in Zn-doped zeolitic imidazolate framework-67 (ZIF-67) for CO2 capture applications. ChemistrySelect 2018, 3, 657–661. [Google Scholar] [CrossRef]
- Sun, J.; Li, Q.; Chen, G.; Duan, J.; Liu, G.; Jin, W. MOF-801 incorporated PEBA mixed-matrix composite membranes for CO2 capture. Sep. Purif. Technol. 2019, 217, 229–239. [Google Scholar] [CrossRef]
- Zhang, G.; Wei, G.; Liu, Z.; Oliver, S.R.J.; Fei, H. A robust sulfonate-based metal-organic framework with permanent porosity for efficient CO2 capture and conversion. Chem. Mater. 2016, 28, 6276–6281. [Google Scholar] [CrossRef]
- Prasad, T.K.; Suh, M.P. Control of interpenetration and gas-sorption properties of metal-organic frameworks by a simple change in ligand design. Chem. Eur. J. 2012, 18, 8673–8680. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Sun, F.; Aguila, B.; Perman, J.A.; Ma, S.; Zhu, G. A bifunctional metal-organic framework featuring the combination of open metal sites and Lewis basic sites for selective gas adsorption and heterogeneous cascade catalysis. J. Mater. Chem. A 2016, 4, 15240–15246. [Google Scholar] [CrossRef]
- Luconi, L.; Mercuri, G.; Islamoglu, T.; Fermi, A.; Bergamini, G.; Giambastiani, G.; Rossin, A. Benzothiazolium-functionalized NU-1000: A versatile material for carbon dioxide adsorption and cyanide luminescent sensing. J. Mater. Chem. C 2020, 8, 7492–7500. [Google Scholar] [CrossRef]
- Lawson, S.; Griffin, C.; Rapp, K.; Rownaghi, A.A.; Rezaei, F. Amine-functionalized MIL-101 monoliths for CO2 removal from enclosed environments. Energy Fuels 2019, 33, 2399–2407. [Google Scholar] [CrossRef]
- Ma, H.; Wang, Z.; Zhang, X.F.; Ding, M.; Yao, J. In situ growth of amino-functionalized ZIF-8 on bacterial cellulose foams for enhanced CO2 adsorption. Carbohydr. Polym. 2021, 270, 118376. [Google Scholar] [CrossRef]
- Yang, F.; Ge, T.; Zhu, X.; Wu, J.; Wang, R. Study on CO2 capture in humid flue gas using amine-modified ZIF-8. Sep. Purif. Technol. 2022, 287, 120535. [Google Scholar] [CrossRef]
- Aliev, S.B.; Samsonenko, D.G.; Maksimovskiy, E.A.; Fedorovskaya, E.O.; Sapchenko, S.A.; Fedin, V.P. Polyaniline-intercalated MIL-101: Selective CO2 sorption and supercapacitor properties. New J. Chem. 2016, 40, 5306–5312. [Google Scholar] [CrossRef]
- Lin, Y.; Yan, Q.; Kong, C.; Chen, L. Polyethyleneimine incorporated metal-organic frameworks adsorbent for highly selective CO2 capture. Sci. Rep. 2013, 3, 1859. [Google Scholar] [CrossRef] [PubMed]
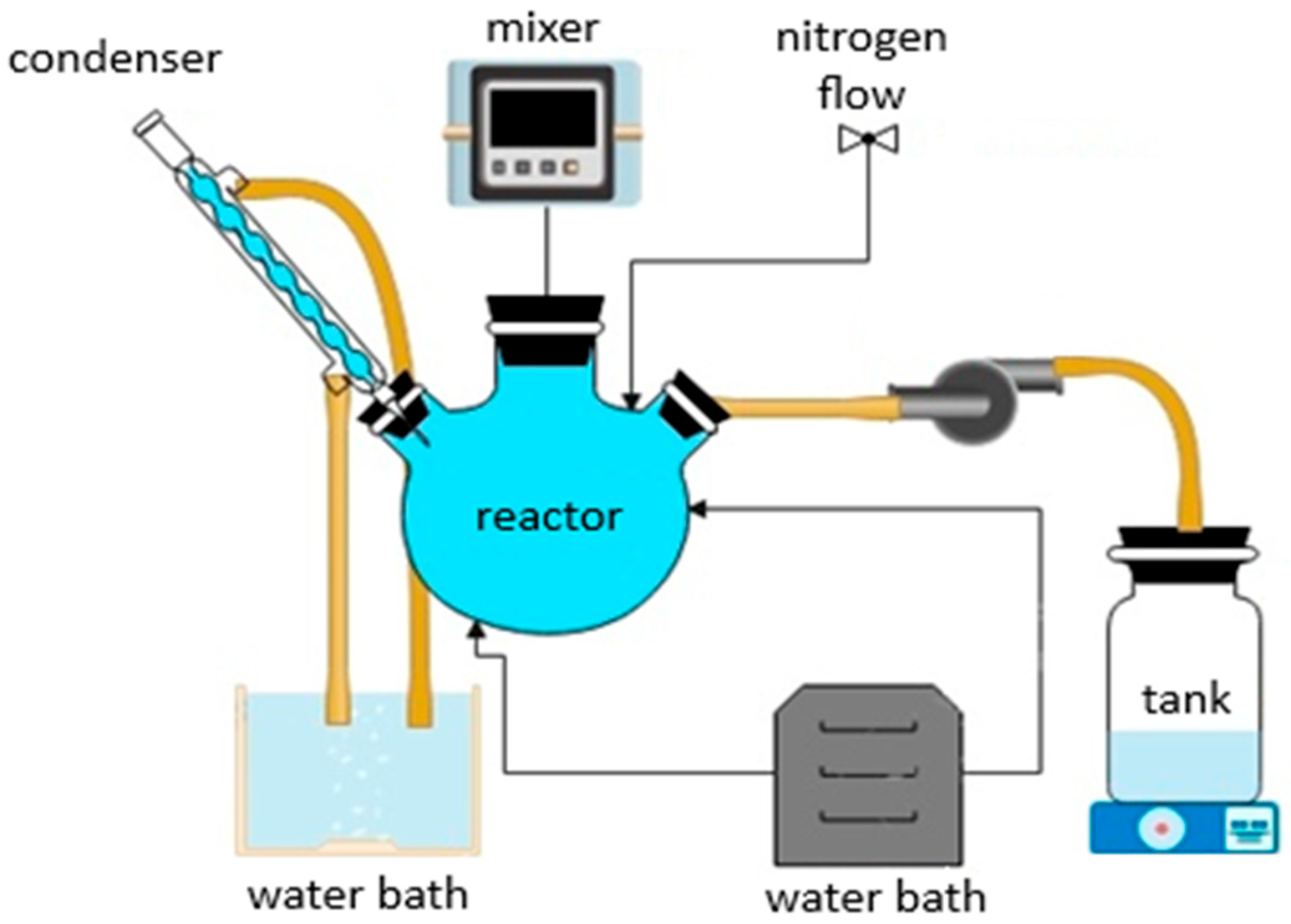

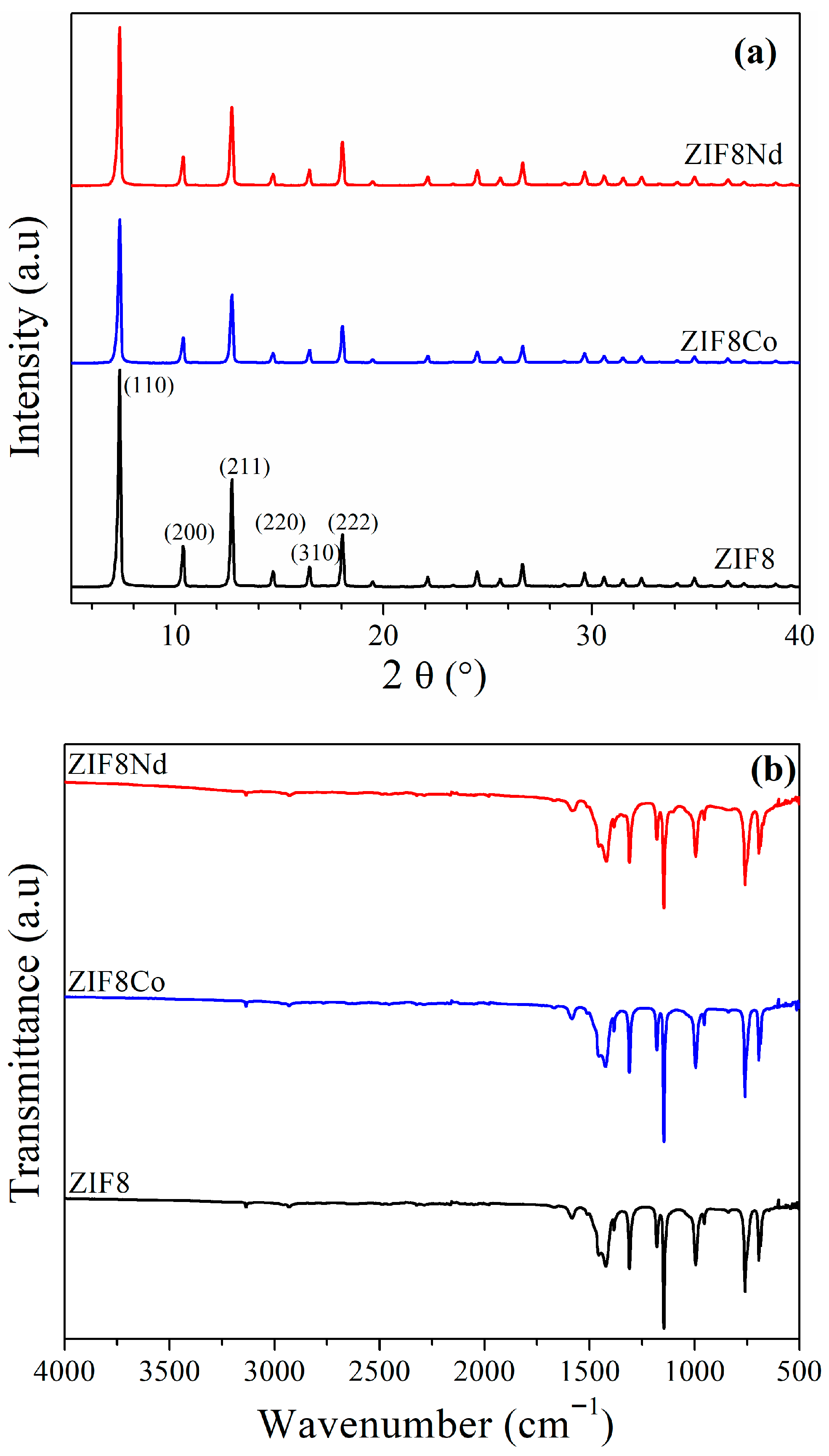
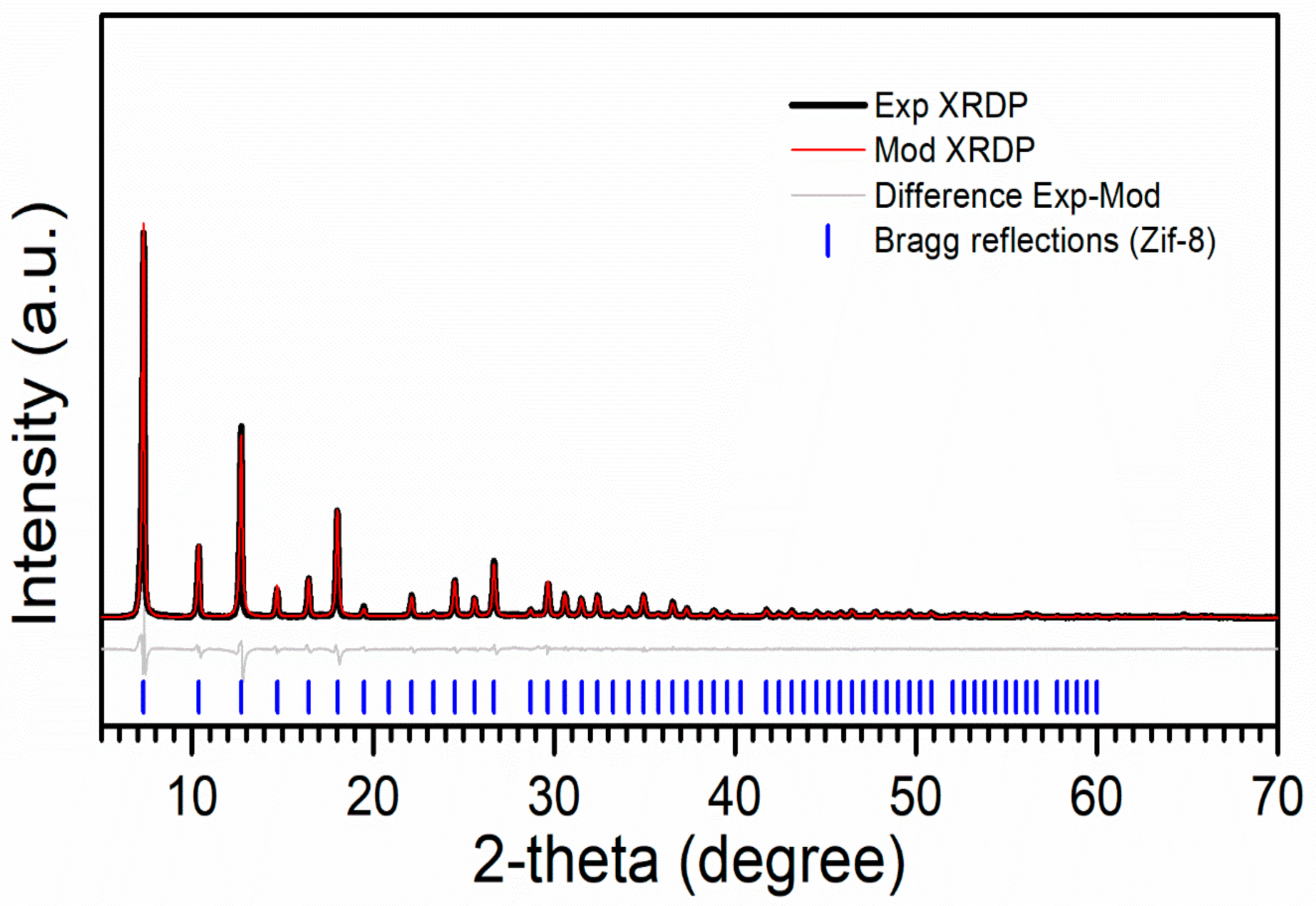

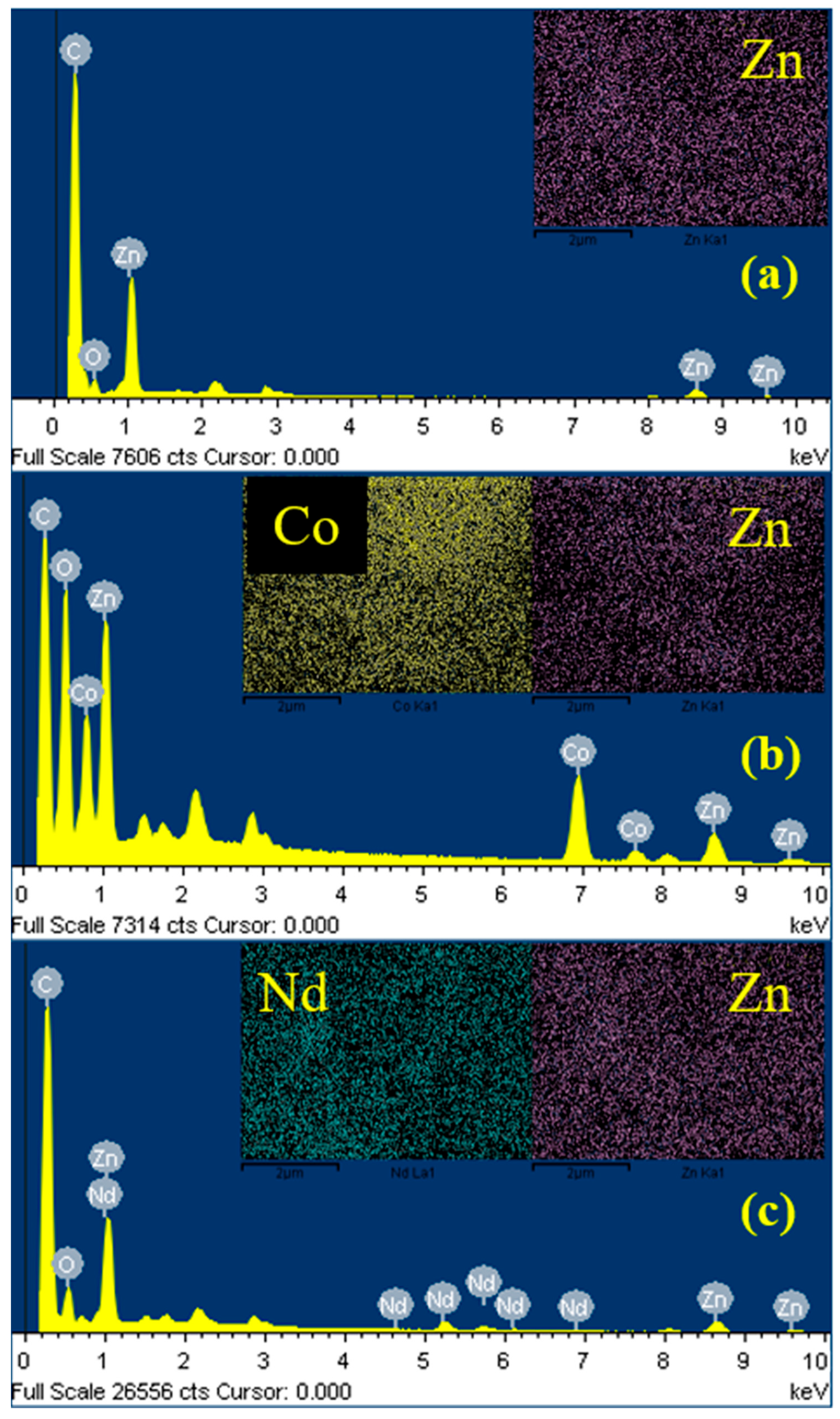
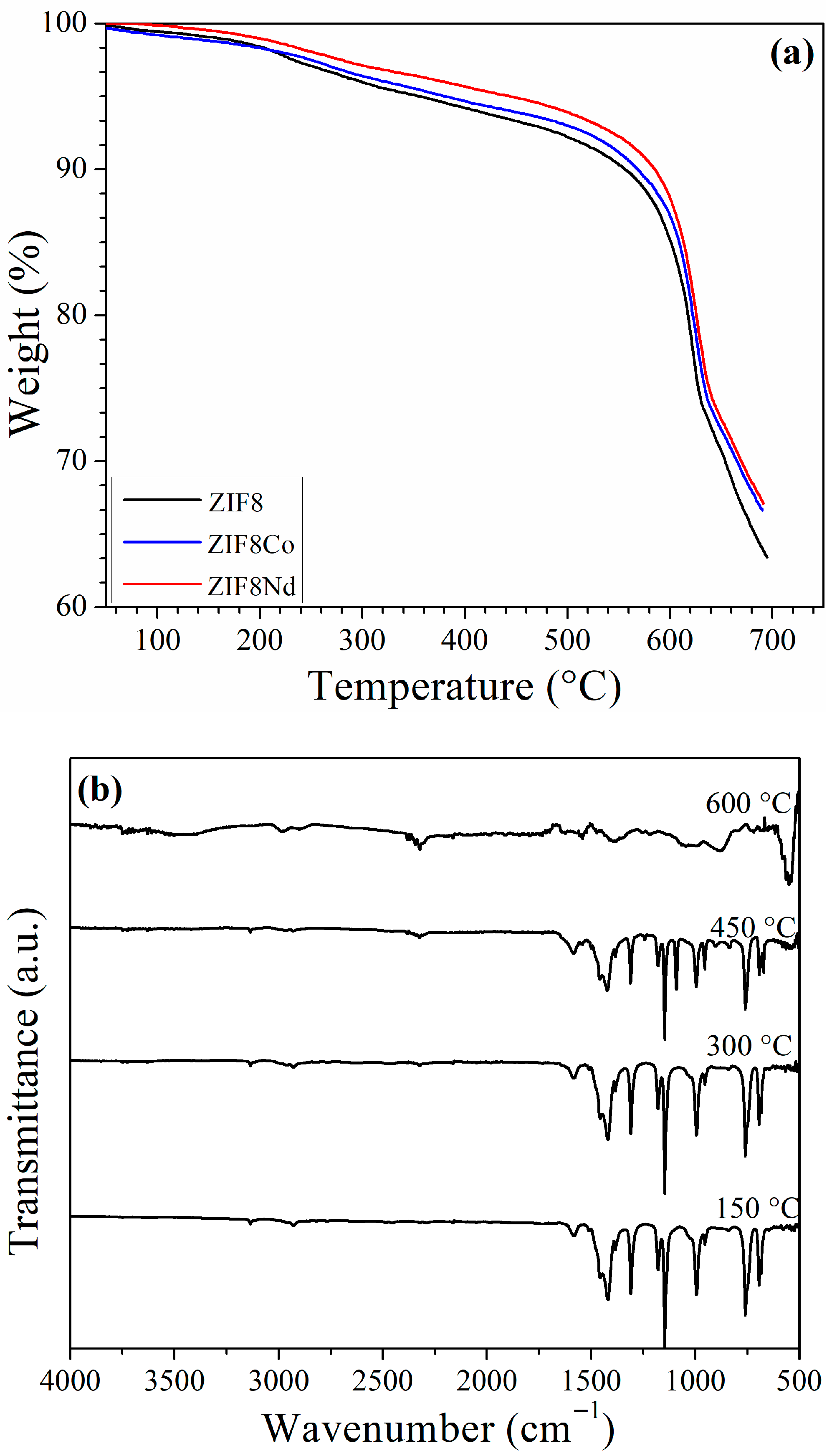
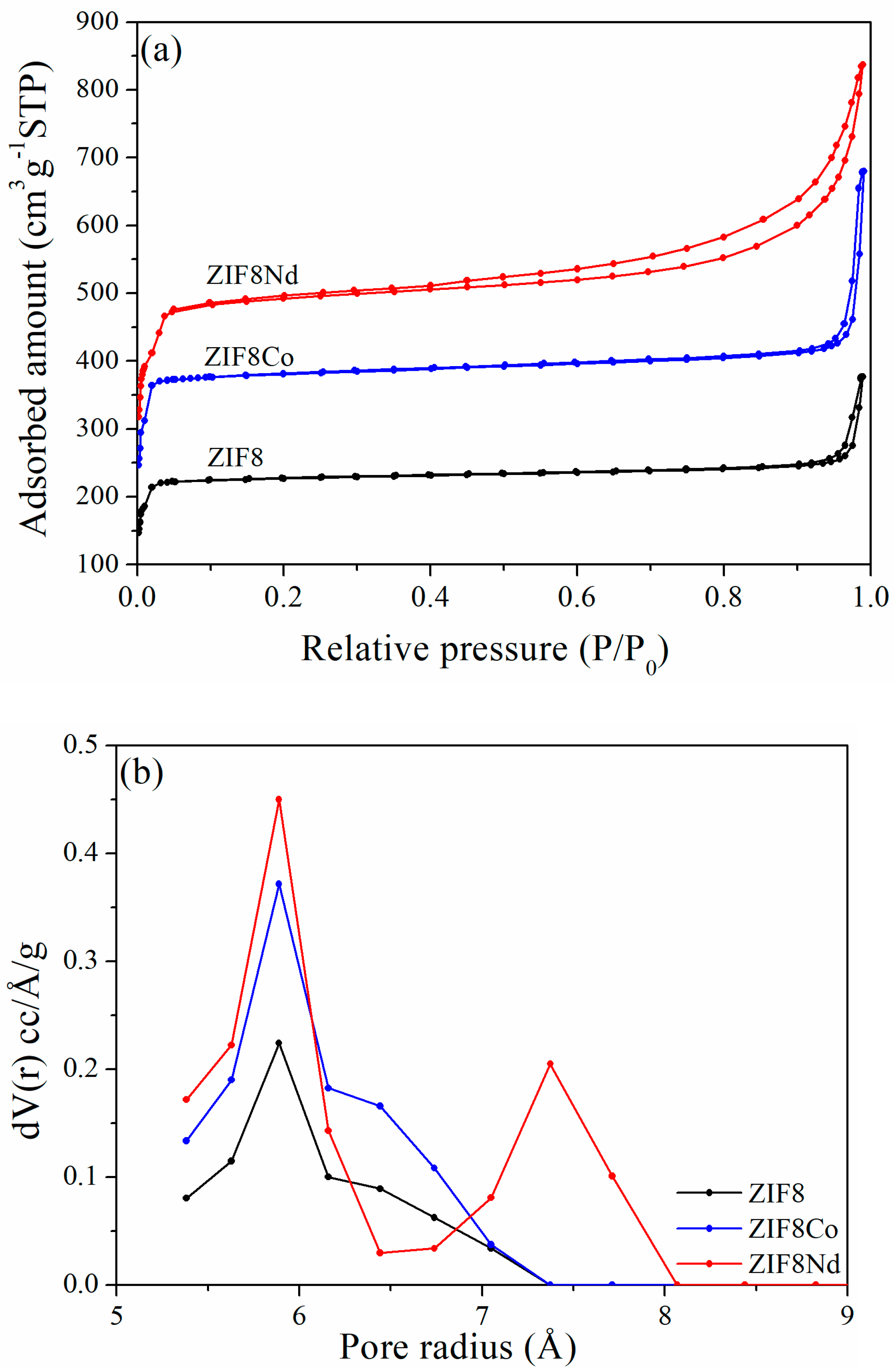
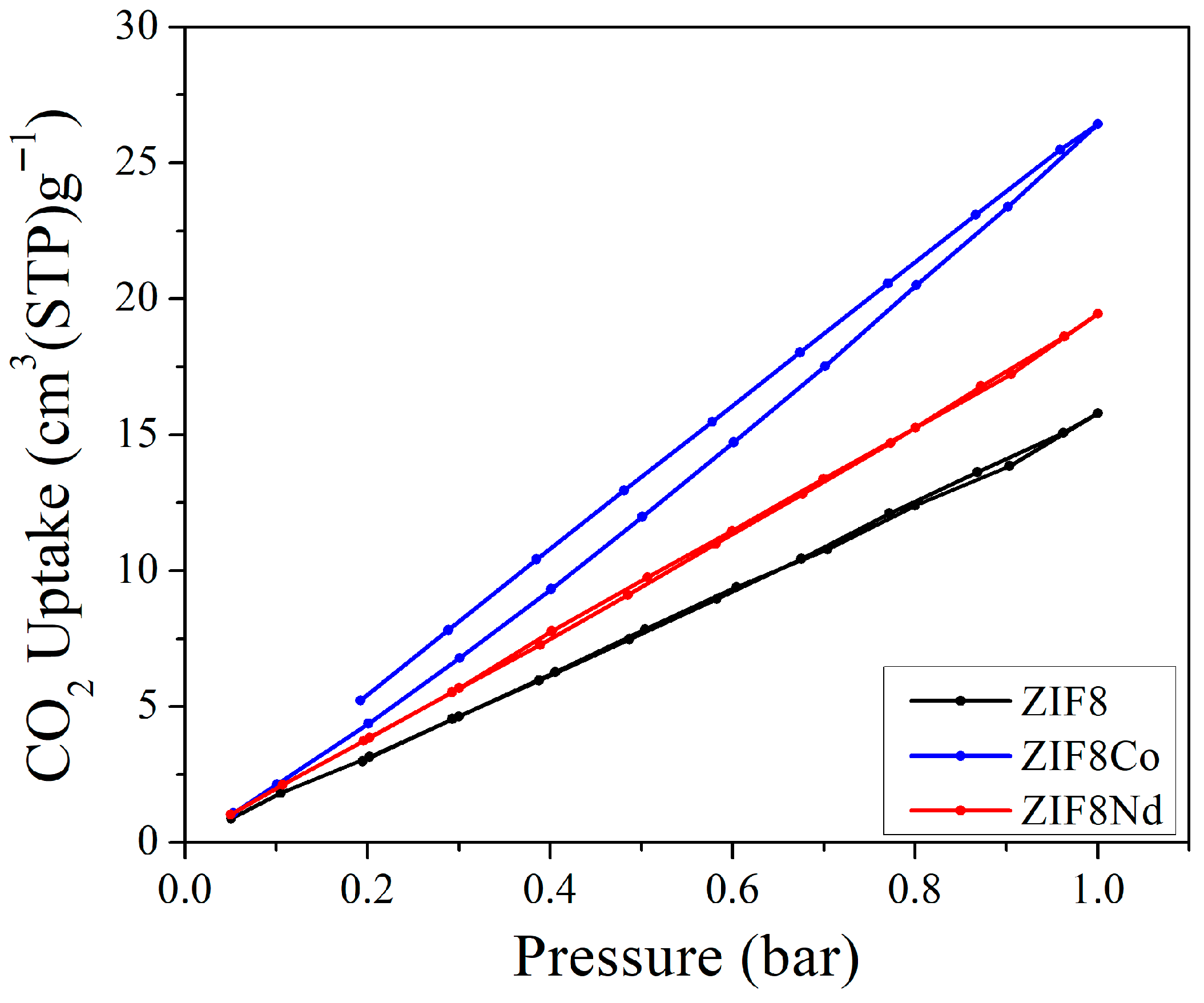
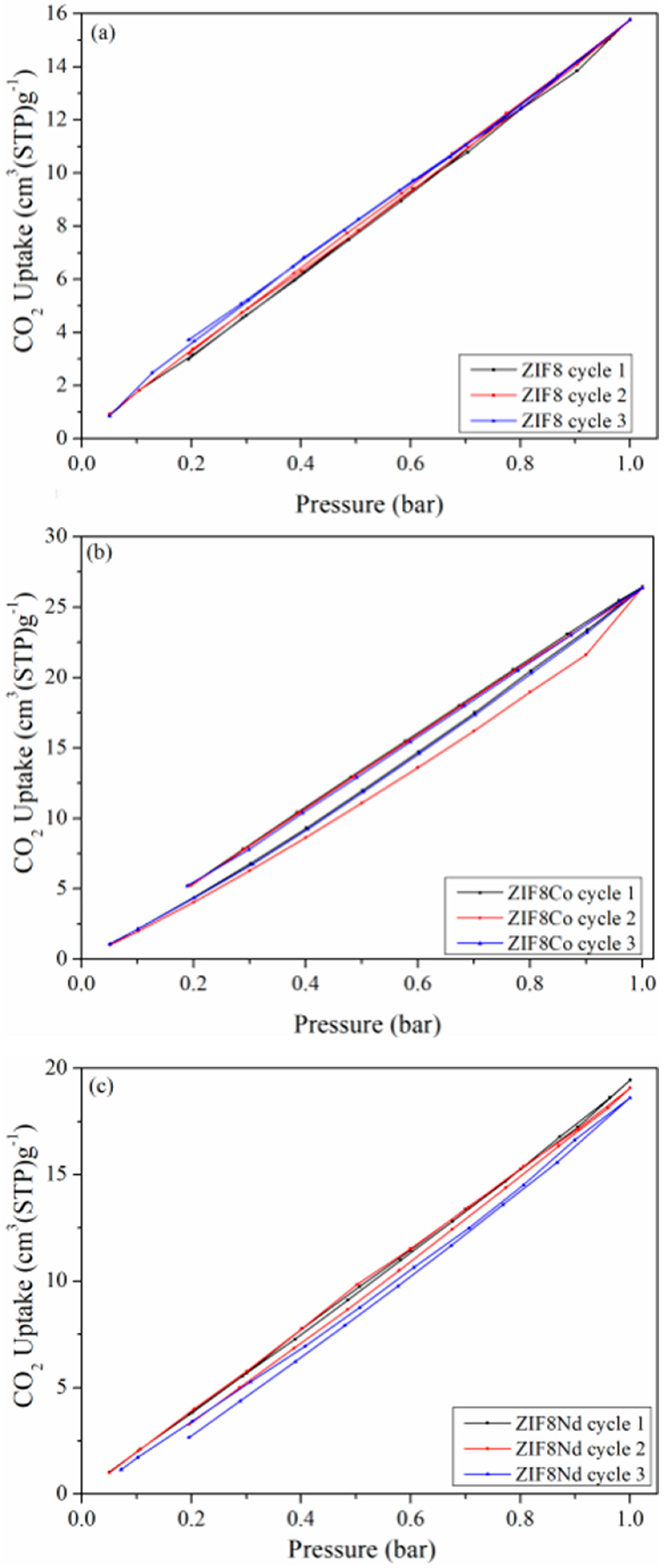
| Reagent | Glass Reactor (g) | Tank (g) |
|---|---|---|
| Surfactant solution A (0.5 wt%) | 0.6 | -- |
| Surfactant solution B (3.73 wt%) | -- | 20 |
| Acrylonitrile monomer | -- | 40 |
| Initiator solution (2.0 wt%) | 1.2 | 6.5 |
| Deionized water | 32 | -- |
| Sample | Cell Parameter a (nm) | Atomic Position (Fracc) | |||
|---|---|---|---|---|---|
| C1 | C2 | C3 | N | ||
| ZIF-8 (Reference [44]) | 1.7033 | x = 0.36910 | x = 0.3760 | x = 0.40520 | x = 0.4100 |
| y = 0.10140 | y = 0.99350 | y = 0.91430 | y = 0.03182 | ||
| z = 0.68660 | z = 0.6230 | z = 0.59470 | z = 0.68241 | ||
| ZIF8 | 1.7031 (31) | x = 0.3673(33) | x = 0.3309(18) | x = 0.4212(15) | x = 0.3720(21) |
| y = 0.1044(17) | y = 1.0213(16) | y = 0.9414(30) | y = 0.0592(35) | ||
| z = 0.6833(26) | z = 0.5930(17) | z = 0.6199(14) | z = 0.6955(16) | ||
| ZIF8Co | 1.7026 (32) | x = 0.3529(38) | x = 0.3351(27) | x = 0.4122(38) | x = 0.3901(22) |
| y = 0.0939(39) | y = 1.0231(10) | y = 0.9221(21) | y = 0.0733(26) | ||
| z = 0.6851(36) | z = 0.5894(20) | z = 0.6131(40) | z = 0.6921(15) | ||
| ZIF8Nd | 1.7033 (34) | x = 0.3775(21) | x = 0.3427(24) | x = 0.3862(24) | x = 0.3994(32) |
| y = 0.0965(29) | y = 1.0067(24) | y = 0.8988(22) | y = 0.0092(33) | ||
| z = 0.6835(25) | z = 0.6006(23) | z = 0.5862(20) | z = 0.7025(34) | ||
| Sample | Cell Volume (nm3) | Crystallite Size (nm) | ||
|---|---|---|---|---|
| (1 1 0) | (2 0 0) | (2 22) | ||
| ZIF8 | 4.940 | 102.8 ± 0.8 | 82.8 ± 1.7 | 111.8 ± 1.5 |
| ZIF8Co | 4.936 | 87.3 ± 0.6 | 72.5 ± 1.3 | 93.8 ± 1.1 |
| ZIF8Nd | 4.941 | 92.5 ± 0.7 | 75.5 ± 1.4 | 100.0 ± 1.2 |
| Sample | Specific Surface Area a SBET (m2·g−1) | Average Pore Size b Pore Diameter (Å) | Pore Volume Vtotal (cm3·g−1) |
|---|---|---|---|
| ZIF8 | 699.6 | 11.72 | 0.38 |
| ZIF8Co | 1178.2 | 11.77 | 0.63 |
| ZIF8Nd | 1474.7 | 11.81 | 1.05 |
| Specific Surface Area (BET Model) (m2/g) | CO2 Uptake (mmol/g) | CO2 Uptake (cm3/g) | Ref. | |
|---|---|---|---|---|
| FJU-88a (FJU-88-derived adsorbent) | 3.68 | 0.49 | 10.98 | [52] |
| ZIF-8 | 1645 | 1.39 | 31.1 | [14] |
| MIL-101 (Cr) | 2166 | 1.17 | 26.22 | [53] |
| UPC-110 | 1384 | 1.08 | 24.21 | [54] |
| MIP-202 | 279 | 0.55 | 12.33 | [55] |
| ZIF-67 | 2189 | 1.19 | 26.6 | [56] |
| MOF-801 | 839 | 1.35 | 30.3 | [57] |
| MOF-508b (Cu) | 364 | 0.58 | 13 | [58] |
| SNU-70 | 5290 | 0.80 | 17.93 | [59] |
| SNU-71 | 1770 | 1.05 | 23.53 | [59] |
| JUC-199 | 821 | 1.78 | 39.90 | [60] |
| NU-1000-BzTz | 1530 | 2.00 | 44.83 | [61] |
| ZIF8 | 699.6 | 0.71 | 15.78 | This work |
| ZIF8Nd | 1474.7 | 0.87 | 19.44 | This work |
| ZIF8Co | 1178.2 | 1.18 | 26.48 | This work |
| Sample | ZIF-8 Loading After Secondary Growth (wt%) | |
|---|---|---|
| Seeded Fibers | Non-Seeded Fibers | |
| ZIF8PAN-f | 27.56 | 21.38 |
| ZIF8CoPAN-f | 25.12 | 20.63 |
| ZIF8NdPAN-f | 24.23 | 19.59 |
| Material | MOF (Filler) | Polymer Matrix | Total Filler Amount (wt%) | CO2 Capture Capacity (mmol/g) | Ref. |
|---|---|---|---|---|---|
| TEPA-MIL-101MA | MIL-101(Cr) | Tetraethylenepentamine (TEPA) | — | 0.3 | [62] |
| BCZ-0.5 | ZIF-8-NH2 | Bacterial cellulose (BC) | — | 0.44 | [63] |
| ZIF8-PEI-10% | ZIF-8 | Polyethanolamine (PEI) | 90 | 0.46 | [64] |
| ZIF8-PEI-30% | ZIF-8 | Polyethanolamine (PEI) | 70 | 1.40 | [64] |
| PANI@MIL-101(Cr) | MIL-101(Cr) | Polyaniline (PANI) | 70 | 1.62 | [65] |
| PEI-MIL-101-50 | MIL-101 | Polyethanolamine (PEA) | 50 | 4.0 | [66] |
| PEI-MIL-101-75 | MIL-101 | Polyethanolamine (PEA) | 75 | 4.64 | [66] |
| ZIF8PAN-f | ZIF-8 | Polyacrylonitrile (PAN) | 30.06 | 0.32 | This work |
| ZIF8CoPAN-f | ZIF-8 (doped Co) | Polyacrylonitrile (PAN) | 27.62 | 0.36 | This work |
| ZIF8NdPAN-f | ZIF-8 (doped Nd) | Polyacrylonitrile (PAN) | 26.73 | 0.34 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargas-Romero, D.; Ovalle-Encinia, O.; Rojas-García, E.; Maubert-Franco, A.M.; Corea, M.; Téllez-Jurado, L.; Ortiz-Landeros, J. Synthesis and CO2 Capture Properties of Co- and Nd-Modified ZIF-8 Materials Loaded onto Electrospun Polyacrylonitrile Fibers. Separations 2025, 12, 248. https://doi.org/10.3390/separations12090248
Vargas-Romero D, Ovalle-Encinia O, Rojas-García E, Maubert-Franco AM, Corea M, Téllez-Jurado L, Ortiz-Landeros J. Synthesis and CO2 Capture Properties of Co- and Nd-Modified ZIF-8 Materials Loaded onto Electrospun Polyacrylonitrile Fibers. Separations. 2025; 12(9):248. https://doi.org/10.3390/separations12090248
Chicago/Turabian StyleVargas-Romero, Daniela, Oscar Ovalle-Encinia, Elizabeth Rojas-García, Ana Marisela Maubert-Franco, Mónica Corea, Lucía Téllez-Jurado, and José Ortiz-Landeros. 2025. "Synthesis and CO2 Capture Properties of Co- and Nd-Modified ZIF-8 Materials Loaded onto Electrospun Polyacrylonitrile Fibers" Separations 12, no. 9: 248. https://doi.org/10.3390/separations12090248
APA StyleVargas-Romero, D., Ovalle-Encinia, O., Rojas-García, E., Maubert-Franco, A. M., Corea, M., Téllez-Jurado, L., & Ortiz-Landeros, J. (2025). Synthesis and CO2 Capture Properties of Co- and Nd-Modified ZIF-8 Materials Loaded onto Electrospun Polyacrylonitrile Fibers. Separations, 12(9), 248. https://doi.org/10.3390/separations12090248







